Introduction
Previous work has determined that aggressive
melanomas express Nodal, an embryonic morphogen belonging to the
TGF-β family of growth factors (1).
It was demonstrated that Nodal expression increases during the
progression of melanoma, from superficial, radial growth phase to
deeper, vertical growth phase to advanced stage, metastatic
melanoma (1). A subsequent report
confirmed initial observations of lower Nodal expression in
superficial melanoma compared to robust expression in deep melanoma
and metastatic melanoma (2).
Furthermore, it has been demonstrated that normal and dysplastic
nevi expressed lower levels of Nodal (2). A well-known risk factor for melanoma is
the number and types of melanocytic nevi, indicating that these may
represent precursor lesions to melanoma (3). In fact, patients diagnosed with familial
dysplastic nevi syndrome who exhibit a large number of melanocytic
nevi, sometimes >50, have shown an increased risk for developing
melanoma (4). It is desirable,
therefore, to characterize the molecular profile of excised nevi
and determine unique expression pattern(s), which may facilitate
screening of patients with an increased likelihood for developing
melanoma.
Since Nodal is a secreted molecule, it may have the
potential for paracrine effects by influencing the homeostasis of
cells that surround or are distant to the secretory source of this
powerful morphogen. This is in agreement with the phenomenon known
as ‘field cancerization’ whereby abnormalities, such as genetic or
epigenetic alterations or changes in growth factor and receptor
expression profiles, have been documented in histologically normal
tissues distant from areas of overt cancer (5). Given the positive ‘feed-forward’
inductive characteristics of Nodal signaling (6), we hypothesize that Nodal secreted from a
melanoma induce its own expression in otherwise nonmalignant nevi
located in regional or distant sites causing phenotypic changes in
these benign lesions, which may lead to increased propensity of
these lesions towards malignant transformation. In this
retrospective study, the immunohistochemical expression patterns of
Nodal were determined, together with Cripto-1, the co-receptor for
Nodal (7), and Notch4, a stem
cell-associated molecule with two RBPJ binding sites on the Nodal
gene shown to be associated with Nodal expression in melanoma
(8). These findings were correlated
with available clinical data, including history of subsequent
melanoma development.
Materials and methods
De-identified slides with tissue sections of human
malignant and nonmalignant melanocytic lesions for
immunohistochemistry (IHC) were collected in compliance with
Institutional Review Board ethics committee approval of study
design and protocols for patient consent from Lurie Children's
Hospital, Northwestern University, Chicago, IL, USA (approval no.
2010-14126), University of Michigan, Ann Arbor, MI, USA (approval
no. HUM00045834) and Istituto Nazionale Tumori (INT) Fondazione
Pascale, Naples, Italy (approval no. DMTMT-OMTI-34-2011). Slides
from a total of 225 individual cases were collected from the
following sources: University of Michigan (nonmalignant negative
history of melanoma, n=12; nonmalignant positive history of
melanoma, n=16; malignant melanoma, N=33); INT Fondazione Pascale
(nonmalignant negative history of melanoma, n=65); histological
slides purchased from Northwestern University Department of
Dermatology Tissue Core (nonmalignant negative history of melanoma
n=50; nonmalignant positive history of melanoma, n=49). The
de-identified slides were stained by IHC for Nodal, Cripto-1 and
Notch4, following the procedure previously described (8). Briefly, following antigen retrieval and
blocking steps, sections were incubated in primary antibody for 60
min [mouse monoclonal anti-Nodal (1:200 dilution; cat no. ab55676;
Abcam, Cambridge, MA, USA); rabbit polyclonal anti-Notch4 (1:80
dilution; cat no. sc-5594; Santa Cruz Biotechnology, Inc., Dallas,
TX, USA); rabbit polyclonal anti-Cripto-1 (1:1500 dilution; cat no.
600-401-997; Rockland Scientific International Inc., Limerick, PA,
USA)], followed by ready-to-use anti-rabbit (cat no. GR602H) or
anti-mouse (cat no. GM601H) biotinylated secondary antibody
(Biocare Medical Inc., Concord, CA, USA), and then
streptavidin-horseradish peroxidase (ThermoFisher Scientific Inc.,
Waltham, MA, USA). Color was developed with 3,3′-diaminobenzidine
substrate (ThermoFisher Scientific Inc.) and sections were
counterstained with hematoxylin (Biocare Medical Inc.). As a
negative control, adjacent serial sections were incubated with
species appropriate irrelevant IgG (Jackson ImmunoResearch Labs) at
the same concentration as primary antibodies. The quality and
intensity of staining were analyzed and scored at low power and
high power in order to calculate an Index Score (IS), as previously
described (2). Student's
t-test, median test and Pearson correlation were used for
statistical analyses of the appropriate data. P<0.05 was
considered to indicate a statistically significant difference.
Results
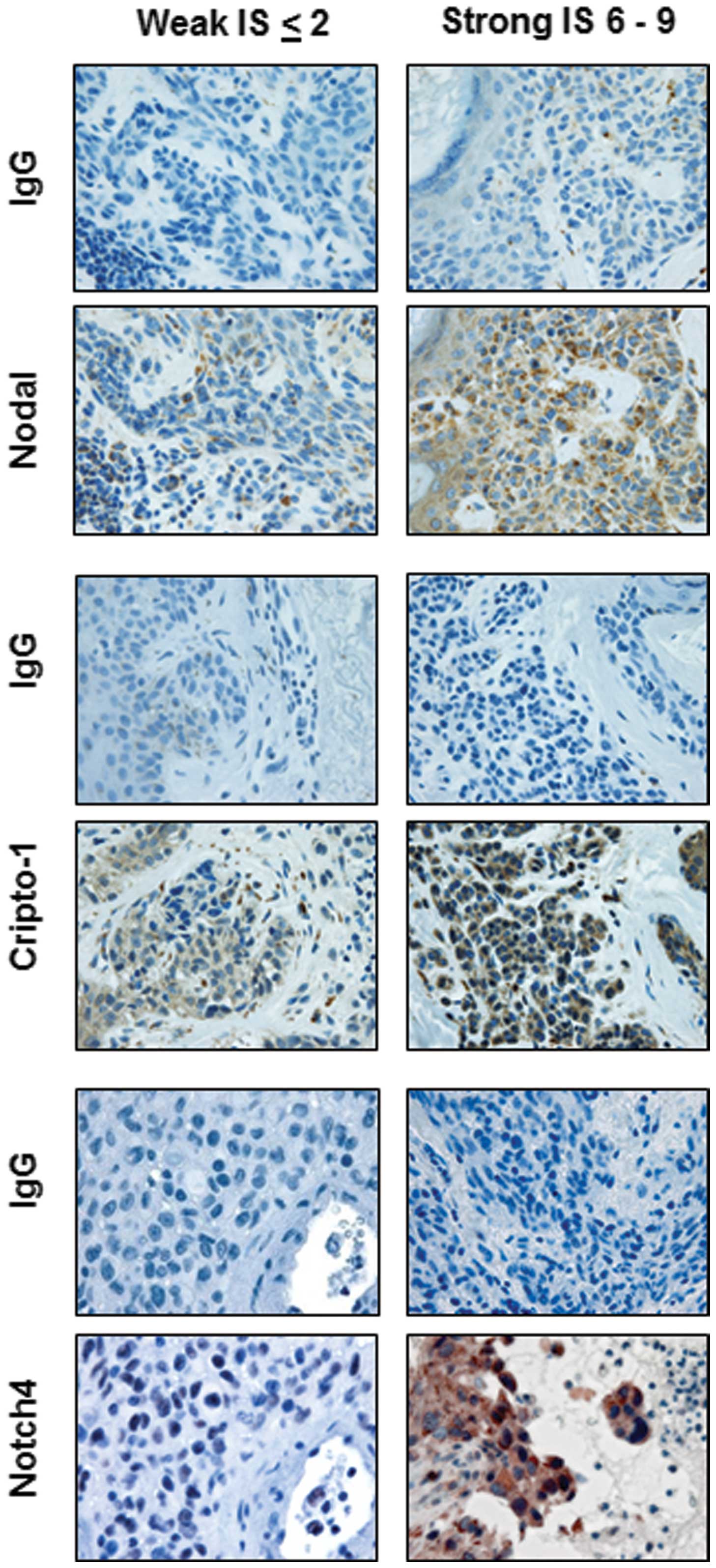
The IHC results demonstrated a broad range of
staining intensities for Nodal, Cripto-1 and Notch4 (Fig. 1). After IHC intensity scores (IS) were
assigned, cases were grouped either as nevi from patients with no
history of melanoma (negative Hx of melanoma) or nevi from patients
that subsequently developed melanoma (positive Hx of melanoma).
Mean IHC IS were calculated for Nodal, Cripto-1 and Notch4 and
overall comparative analysis was performed to determine differences
in expression levels among 4 groups for each marker, as summarized
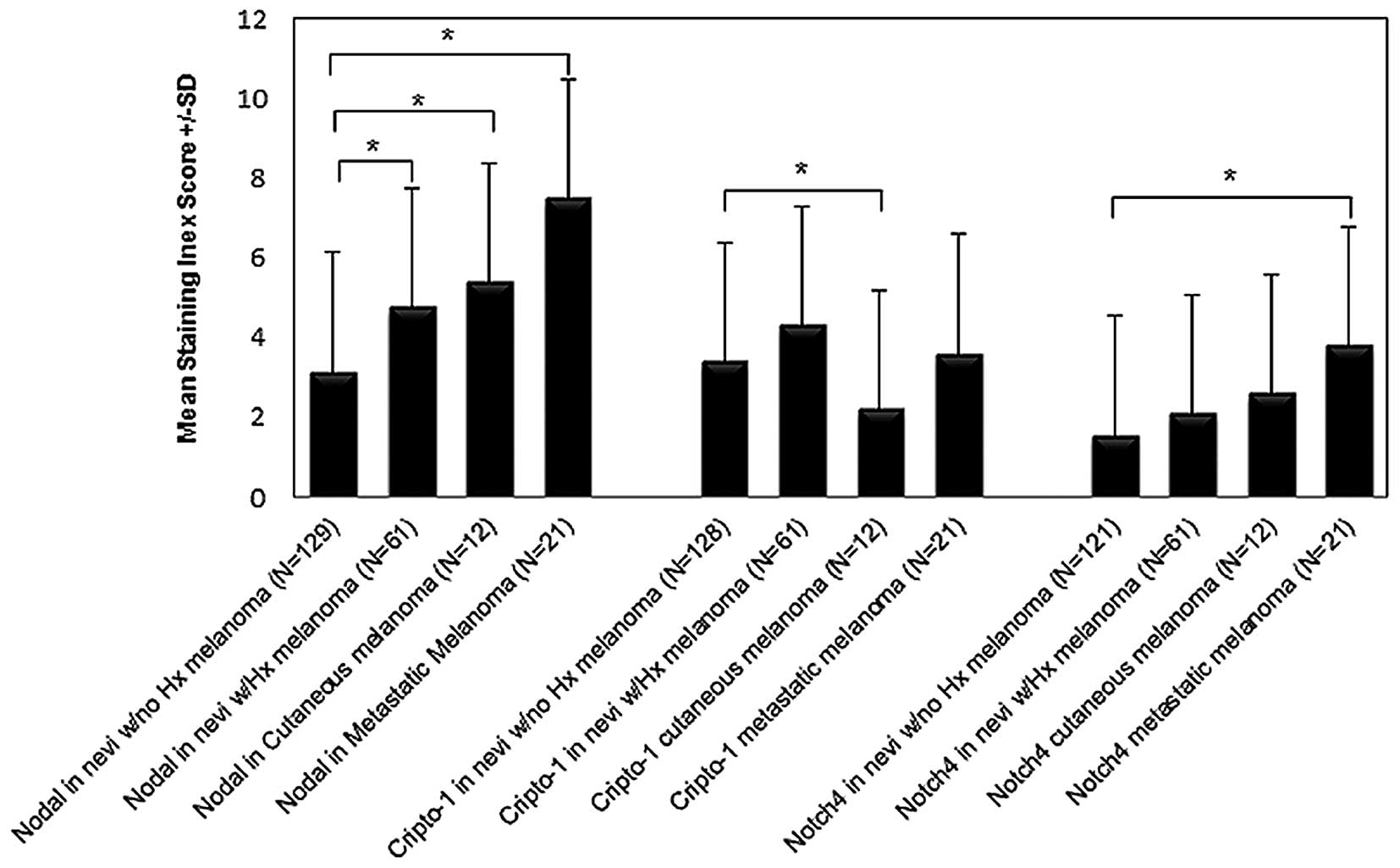
in Table I. The mean level for Nodal
IS progressively increased from nevi with a negative history of
melanoma to nevi with a positive history of melanoma to cutaneous
melanoma to metastatic melanoma. The level of Nodal IS was
significantly different among these groups (Fig. 2: all P<0.05 versus negative history
of melanoma). The same trend of progressive increase from nevi with
a negative history of melanoma to nevi with a positive history of
melanoma to cutaneous melanoma to metastatic melanoma was observed
for Notch4 IS with a statistically significant difference for the
highest mean levels of Notch4 IS in the metastatic melanoma group
(Fig. 2; P<0.05 metastatic
melanoma versus negative history of melanoma). Interestingly, mean
Cripto-1 IS level was significantly greater only in the group of
nevi from patients with a positive history of melanoma compared to
the group of nevi from patients with a negative history of melanoma
(Fig. 2; P<0.05). There was no
significant difference observed in the mean Cripto-1 IS between
melanoma and the other groups. In addition, the cutaneous melanoma
group showed the lowest mean Cripto-1 IS compared with the other
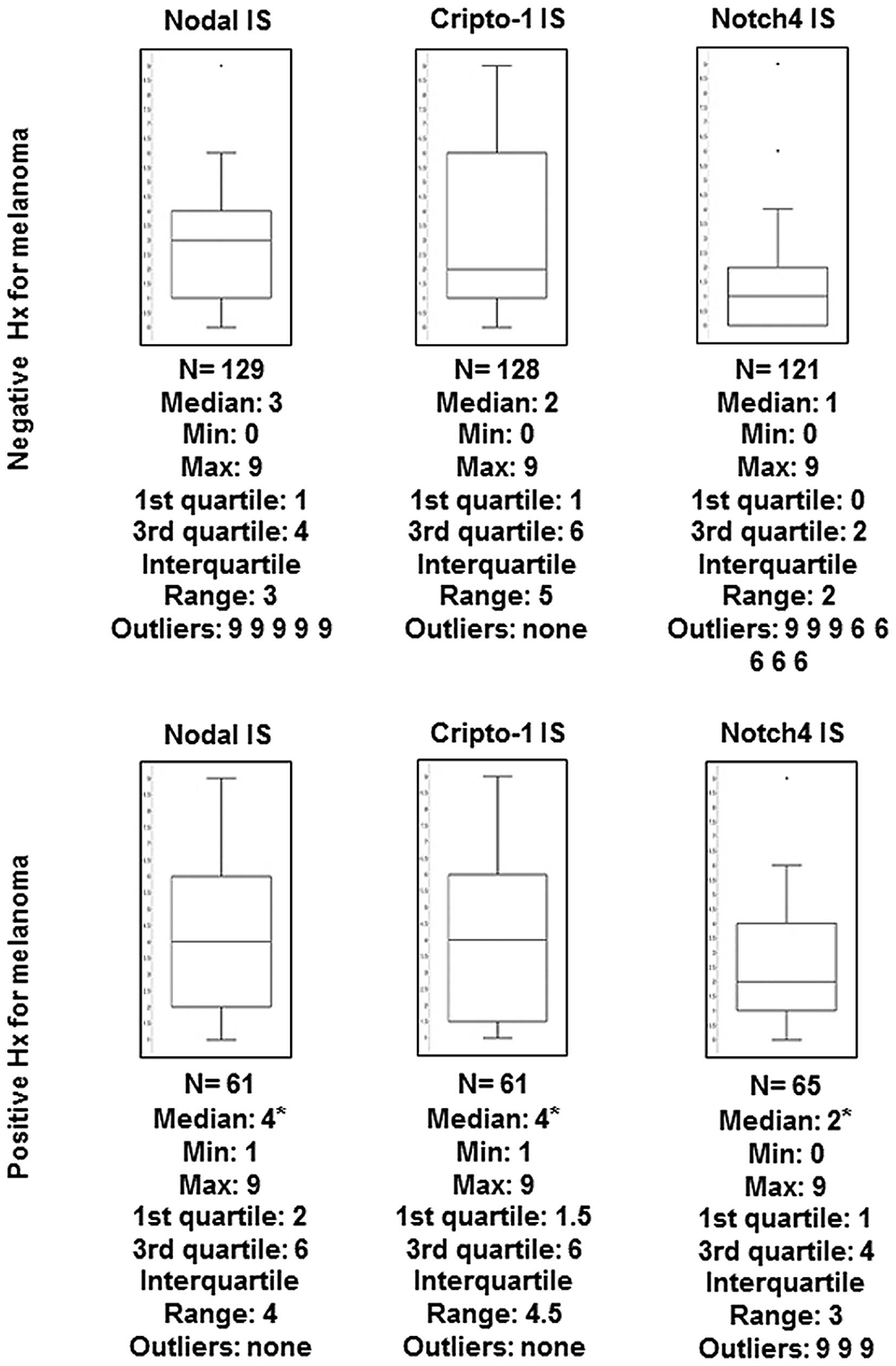
groups (Fig. 2). Since calculation of
mean values can be distorted by outliers, the median IS was also
determined among the different groups for each marker. In fact,
this analysis shows a significantly higher median IS for Nodal,
Cripto-1 and Notch4 in the group of nevi from patients with a
positive history of melanoma compared with the group of nevi from
patients with no history of melanoma (Fig. 3).
 | Table I.Results of intensity of
immunohistochemistry staining for Nodal, Cripto-1 and Notch4,
represented as mean index score, in nevi from patients with a
negative or positive history of melanoma, and cutaneous and
metastatic melanoma |
Table I.
Results of intensity of
immunohistochemistry staining for Nodal, Cripto-1 and Notch4,
represented as mean index score, in nevi from patients with a
negative or positive history of melanoma, and cutaneous and
metastatic melanoma
| Gene and type of
lesion | Mean IS | N | P-valuea |
|---|
| Nodal |
|
|
|
| Nevi,
negative Hx of melanoma | 3.14+/-2.1 | 129 |
|
| Nevi,
positive Hx of melanoma | 4.77+/-2.45 | 61 | <0.01 |
| Cutaneous
melanoma | 5.42+/-2.17 | 12 | <0.01 |
|
Metastatic melanoma | 7.52+/-3.57 | 21 | <0.01 |
| Cripto-1 |
|
|
|
| Nevi,
negative Hx of melanoma | 3.38+/-2.65 | 128 |
|
| Nevi,
positive Hx of melanoma | 4.3 +/-2.98 | 61 | 0.03 |
| Cutaneous
melanoma | 2.17+/-2.41 | 12 | NS |
|
Metastatic melanoma | 3.57+/-3.03 | 21 | NS |
| Notch4 |
|
|
|
| Nevi,
negative Hx of melanoma | 1.55+/-1.96 | 121 |
|
| Nevi,
positive Hx of melanoma | 2.08+/-1.87 | 61 | NS |
| Cutaneous
melanoma | 2.58+/-2.71 | 12 | NS |
|
Metastatic melanoma | 3.81+/-3.03 | 21 | <0.01 |
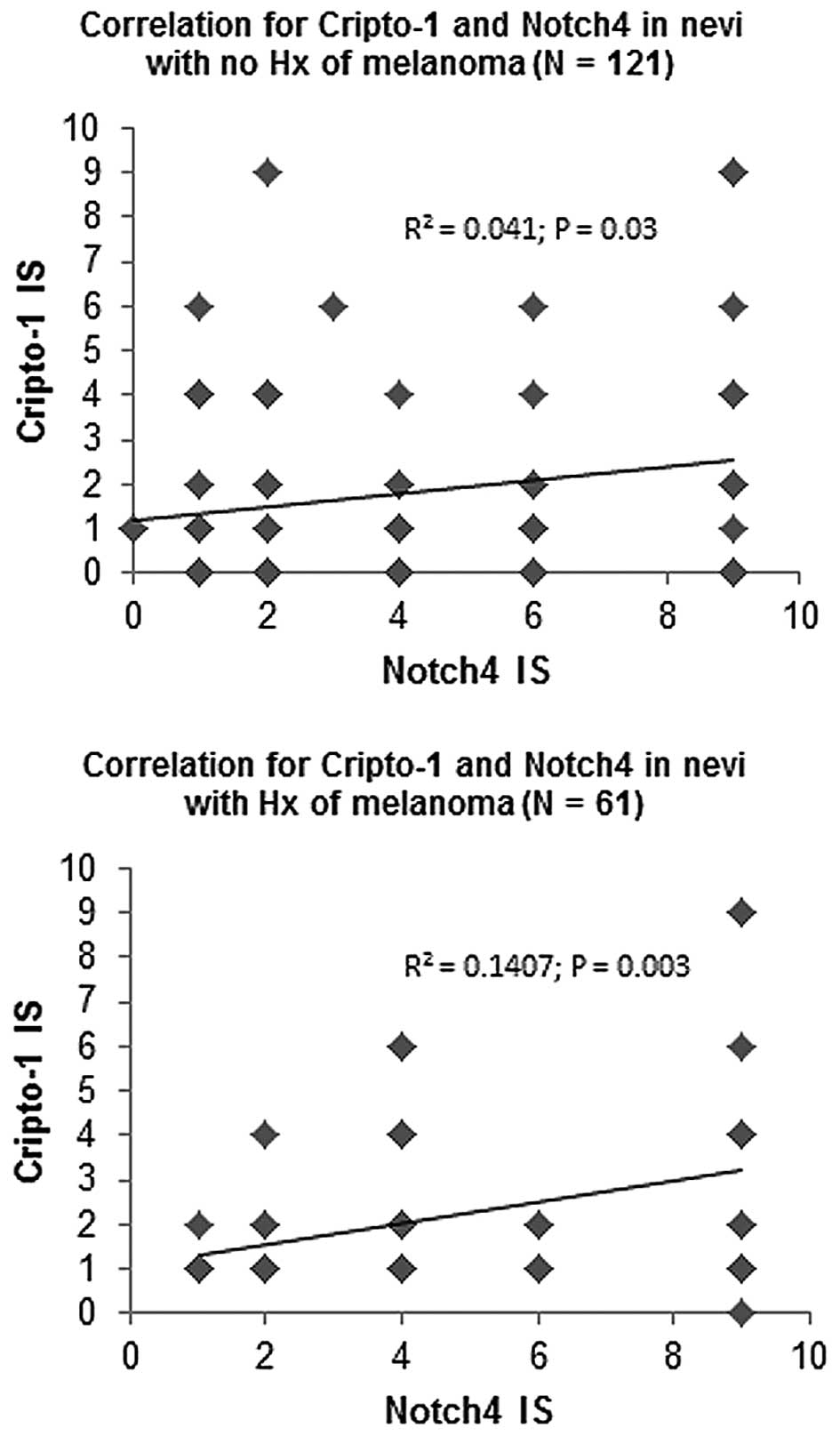
Pearson's correlation analysis was performed to
determine associations between Nodal, Cripto-1 and Notch4
expression levels for each group of nevi. Nodal levels did not
correlate with Cripto-1 or Notch4 in either group of nevi (data not
shown). Notably, a significant positive correlation was observed
for Cripto-1 and Notch4 expression levels in the group of nevi from
patients with no history of melanoma (R2=0.041; P=0.03;
n=121) and in the group of nevi from patients with a positive
history of melanoma (R2=0.141; P=0.003; n=61) (Fig. 4). In the group of nevi from patients
with a positive history of melanoma, no significant correlation was
observed between IS for Nodal, Cripto-1 or Notch4 and patient age,
time elapsed between surgical removal of the nevus and diagnosis of
melanoma, and anatomical site (data not shown). When analyzing high
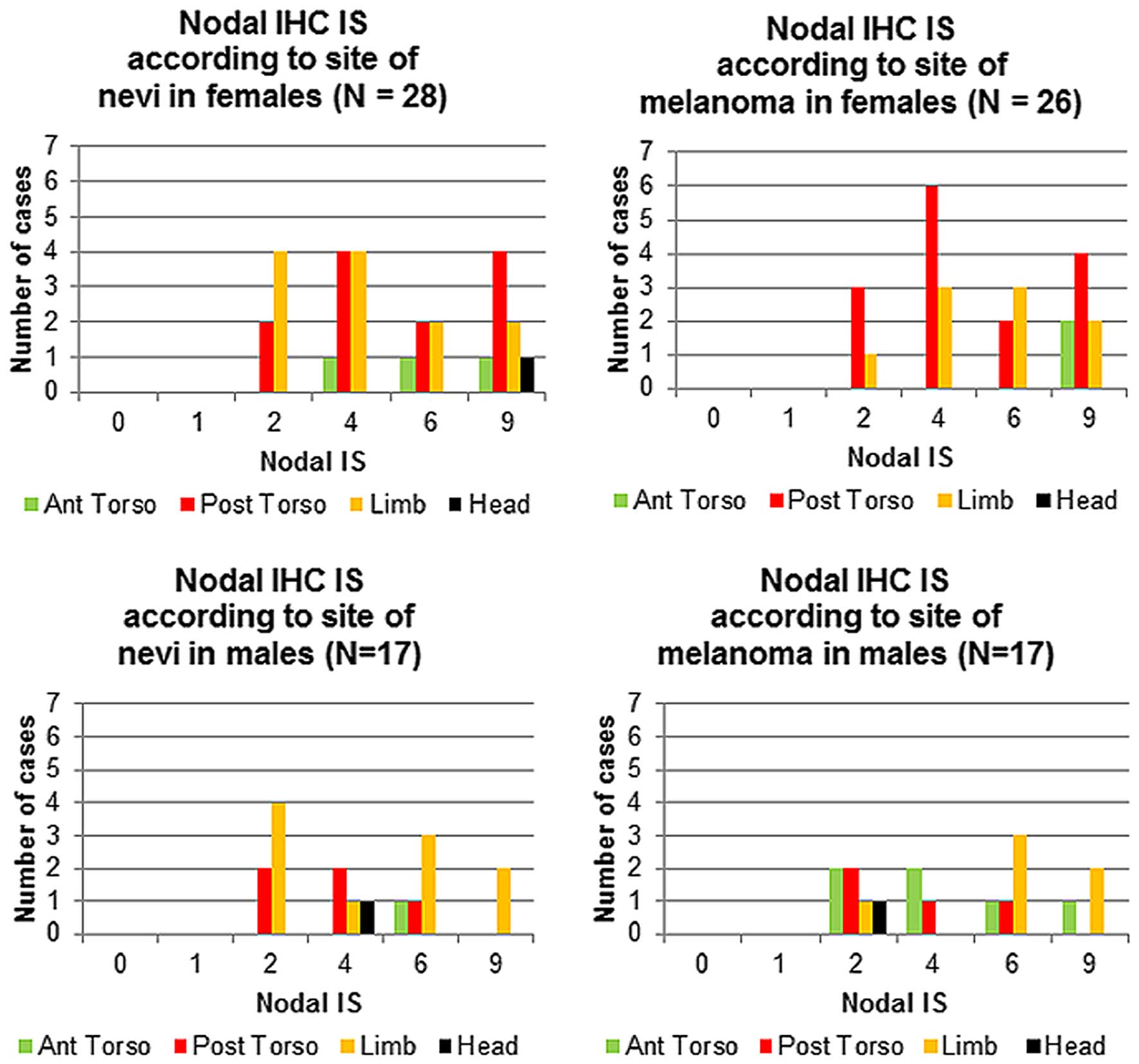
Nodal expression and anatomical sites in the group of nevi from
female patients that subsequently developed melanoma, the highest
Nodal IS (9) for both the nevi and
the subsequent melanomas was predominantly associated with lesions
located on the posterior torso (Fig.
5). In contrast, male patients showed the highest Nodal IS
(9) for both the nevi and the
subsequent melanomas in lesions located on the limbs (Fig. 5).
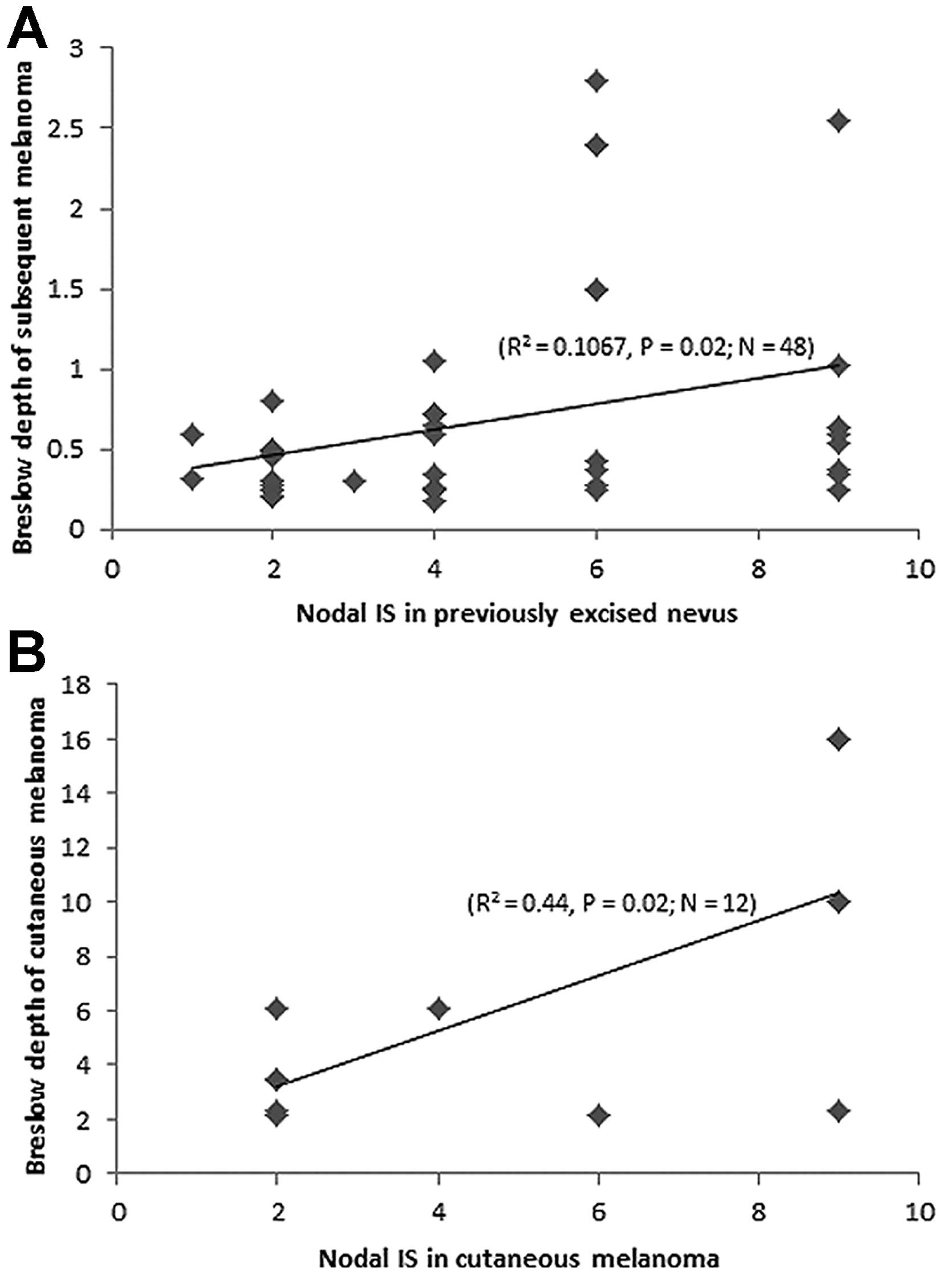
Finally, in patients with a positive history of
melanoma, a significant positive correlation was observed between
Nodal IS in the preceding nevus and Breslow depth in the subsequent
melanomas (R2=0.1067; P=0.02; n=48) (Fig. 6A). The same significant positive
correlation was found between Nodal IS and Breslow depth in the
cutaneous melanoma cases (R2=0.44; P=0.02; n=12)
(Fig. 6B).
Discussion
The present study demonstrated that there is a wide
range of variability in the intensity of IHC staining of Nodal,
Cripto-1 and Notch4 in nonmalignant melanocytic lesions regardless
of whether or not the nevi were from patients with or without a
history of melanoma, as reflected by the means of the IHC scores
calculated for the different groups analyzed. Calculating the mean
from a grading system of low to high expression levels, however,
can be distorted by outlier cases having values at either end of
the spectrum. Calculation of median levels with corresponding
interquartile ranges provides an improved representation of the
distribution of IHC scores for individual cases. In fact, this
method showed a significantly higher median value for all three
markers in the group of nevi from patients with a history of
melanoma compared with nevi from patients with no melanoma history.
However, on a case-by-case basis, no significant correlation was
observed in IS between Nodal and Cripto-1 or Nodal and Notch4 for
either group of nevi. This was unexpected since Cripto-1 is a
well-established co-receptor for canonical Nodal signaling
(7) and Notch4 has been shown to be
associated with Nodal expression in aggressive melanoma (8), and thus one would anticipate a
relationship between these two protein markers. These data may
indicate that either Nodal biological effects, assuming that nevi
are responsive to Nodal, may be mediated independently of Cripto-1
or Notch4, or that Nodal's effect may become relevant only when
Cripto-1 and Notch4 reach critical levels. In fact, Nodal, Cripto-1
and Notch4 have been shown to reach significantly high expression
levels in advanced stage melanoma (8,9).
Similarly, the data in the present study also show that Nodal,
Cripto-1 and Notch4 reach higher levels in metastatic melanoma.
Notably, a significant correlation was not observed between
Cripto-1 and Notch4 expression in nevi regardless of the previous
history of melanoma. Indeed, an earlier study demonstrated the
close association between Cripto-1 and all 4 Notch proteins by
describing the role for Cripto-1 in facilitating the
post-translational maturation of Notch receptors (10). Perhaps this maturation process must
occur in these nevi for Nodal to exert a more effective biological
role. Further studies are required to determine the combined roles
of Cripto-1 and Notch4 during melanomagenesis and how this may
relate to Nodal expression and function in melanoma.
Since the nevi in the present study were surgically
excised, a direct association between the high levels of Nodal,
Cripto-1 and Notch4 and potential melanomagenesis in the excised
nevus cannot be experimentally established. Nevertheless, a
proportion of these patients eventually developed a melanoma
following excision of the nevus, i.e. in the group of patients with
a positive history of melanoma. Perhaps in this group, the
melanomas may have developed from the malignant transformation of
other high Nodal expressing nevi that were not yet excised.
Alternatively, high Nodal expressing melanomas may secrete a level
of Nodal capable of inducing additional Nodal expression in other
nevi at locoregional sites in the same patient, since feedforward
autoinduction from paracrine signaling is one of several
established mechanisms underlying Nodal's biological effects
(6,11). In fact, in the group of nevi from
patients that subsequently developed melanoma, the majority of
these nevi showing highest Nodal expression (i.e. posterior torso)
were from the same anatomical sites in which the melanoma
subsequently developed-in both female and male patients. Perhaps
the surrounding melanocytic cells/lesions in the posterior torso
may have become more responsive to Nodal as a consequence of
accumulated effects of other melanogenic factors, such as exposure
to UV radiation. In fact, the posterior torso is a common site in
both men and women for increased sun exposure. This also raises the
possibility of a potential dosage effect of Nodal whereby, more
Nodal would presumably exert more autoinductive effects. To this
regard, it would be interesting to study the circulating Nodal
levels and number of nevi to determine whether higher levels of
Nodal can be detected in patients with increased numbers of nevi.
Unfortunately, this data was not available since an exact count of
all nevi present in each patient was not performed during whole
body inspection prior to excision of the nevus. Notably, in the
group of nevi from patients with a prior history of melanoma, for
which accompanying Breslow depths were available for the melanomas
that subsequently developed, a significant positive correlation was
identified between Nodal expression in those nevi and thicker
melanomas. These observations suggest that high Nodal expression in
nevi may have the potential to predict approximate anatomical site
and aggressiveness of a subsequent melanoma. No other clinical
parameter, including patient age, anatomical site of the nevus or
time elapsed between excision of the nevus and subsequent
development of melanoma, correlated with any of the markers
analyzed.
As in all retrospective studies such as this one, it
is difficult to determine direct cause and effect. However, the
significantly high levels of Nodal, Cripto-1 and Notch4 in nevi
from patients that subsequently developed melanoma compared to the
levels detected in the nevi from patients with no history of
melanoma strongly suggests that Nodal signaling may serve a key
role during melanoma development. Another limitation of the present
study is the relatively small sample size due to the difficulty in
collecting sufficient numbers of excised nevi from patients that
subsequently develop melanoma, since the majority of these patients
are lost to follow-up following nevi removal and the establishment
of a benign diagnosis. Nevertheless, these data provide a strong
evidence-based rationale for developing future, long term, and
prospective follow-up studies of a large patient cohort with high
Nodal expressing nevi, which will better determine the likelihood
of these patients, with or without a history of melanoma, to
subsequently develop a recurring or novel melanoma,
respectively.
Nodal expression in melanoma is associated with
aggressive, metastatic disease. The ability for early detection of
Nodal in patients with benign melanocytic lesions could represent a
powerful tool for screening and prevention of melanoma. The results
of this study indicate that nevi with high Nodal expression are
associated with the development of aggressive melanoma, suggesting
that patients from whom these types of nevi are removed should be
closely monitored for potential melanomagenesis.
Acknowledgements
The present study was supported by grants from the
National Institutes of Health (Bethesda, MD, USA; grant nos.
RO1CA121205 and R37CA59702) and H. Foundation and Dixon
Translational Research Grants to Dr Mary J.C. Hendrix (Ann &
Robert H. Lurie Children's Hospital of Chicago, Northwestern
University, Chicago, IL USA); and a grant from the American Cancer
Society Institutional Research Grant (grant no. 93-037-18) to Dr
Luigi Strizzi (Northwestern University).
References
|
1
|
Postovit LM, Margaryan NV, Seftor EA,
Kirschmann DA, Lipavsky A, Wheaton WW, Abbott DE, Seftor RE and
Hendrix MJ: Human embryonic stem cell microenvironment suppresses
the tumorigenic phenotype of aggressive cancer cells. Proc Natl
Acad Sci USA. 105:4329–4334. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yu L, Harms PW, Pouryazdanparast P, Kim
DS, Ma L and Fullen DR: Expression of the embryonic morphogen Nodal
in cutaneous melanocytic lesions. Mod Pathol. 23:1209–1214. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Seykora J and Elder D: Dysplastic nevi and
other risk markers for melanoma. Semin Oncol. 23:682–687.
1996.PubMed/NCBI
|
|
4
|
Slade J, Marghoob AA, Salopek TG, Rigel
DS, Kopf AW and Bart RS: Atypical mole syndrome: Risk factor for
cutaneous malignant melanoma and implications for management. J Am
Acad Dermatol. 32:479–494. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dakubo GD, Jakupciak JP, Birch-Machin MA
and Parr RL: Clinical implications and utility of field
cancerization. Cancer Cell Int. 7:22007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
D'Andrea D, Liguori GL, Le Good JA,
Lonardo E, Andersson O, Constam DB, Persico MG and Minchiotti G:
Cripto promotes A-P axis specification independently of its
stimulatory effect on Nodal autoinduction. J Cell Biol.
180:597–605. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shen MM: Nodal signaling: Developmental
roles and regulation. Development. 134:1023–1034. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hardy KM, Kirschmann DA, Seftor EA,
Margaryan NV, Postovit LM, Strizzi L and Hendrix MJ: Regulation of
the embryonic morphogen Nodal by Notch4 facilitates manifestation
of the aggressive melanoma phenotype. Cancer Res. 70:10340–10350.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
De Luca A, Lamura L, Strizzi L, Roma C,
D'Antonio A, Margaryan N, Pirozzi G, Hsu MY, Botti G, Mari E, et
al: Expression and functional role of CRIPTO-1 in cutaneous
melanoma. Br J Cancer. 105:1030–1038. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Watanabe K, Nagaoka T, Lee JM, Bianco C,
Gonzales M, Castro NP, Rangel MC, Sakamoto K, Sun Y, Callahan R and
Salomon DS: Enhancement of Notch receptor maturation and signaling
sensitivity by Cripto-1. J Cell Biol. 187:343–353. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Le Good JA, Joubin K, Giraldez AJ,
Ben-Haim N, Beck S, Chen Y, Schier AF and Constam DB: Nodal
stability determines signaling range. Curr Biol. 15:31–36. 2005.
View Article : Google Scholar : PubMed/NCBI
|