Introduction
Esophageal cancer (EC) is one of the most common
cancers in the world (1). Esophageal
squamous cell carcinoma (ESCC) and esophageal adenocarcinoma (EAC)
are the two main types of EC (2),
with ESCC being the main form of EC in Asian countries, and EAC
being the most common type of EC in Western countries (3). The incidence of EC is increasing
worldwide (4). In the USA in 2012,
17,460 patients were diagnosed with EC, and 15,070 patients
succumbed to the disease (5). Upon
initial diagnosis, the majority of EC patients already present with
metastasis, which results in poor prognosis (6,7).
Accumulating evidence indicates that numerous
molecular changes are associated with EC tumorigenesis, including
epidermal growth factor receptor (EGFR) amplification,
phosphoinositide 3-kinase, catalytic subunit alpha (PIK3CA)
amplification and mutation (8–10), and
phosphatase and tensin homolog (PTEN) mutation or loss (11,12).
Alteration of these molecular events contributes to downstream
pathway activation (8–12). The phosphoinositide 3-kinase
(PI3K)/AKT and mitogen-activated protein kinase kinase (MEK)/ERK
signaling pathways are two important pathways that can be activated
by EGFR amplification and PTEN loss, which may ultimately lead to
tumorigenesis (13). ERK is a
downstream component of an evolutionarily conserved signaling
module that is activated by Raf serine/threonine kinases (14). Raf is activated by growth factor
stimulation, eventually leading to the activation of ERK (14). ERK then can mediate cell proliferation
and oncogenesis through downstream targeting (15). ERK can activate the pro-apoptotic
protein B-cell lymphoma-2 (Bcl-2)-associated death promoter (BAD)
at Ser112 and phosphorylate the transcription factor cyclic
adenosine monophosphate response element-binding protein (CREB) at
Ser133 to promote cell survival by activating the ribosomal S6
kinase (RSK) family of serine/threonine kinases (16). Previously, inhibitors targeting this
pathway were developed and tested in clinical trials (15,17) with
certain success.
Tumorigenesis frequently occurs during PI3K/AKT
signal pathway activation following EGFR amplification and PTEN
loss (18). Activation of AKT results
from Thr308 phosphorylation by 3-phosphoinositide-dependent kinase
1 (PDK1) and Ser473 phosphorylation by mechanistic target of
rapamycin complex 2 (mTORC2) (18).
AKT regulates CREB activity (19,20) by
phosphorylating CREB at Ser133, which induces the binding of
accessory proteins, thereby regulating anti-apoptotic genes,
including Bcl-2 and myeloid cell leukemia 1 (21,22). AKT
can suppress mouse double minute 2 homolog (MDM2)
self-ubiquitination, thereby inhibiting p53-mediated apoptosis
(23). AKT also promotes the cell
cycle by phosphorylating p21Waf1/Cip1, also known
as cyclin-dependent kinase inhibitor 1 or CDK-interacting protein
1, at Thr145 (24). In
addition, AKT directly controls the activation of glycogen synthase
kinase (GSK)3β. which phosphorylates cyclin D1 at Thr286 (25) and Myc at Thr58 (26). GSK3β promotes the nuclear export and
ubiquitin-pathway degradation of AKT, which could in turn regulate
the cell cycle by regulating GSK3β activity (25). Therefore, blocking the activation of
PI3K/AKT signaling may be a promising strategy for cancer
treatment.
Aloe-emodin has anti-proliferative effects and
induces cellular apoptosis (27–30).
Aloe-emodin has anti-cancer activity in neuroectodermal tumors
(31), nasopharyngeal carcinoma
(32), lung squamous cell carcinoma
(33), hepatoma cells (34), gastric cancer (35) and prostate cancer (36). Aloe-emodin induces apoptotic cell
death by oxidative stress and sustained c-Jun N-terminal kinase
(JNK) activation (37). Previous
studies have demonstrated that aloe-emodin induces cell death
through S-phase arrest in human tongue squamous cancer SCC-4 cells
(38). A previous study by the
present authors also indicated that mTORC2 is a target of
aloe-emodin, and aloe-emodin can strongly inhibit the AKT
activation caused by PTEN loss (36).
Aloe-emodin is a natural compound from aloe or Rheum palmatum
(39,40). The present study aimed to determine
the efficacy of aloe-emodin in the treatment of EC.
The present results demonstrate that both ERK and
AKT are activated in EC cells. Aloe-emodin can suppress the
proliferation and anchor-independent cell growth of the EC cell
line TE1. Western blot data revealed that aloe-emodin inhibits both
AKT and ERK phosphorylation and their downstream activation. The
inhibition of these pathways results in cell cycle arrest at S
phase and decreased cyclin D1 transcription. These results suggest
that aloe-emodin could prevent and even reverse the development of
EC, thus identifying it as a candidate compound for EC
chemoprevention.
Materials and methods
Materials
Aloe-emodin (>95% purity) was purchased from
Sigma-Aldrich (St. Louis, MO, USA). Other chemical reagents,
including Tris, NaCl, acrylamide, glycine and sodium dodecyl
sulfate (SDS), were purchased from Sigma-Aldrich or Fluka
(Sigma-Aldrich). AKT [rabbit polyclonal immunoglobulin G (IgG);
sc-8312], p-AKT (mouse monoclonal IgG1; sc-293125), ERK (rabbit
polyclonal IgG; sc-94) and p-ERK (rabbit polyclonal IgG; sc-23759)
primary antibodies, and horseradish peroxidase (HRP)-conjugated
goat anti-rabbit IgG (sc-2004) and HRP-conjugated rabbit anti-mouse
IgG (sc-358914) secondary antibodies were obtained from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA). α-tubulin (rabbit
polyclonal; #2148) antibody was obtained from Cell Signaling
Technology, Inc. (Danvers, MA, USA).
Cell culture
The esophageal cancer cell lines TE1 and KYSE140
were purchased from Nanjing KeyGen Biotech Co., Ltd. (Nanjing,
China). Eca109 and EC9706 cells were provided by the State Key
Laboratory of Molecular Oncology, Chinese Academy of Medical
Science (Shanghai, China). TE1 cells were cultured in Dulbecco's
modified Eagle's medium (DMEM)-high glucose (HyClone; GE Healthcare
Life Sciences, Logan, UT, USA) containing 10% fetal bovine serum
(FBS) (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA), 10
IU penicillin/ml and 10 IU streptomycin/ml at 37°C in a humidified
incubator with 5% CO2.
Cell counting kit-8 (CCK-8) assay
TE1 cells (2×104) were seeded into 96-well plates in
100 µl of 10% FBS-DMEM, and incubated in a 37°C, 5% CO2
incubator overnight. The cells were treated with increasing doses
of aloe-emodin (1, 5, 10, 25 and 50 µM), and cytotoxicity was
analyzed at 24 h and 48 h using a CCK-8 (Beyotime Institute of
Biotechnology, Haimen, China) according to the manufacturer's
protocol. TE1 cells (5×103) were seeded in 96-well
plates in 100 µl of 10% FBS-DMEM and cultured in a 37°C, 5%
CO2 incubator. After 12 h, the medium was changed for
medium containing different concentrations of aloe-emodin (0, 2.5,
5, 10 and 20 µM). Cells were cultured for an additional 24, 48, 72
and 96 h, and 10 µl of CCK-8 was then added to each well. The cells
were incubated for 2 h and the absorbance was measured at 450
nm.
Soft agar assay
The soft agar assay was performed in 6-well plates
containing two layers of agar (Bacto Agar; BD Biosciences, Franklin
Lakes, NJ, USA) and different concentrations of aloe-emodin (0,
2.5, 5, 10 and 20 µM). The bottom layer consisted of 0.5% agar in 1
ml of basal medium Eagle (BEM; Sigma-Aldrich) with 10% FBS. The top
layer consisted of 0.33% agar in 1 ml of BEM with 10% FBS
containing 8×103 TE1 cells. The TE1 cells embedded in
agar were incubated in a 37°C humid incubator for 14 days, and
colonies were imaged using a microscope with the aid of Image-Pro
Plus software (version 6; Media Cybernetics, Inc., Rockville, MD,
USA) and MicroPublisher 5.0 RTV camera (Olympus Corporation, Tokyo,
Japan).
Cell cycle analysis
For flow cytometric analysis of cell cycle, TE1
cells (1.5×106) were seeded into a 60-mm dish. After 12
h of culture, the cells were washed three times with Dulbecco's
phosphate-buffered saline (DPBS, pH 7.2). The cells were next
cultured for 48 h in DMEM without FBS. Then, the FBS-free DMEM was
removed, and different concentrations of aloe-emodin (0, 2.5, 5, 10
and 20 µM) in DMEM containing 10% FBS were added to each dish.
After culturing for additional 48 h, the cells were trypsinized,
washed with ice-cold DPBS and fixed with ice-cold 70% ethanol at
−20°C overnight. Cells were then washed twice with DPBS, incubated
with 0.5 mg/ml RNase A and 200 µg/ml propidium iodide in DPBS at
room temperature for 30 min in the dark, and subjected to flow
cytometry using a FACSCalibur flow cytometer (BD Biosciences). The
percentages of cells in different cell cycle phases (G0/G1, S or
G2/M) were calculated.
Western blotting
A total of 1.5×106 TE1 cells were seeded
and cultured in a 10-cm dish for 24 h. The cells were treated with
various concentrations of aloe-emodin (0, 2.5, 5, 10 and 20 µM) for
additional 24 h. The cells were then harvested, and cell lysates
were collected in modified radioimmunoprecipitation assay buffer
(50 mM Tris base, 1% NP-40, 0.25% superoxide dismutase, 150 mM
NaCl, 1 mM ethylenediaminetetraacetic acid and 0.1% SDS). A total
of 50 µg proteins were subjected to 12% SDS-polyacrylamide gel
electrophoresis. The nitrocellulose membranes (Hybond-c pure; GE
Healthcare Life Sciences, Chalfont, UK) with transferred protein
were incubated with a specific primary antibody [anti-AKT (1:200),
p-AKT (1:200), ERK (1:200), p-ERK (1:200) or α-tubulin (1:1,000)]
at 4°C overnight, followed by incubation with the appropriate
secondary antibody (1:1,000) for 2 h at room temperature. Protein
bands were detected with an enhanced chemiluminescence (ECL) kit
(Beyo ECL Plus; Beyotime Institute of Biotechnology) after
hybridization with a specific secondary antibody.
Cyclin D1 luciferase assay
TE1 cells (600×103 cells/well) were
seeded and cultured in 24-well plates for 12 h, prior to be
transfected with 400 ng of the pGL4.29/Luc/Cyclin D1 reporter gene
plasmid (generously donated by Dr Chris Albanese, Department of
Developmental and Molecular Biology, Albert Einstein Cancer Center,
Albert Einstein College of Medicine, New York, NY, USA) via a DNA
transfection method (jetPRIME®,
Polyplus-transfection® SA, Illkirch, France). This
reporter gene was constructed from the −1,715 to +134 region of the
human cyclin D1 promoter (41). The
medium was replaced with medium containing different aloe-emodin
concentrations (0, 2.5, 5, 10 and 20 µM) after 6 h transfection.
For luciferase detection, the cells were incubated for another 24
h. Luciferase assays were performed with the
Dual-Luciferase® Reporter (DLR™) Assay System (Promega
Corporation, Madison, WI, USA) according to the manufacturer's
protocol. Detection was conducted with the Centro LB 960 microplate
luminometer (Berthold Technologies GmbH & Co. KG, Bad Wildbad,
Germany), and the data were calculated as the mean of three
independent experiments.
Statistical analysis
All the data were reported as means ± standard error
or standard deviation, as calculated with the statistical software
SPSS version 17.0 (SPSS, Inc., Chicago, IL, USA). Single factor
analysis of variance was used for statistical analysis. P<0.05
was considered to indicate a statistically significant
difference.
Results
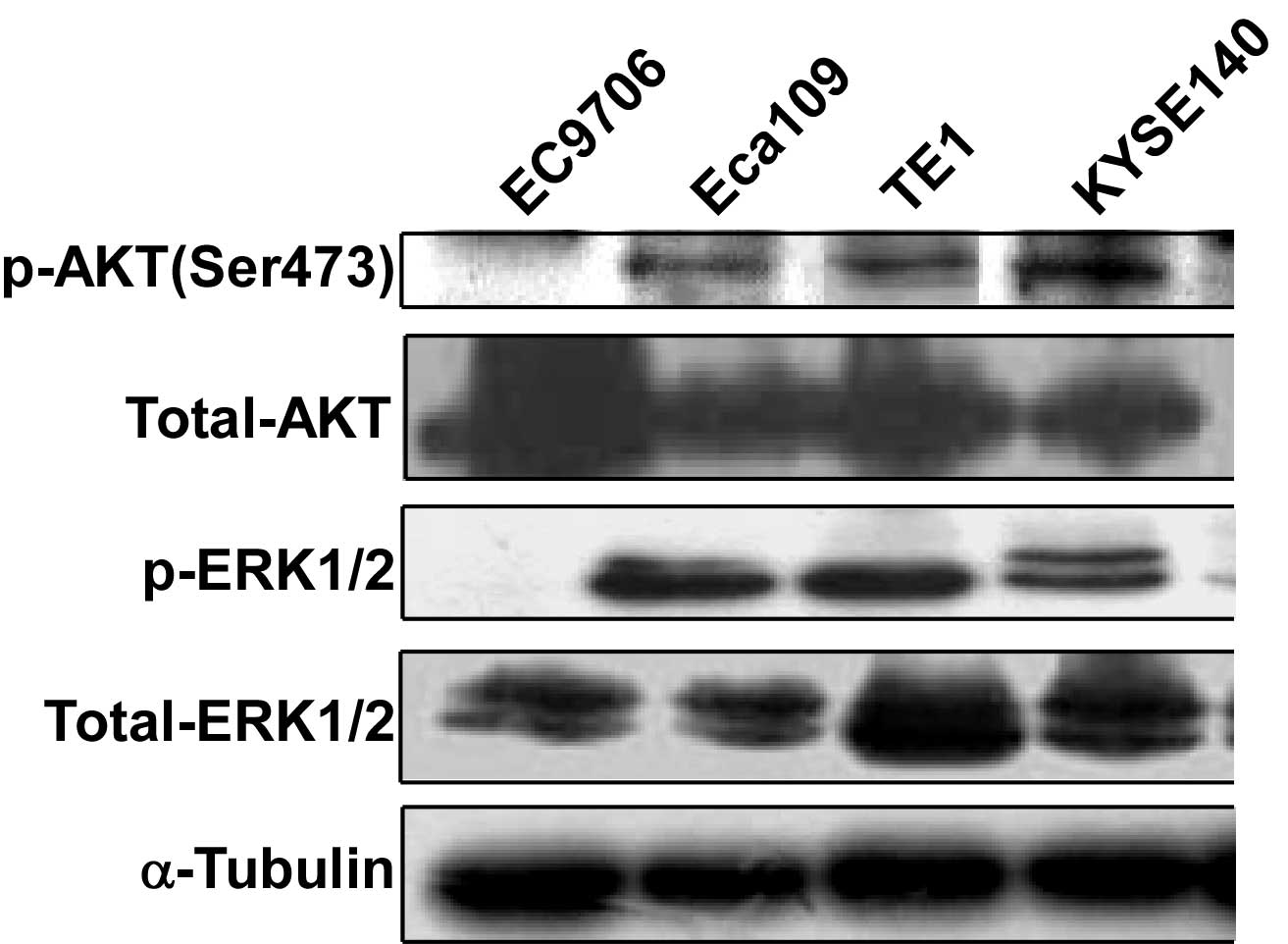
AKT and ERK are activated in EC cell
lines
Since the MEK/ERK and PI3K/AKT signaling pathways
are important in tumorigenesis, the level of activation of these
pathways in EC cells was investigated. Cell lysates of different
human EC cell lines were collected and subjected to western blot
analysis to detect AKT and ERK phosphorylation. AKT phosphorylation
at Ser473 was detected in Eca109, TE1 and KYSE140 cells. ERK
phosphorylation was detected in Eca109, TE1 and KYSE140 cells.
Phosphorylation could not be detected in either AKT or ERK in the
EC9706 cell line (Fig. 1).
Aloe-emodin suppresses TE1 cell
proliferation and anchor-independent cell growth
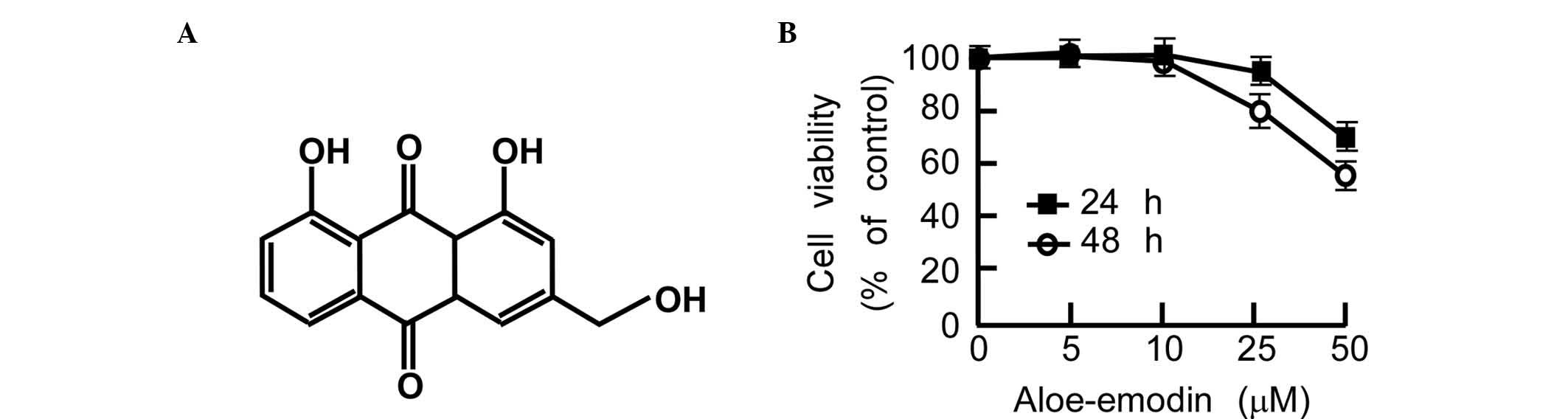
Aloe-emodin is a substance derived from aloe or
Rheum palmatum that is effective at suppressing cancer cell
growth in gastric cancer, prostate cancer and colon cancer cells
(Fig. 2A) (42,43). To
investigate the degree to which aloe-emodin can suppress EC cell
proliferation, TE1 cell growth and anchor-independent cell growth
was examined. In the cytotoxicity assay, 90% of the cells survived
after treatment with 20 µM aloe-emodin for 48 h (Fig. 2B). To investigate the level to which
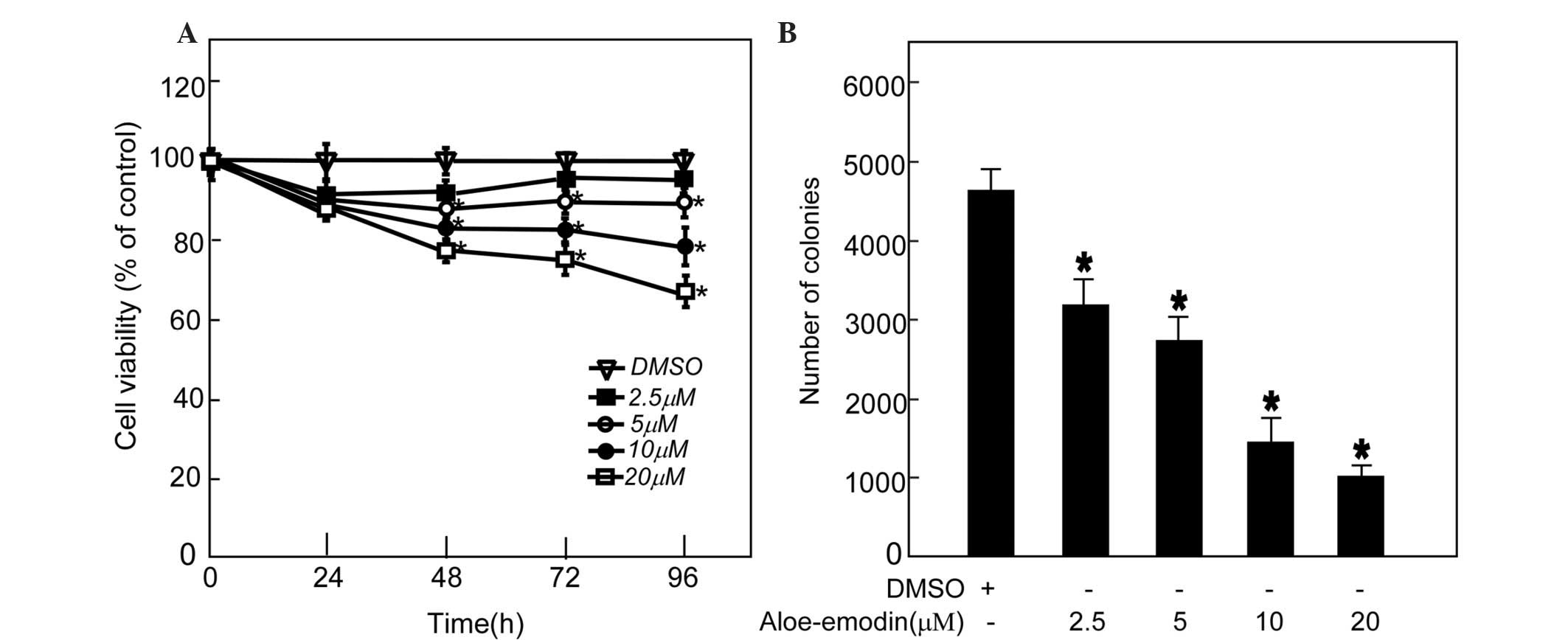
aloe-emodin can inhibit TE1 cell proliferation, 2.5, 5, 10 and 20
µM aloe-emodin was added to the medium of TE1 cells, and CCK-8
assay was performed. The data indicated that aloe-emodin suppressed
TE1 cell proliferation in a dose-dependent manner (Fig. 3A). An anchor-independent cell growth
assay was performed on TE1 cells in the presence of aloe-emodin.
The results indicated that aloe-emodin could suppress colony
formation of TE1 cells in a dose-dependent manner (Fig. 3B).
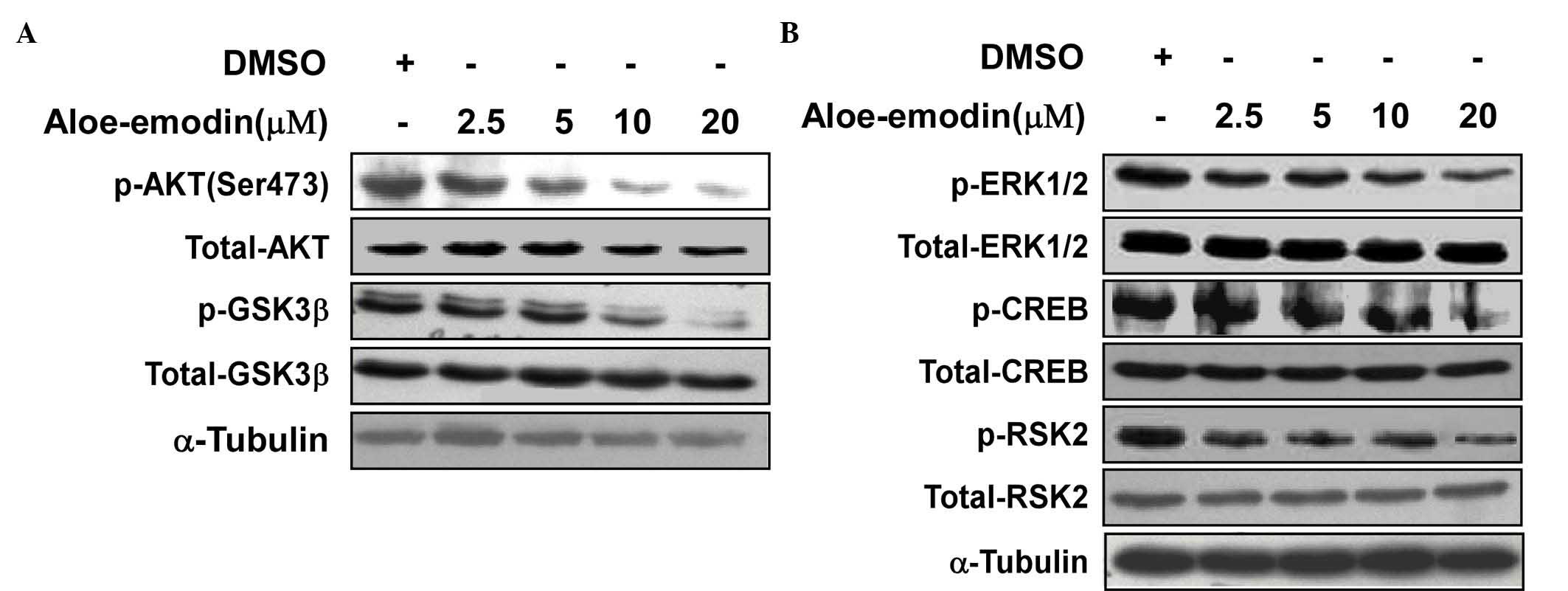
Aloe-emodin inhibits AKT and ERK
activity
Aloe-emodin was used to inhibit the ERK and
AKT-related signaling pathways activated in TE1 cells. The western
blot data indicated that aloe-emodin inhibited the phosphorylation
of AKT at Ser473 (Fig. 4A).
Downstream of AKT, Ser9 phosphorylation of GSK3β also decreased in
a dose-dependent manner. In addition, the phosphorylation of ERK
and its downstream target, RSK2, were also investigated. The
results indicated that the phosphorylation of ERK at Thr202/Tyr204,
RSK2 at Ser360 and CREB at Ser133 was also inhibited by aloe-emodin
treatment (Fig. 4B).
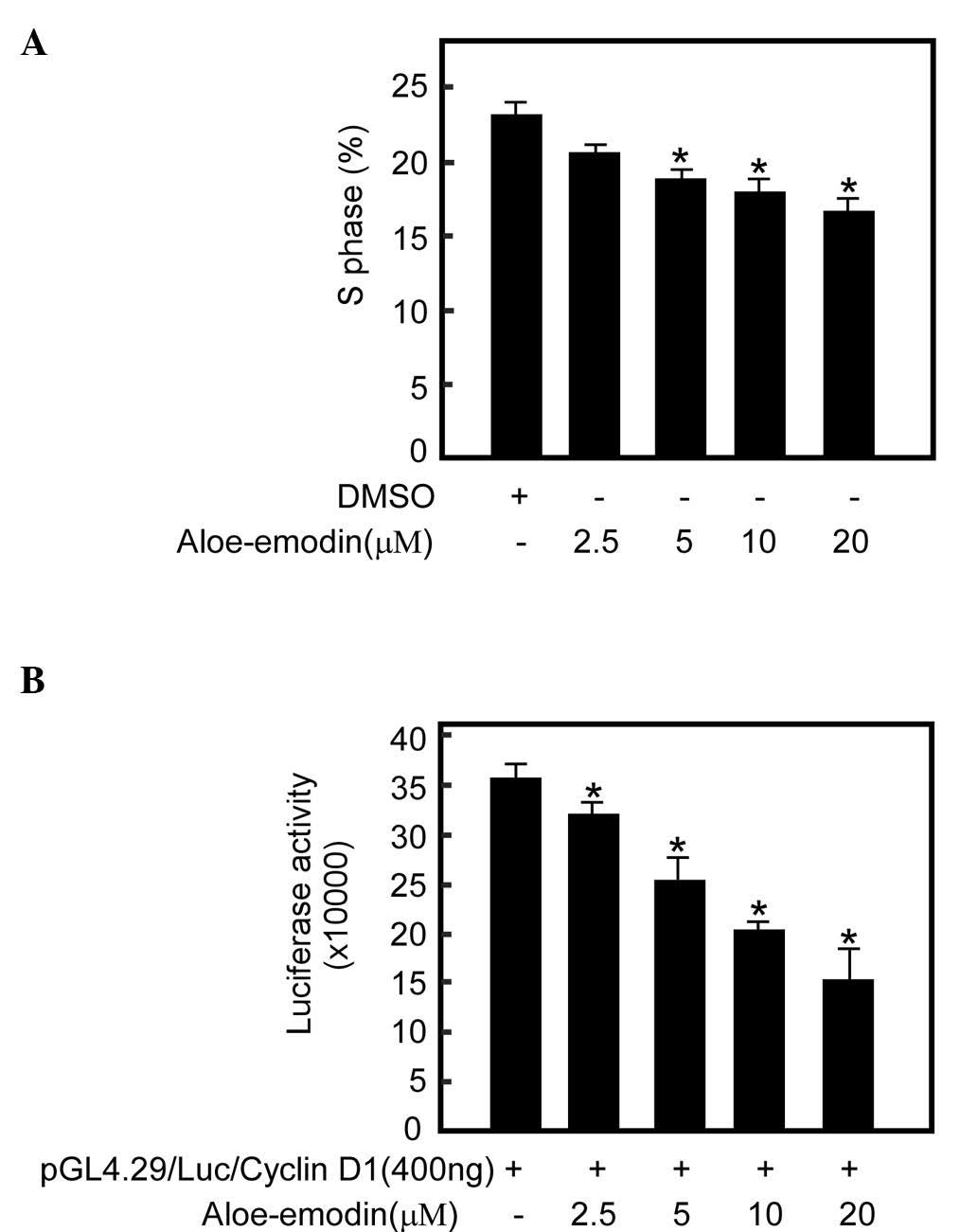
Aloe-emodin inhibits TE1 cell growth
by decreasing the number of cells in S phase
To investigate the extent to which the
aloe-emodin-mediated TE1 cell growth was associated with cell cycle
arrest, cell cycle analysis was performed. The data revealed that
treatment with increasing concentrations of aloe-emodin for 48 h
resulted in a dose-dependent decrease in the number of cells in S
phase (Fig. 5A).
Aloe-emodin inhibits cyclin D1
expression in TE1 cells
AKT and its downstream kinase GSK3β regulate cyclin
D1 transcription, which regulates cell transition from G1 to S
phase (44). To investigate the
degree to which aloe-emodin-mediated S phase reduction is
associated with cyclin expression, a cyclin D1 reporter gene assay
was performed with aloe-emodin treatment. The cyclin D1 reporter
gene assay demonstrated that aloe-emodin could inhibit cyclin D1
transcription activity in a dose-dependent manner (Fig. 5B).
Discussion
Signal transduction pathways have an important role
in tumorigenesis (45). Both AKT and
ERK are important molecules in the MEK/ERK and PI3K/AKT signal
transduction pathways (46,47). In the present study, ERK and AKT were
activated in EC cell lines, including TE1, Eca109 and KYSE 140,
which implies that these two activation pathways are important in
esophageal tumorigenesis and development. Previous studies have
also indicated that both MEK/ERK and PI3K/AKT signaling are
activated in ESCC (8,48,49).
Therefore, blocking these two pathways is a promising strategy for
EC treatment and chemoprevention.
Previous research on cancer cells has revealed that
aloe-emodin has anti-proliferative effects and can induce apoptosis
at high doses (50). Aloe-emodin
suppresses prostate cancer by targeting mTORC2 and inhibiting
growth in a dose-dependent manner, with a maximal inhibitory effect
at a concentration of 15 µM (36). By
contrast, other studies have indicated that aloe-emodin has
anti-proliferative effects at 75 µM and induces apoptosis of human
hepatoma Huh-7 cells via downregulation of calpain-2 and
ubiquitin-protein ligase E3A (51).
In the present study, aloe-emodin had a cytotoxic effect on EC
cells. Thus, at lower doses than those previously reported (<20
µM), aloe-emodin inhibited TE1 cell proliferation and
anchor-independent cell growth in a dose-dependent manner. Cell
cycle analysis indicated that aloe-emodin inhibited cell entry into
S phase. These data demonstrated that aloe-emodin is a promising
compound for suppressing the growth of EC cells.
Aloe-emodin has been reported to inhibit tumor cell
proliferation through various mechanisms (35,50).
Aloe-emodin induced G2/M arrest and differentiation of cervical
cancer cells (52), while induced
apoptosis of retina ganglion cells in glaucomatous patients through
regulation of ERK phosphorylation (53). Aloe-emodin induced apoptosis,
autophagy and differentiation of glioma cells by inhibiting the
action of ERK (54). In the current
study, aloe-emodin at low doses inhibited AKT and ERK
phosphorylation, which is consistent with a previous study by the
present authors on prostate cancer cells (PC3) (36). However, PTEN loss caused strong
activation of AKT, which, in turn, attenuated the phospho-ERK
signal. This may be the explanation for the lack of an effect of
ERK (55). In the current study,
GSK3β and RSK2, the downstream kinases of AKT, were also inhibited,
as was the phosphorylation of the transcription factor CREB. This
suggests that aloe-emodin can suppress the MEK/ERK and PI3K/AKT
signaling pathways. However, further investigation is required to
determine if this is caused by one or several targets. Furthermore,
aloe-emodin inhibited cyclin D1 transcription in the present study,
which may be associated with AKT and ERK pathway inhibition.
In conclusion, the present study demonstrated that
AKT and ERK were activated in EC cells. Aloe-emodin suppressed EC
TE1 cell proliferation through the inhibition of AKT, ERK and their
downstream molecules, thus regulating cyclin D1 transcription.
Further studies on aloe-emodin and its potential use as a
therapeutic agent for EC should be conducted.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (Beijing, China; grant nos.
81372269 and U1304813), the Science Foundation of the Henan
Province of China (Zhengzhou, China; grant nos. 112106000039,
13HASTIT022, 2011A310009, 12B310022, 13A310553 and 14A310006) and
the Key Science and Technology Program of Zhengzhou City
(Zhengzhou, China; grant no. 141PPTGG449).
References
|
1
|
Ghasemi-Kebria F, Roshandel G, Semnani S,
Shakeri R, Khoshnia M, Naeimi-Tabiei M, Merat S and Malekzadeh R:
Marked increase in the incidence rate of esophageal adenocarcinoma
in a high-risk area for esophageal cancer. Arch Iran Med.
16:320–323. 2013.PubMed/NCBI
|
|
2
|
Zhang XM and Guo MZ: The value of
epigenetic markers in esophageal cancer. Front Med China.
4:378–384. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pohl H, Sirovich B and Welch HG:
Esophageal adenocarcinoma incidence: Are we reaching the peak?
Cancer Epidemiol Biomarkers Prev. 19:1468–1470. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics for Hispanics/Latinos, 2012. CA Cancer J Clin.
62:283–298. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pennathur A, Gibson MK, Jobe BA and
Luketich JD: Oesophageal carcinoma. Lancet. 381:400–412. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pennathur A, Farkas A, Krasinskas AM,
Ferson PF, Gooding WE, Gibson MK, Schuchert MJ, Landreneau RJ and
Luketich JD: Esophagectomy for T1 esophageal cancer: Outcomes in
100 patients and implications for endoscopic therapy. Ann Thorac
Surg. 87:1048–1054; discussion 1054–1055. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Akagi I, Miyashita M, Makino H, Nomura T,
Hagiwara N, Takahashi K, Cho K, Mishima T, Ishibashi O, Ushijima T,
et al: Overexpression of PIK3CA is associated with lymph node
metastasis in esophageal squamous cell carcinoma. Int J Oncol.
34:767–775. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Okines A, Cunningham D and Chau I:
Targeting the human EGFR family in esophagogastric cancer. Nat Rev
Clin Oncol. 8:492–503. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bettstetter M, Berezowska S, Keller G,
Walch A, Feuchtinger A, Slotta-Huspenina J, Feith M, Drecoll E,
Höfler H and Langer R: Epidermal growth factor receptor,
phosphatidylinositol-3-kinase catalytic subunit/PTEN, and
KRAS/NRAS/BRAF in primary resected esophageal adenocarcinomas: Loss
of PTEN is associated with worse clinical outcome. Hum Pathol.
44:829–836. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hou G, Lu Z, Liu M, Liu H and Xue L:
Mutational analysis of the PTEN gene and its effects in esophageal
squamous cell carcinoma. Dig Dis Sci. 56:1315–1322. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cho MY, Kim HS, Eng C, Kim DS, Kang SJ,
Eom M, Yi SY and Bronner MP: First report of ovarian dysgerminoma
in Cowden syndrome with germline PTEN mutation and PTEN-related 10q
loss of tumor heterozygosity. Am J Surg Pathol. 32:1258–1264. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lugli A, Zlobec I, Minoo P, Tornillo L,
Terracciano L and Jass JR: Role of the mitogen-activated protein
kinase and phosphoinositide 3-kinase/AKT pathways downstream
molecules, phosphorylated extracellular signal-regulated kinase,
and phosphorylated AKT in colorectal cancer - a tissue
microarray-based approach. Hum Pathol. 37:1022–1031. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kocieniewski P and Lipniacki T: MEK1 and
MEK2 differentially control the duration and amplitude of the ERK
cascade response. Phys Biol. 10:0350062013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chappell WH, Steelman LS, Long JM, Kempf
RC, Abrams SL, Franklin RA, Bäsecke J, Stivala F, Donia M, Fagone
P, et al: Ras/Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR inhibitors:
Rationale and importance to inhibiting these pathways in human
health. Oncotarget. 2:135–164. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mebratu Y and Tesfaigzi Y: How ERK1/2
activation controls cell proliferation and cell death: Is
subcellular localization the answer? Cell Cycle. 8:1168–1175. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Santarpia L, Lippman SM and El-Naggar AK:
Targeting the MAPK-RAS-RAF signaling pathway in cancer therapy.
Expert Opin Ther Targets. 16:103–119. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Martelli AM, Tabellini G, Bressanin D,
Ognibene A, Goto K, Cocco L and Evangelisti C: The emerging
multiple roles of nuclear Akt. Biochim Biophys Acta.
1823:2168–2178. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li XY, Zhan XR, Liu XM and Wang XC: CREB
is a regulatory target for the protein kinase Akt/PKB in the
differentiation of pancreatic ductal cells into islet β-cells
mediated by hepatocyte growth factor. Biochem Biophys Res Commun.
404:711–716. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Du K and Montminy M: CREB is a regulatory
target for the protein kinase Akt/PKB. J Biol Chem.
273:32377–32379. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nicholson KM and Anderson NG: The protein
kinase B/Akt signalling pathway in human malignancy. Cell Signal.
14:381–395. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang JM, Chao JR, Chen W, Kuo ML, Yen JJ
and Yang-Yen HF: The antiapoptotic gene mcl-1 is up-regulated by
the phosphatidylinositol 3-kinase/Akt signaling pathway through a
transcription factor complex containing CREB. Mol Cell Biol.
19:6195–6206. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Feng J, Tamaskovic R, Yang Z, Brazil DP,
Merlo A, Hess D and Hemmings BA: Stabilization of Mdm2 via
decreased ubiquitination is mediated by protein kinase
B/Akt-dependent phosphorylation. J Biol Chem. 279:35510–35517.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhou BP, Liao Y, Xia W, Spohn B, Lee MH
and Hung MC: Cytoplasmic localization of p21Cip1/WAF1 by
Akt-induced phosphorylation in HER-2/neu-overexpressing cells. Nat
Cell Biol. 3:245–252. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Diehl JA, Cheng M, Roussel MF and Sherr
CJ: Glycogen synthase kinase-3beta regulates cyclin D1 proteolysis
and subcellular localization. Genes Dev. 12:3499–3511. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gregory MA, Qi Y and Hann SR:
Phosphorylation by glycogen synthase kinase-3 controls c-myc
proteolysis and subnuclear localization. J Biol Chem.
278:51606–51612. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Popadic D, Savic E, Ramic Z, Djordjevic V,
Trajkovic V, Medenica L and Popadic S: Aloe-emodin inhibits
proliferation of adult human keratinocytes in vitro. J Cosmet Sci.
63:297–302. 2012.PubMed/NCBI
|
|
28
|
Tabolacci C, Oliverio S, Lentini A, Rossi
S, Galbiati A, Montesano C, Mattioli P, Provenzano B, Facchiano F
and Beninati S: Aloe-emodin as antiproliferative and
differentiating agent on human U937 monoblastic leukemia cells.
Life Sci. 89:812–820. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Suboj P, Babykutty S, Srinivas P and
Gopala S: Aloe emodin induces G2/M cell cycle arrest and apoptosis
via activation of caspase-6 in human colon cancer cells.
Pharmacology. 89:91–98. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Radovic J, Maksimovic-Ivanic D,
Timotijevic G, Popadic S, Ramic Z, Trajkovic V, Miljkovic D,
Stosic-Grujicic S and Mijatovic S: Cell-type dependent response of
melanoma cells to aloe emodin. Food Chem Toxicol. 50:3181–3189.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pecere T, Gazzola MV, Mucignat C, Parolin
C, Vecchia FD, Cavaggioni A, Basso G, Diaspro A, Salvato B, Carli M
and Palù G: Aloe-emodin is a new type of anticancer agent with
selective activity against neuroectodermal tumors. Cancer Res.
60:2800–2804. 2000.PubMed/NCBI
|
|
32
|
Lin ML, Lu YC, Chung JG, Li YC, Wang
SGNGSH, Wu CY, Su HL and Chen SS: Aloe-emodin induces apoptosis of
human nasopharyngeal carcinoma cells via caspase-8-mediated
activation of the mitochondrial death pathway. Cancer Lett.
291:46–58. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lee HZ: Protein kinase C involvement in
aloe-emodin- and emodin-induced apoptosis in lung carcinoma cell.
Br J Pharmacol. 134:1093–1103. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kuo PL, Lin TC and Lin CC: The
antiproliferative activity of aloe-emodin is through p53-dependent
and p21-dependent apoptotic pathway in human hepatoma cell lines.
Life Sci. 71:1879–1892. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Guo J, Xiao B, Zhang S, Liu D, Liao Y and
Sun Q: Growth inhibitory effects of gastric cancer cells with an
increase in S phase and alkaline phosphatase activity repression by
aloe-emodin. Cancer Biol Ther. 6:85–88. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liu K, Park C, Li S, Lee KW, Liu H, He L,
Soung NK, Ahn JS, Bode AM, Dong Z, et al: Aloe-emodin suppresses
prostate cancer by targeting the mTOR complex 2. Carcinogenesis.
33:1406–1411. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lu GD, Shen HM, Chung MC and Ong CN:
Critical role of oxidative stress and sustained JNK activation in
aloe-emodin-mediated apoptotic cell death in human hepatoma cells.
Carcinogenesis. 28:1937–1945. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chiu TH, Lai WW, Hsia TC, Yang JS, Lai TY,
Wu PP, Ma CY, Yeh CC, Ho CC, Lu HF, et al: Aloe-emodin induces cell
death through S-phase arrest and caspase-dependent pathways in
human tongue squamous cancer SCC-4 cells. Anticancer Res.
29:4503–4511. 2009.PubMed/NCBI
|
|
39
|
Wang ZW, Wang JS, Yang MH, Luo JG and Kong
LY: Developmental Changes in the composition of five anthraquinones
from rheum palmatum as quantified by (1) H-NMR. Phytochem Anal.
24:329–335. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Di Luccia B, Manzo N, Vivo M, Galano E,
Amoresano A, Crescenzi E, Pollice A, Tudisco R, Infascelli F and
Calabrò V: A biochemical and cellular approach to explore the
antiproliferative and prodifferentiative activity of Aloe
arborescens leaf extract. Phytother Res. 27:1819–1828. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Albanese C, Johnson J, Watanabe G, Eklund
N, Vu D, Arnold A and Pestell RG: Transforming p21ras mutants and
c-Ets-2 activate the cyclin D1 promoter through distinguishable
regions. J Biol Chem. 270:23589–23597. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Guo J, Xiao B, Liu Q, Gong Z and Le Y:
Suppression of C-myc expression associates with anti-proliferation
of aloe-emodin on gastric cancer cells. Cancer Invest. 26:369–374.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Suboj P, Babykutty S, Valiyaparambil Gopi
DR, Nair RS, Srinivas P and Gopala S: Aloe emodin inhibits colon
cancer cell migration/angiogenesis by downregulating MMP-2/9, RhoB
and VEGF via reduced DNA binding activity of NF-κB. Eur J Pharm
Sci. 45:581–591. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Shimura T: Acquired radioresistance of
cancer and the AKT/GSK3β/cyclin D1 overexpression cycle. J Radiat
Res. 52:539–544. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Fonar Y and Frank D: FAK and WNT
signaling: The meeting of two pathways in cancer and development.
Anticancer Agents Med Chem. 11:600–606. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Xia P and Xu XY: PI3K/Akt/mTOR signaling
pathway in cancer stem cells: From basic research to clinical
application. Am J Cancer Res. 5:1602–1609. 2015.PubMed/NCBI
|
|
47
|
Steelman LS, Chappell WH, Abrams SL, Kempf
RC, Long J, Laidler P, Mijatovic S, Maksimovic-Ivanic D, Stivala F,
Mazzarino MC, et al: Roles of the Raf/MEK/ERK and
PI3K/PTEN/Akt/mTOR pathways in controlling growth and sensitivity
to therapy-implications for cancer and aging. Aging (Albany NY).
3:192–222. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Shigaki H, Baba Y, Watanabe M, Murata A,
Ishimoto T, Iwatsuki M, Iwagami S, Nosho K and Baba H: PIK3CA
mutation is associated with a favorable prognosis among patients
with curatively resected esophageal squamous cell carcinoma. Clin
Cancer Res. 19:2451–2459. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Roberts PJ and Der CJ: Targeting the
Raf-MEK-ERK mitogen-activated protein kinase cascade for the
treatment of cancer. Oncogene. 26:3291–3310. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Acevedo-Duncan M, Russell C, Patel S and
Patel R: Aloe-emodin modulates PKC isozymes, inhibits
proliferation, and induces apoptosis in U-373MG glioma cells. Int
Immunopharmacol. 4:1775–1784. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Jeon W, Jeon YK and Nam MJ: Apoptosis by
aloe-emodin is mediated through down-regulation of calpain-2 and
ubiquitin-protein ligase E3A in human hepatoma Huh-7 cells. Cell
Biol Int. 36:163–167. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Guo JM, Xiao BX, Liu Q, Zhang S, Liu DH
and Gong ZH: Anticancer effect of aloe-emodin on cervical cancer
cells involves G2/M arrest and induction of differentiation. Acta
Pharmacol Sin. 28:1991–1995. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Lin HJ, Chao PD, Huang SY, Wan L, Wu CJ
and Tsai FJ: Aloe-emodin suppressed NMDA-induced apoptosis of
retinal ganglion cells through regulation of ERK phosphorylation.
Phytother Res. 21:1007–1014. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Mijatovic S, Maksimovic-Ivanic D, Radovic
J, Miljkovic Dj, Harhaji Lj, Vuckovic O, Stosic-Grujicic S,
Stojkovic Mostarica M and Trajkovic V: Anti-glioma action of aloe
emodin: The role of ERK inhibition. Cell Mol Life Sci. 62:589–598.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Selvaraj N, Budka JA, Ferris MW, Jerde TJ
and Hollenhorst PC: Prostate cancer ETS rearrangements switch a
cell migration gene expression program from RAS/ERK to PI3K/AKT
regulation. Mol Cancer. 13:612014. View Article : Google Scholar : PubMed/NCBI
|