Introduction
Aspirin is a very commonly used clinical drug with
antipyretic, analgesic and antiplatelet functions (1). Recently, animal studies and clinical
observations (2,3) have found that regular doses of aspirin
play a role in the prevention and treatment of colitis-associated
colorectal cancer, and could reduce mortality in patients with
liver metastasis. In addition, aspirin was found to have an
inhibitory effect on the growth of esophageal cancer, ovarian
cancer, liver cancer, breast cancer, lung cancer, lymphoma and
endometrial cancer (4–6). However, the specific mechanism of action
remains to be further explored. The present study analyzed whether
aspirin also has an inhibitory effect on gastric cancer
proliferation in p53 gene-knockout (p53−/−) mouse with
gastric cancer to provide a basis for its use in clinical
treatment.
Materials and methods
Animals
Twenty p53−/− male mice aged 6 to 7 weeks
were used in the study. The average weight was 20±3 g. Mice were
purchased from Shanghai BangYao Biological Technology Co., Ltd.
(Shanghai, China). Mice were given food and drinking water ad
libitum and maintained at a constant temperature of 22±0.5°C
with a 12-h light/dark cycle (license no., SYXK< Shanghai
>2015-0032).
Reagents and instruments
Mouse forestomach carcinoma cell line (MFC) was
purchased from the Cell Bank of the Chinese Academy of Sciences
(Shanghai); aspirin was purchased from Sigma (St. Louis, MO, USA).
RPMI-1640 medium (containing 15% fetal bovine serum, 100 µ/ml
penicillin and 100 µ/ml streptomycin) were obtained from HyClone
(Logan, UT, USA). Trypsin was obtained from Gibco (Grand Island,
NY, USA). PBS was purchased from Shanghai Biological Engineering
Company (Shanghai, China). MTT assay (5 mg/ml) was obtained from
Sigma (St. Louis, MO, USA). Polyclonal rabbit anti-human E-cadherin
was purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA,
USA). FITC-labeled goat anti-rabbit IgG was from Beijing Zhong Shan
Jinqiao Biological Technology Co., Ltd. (Beijing, China).
Centrifuge was obtained from Thermo Fisher Scientific (Waltham, MA,
USA) and syringes were from Bio-Rad (Berkeley, CA, USA). Inverted
microscope was from Olympus (Tokyo Japan), Eppendorf tubes were
obtained from Eppendorf (Hauppauge, NY, USA), super clean bench was
from Thermo Fisher Scientific (Waltham, MA, USA), and cell counting
chamber was from Shanghai Optical Instrument Factory (Shanghai,
China). Paraffin slicing machine and tissue embedder were obtained
from Leica (Mannheim, Germany) and ELISA plate reader was from
Bio-Rad.
Establishment of a mouse model of
gastric cancer
MFC cells were cultured in RPMI-1640 medium and
placed in the incubator with saturated humidity and 5%
CO2 at 37°C, and passaged every 3 or 4 days. MCF cells
in the logarithmic growth phase were trypsinized with 1 ml of 0.25%
trypsin and observed under an inverted microscope. When the
adherent cells gradually became round, 3 ml of medium was used to
terminate the digestion. Culture flasks were agitated left and
right, gently to make the cells detach from the bottom of the
bottle. The cell suspension was then transferred into a centrifuge
tube for centrifugation at 1,000 rpm for 5 min. The supernatant was
discarded and PBS was added to resuspend the cells. A cell counting
plate was used for counting, and the cell concentration was
adjusted to 1.0×107/ml.
After skin disinfection at the back of the neck of
the mice, 1 ml syringes were used to aspirate MFC cell suspensions,
and were then inserted into the skin at an angle of roughly 30
degrees, withdrawing without liquid, and then the cells were
injected. Each mouse was injected with 0.2 ml of cell suspension.
After inoculation, the mice were fed with normal diet and
water.
After successful establishment of the cancer model,
mice were randomly divided into a control group (n=10) and an
experimental group (n=10). Mice in the experimental group were
treated with aspirin at the dose of 250 mg/kg daily one day before
cancer model establishment, until the end of the experiment; the
control group was fed without aspirin. Ten p53−/− male
mice were chosen as the control group, and given regular food.
Three months later, all mice were sacrificed to harvest gastric
cancer tissues. MTT assay was used to detect cell proliferation.
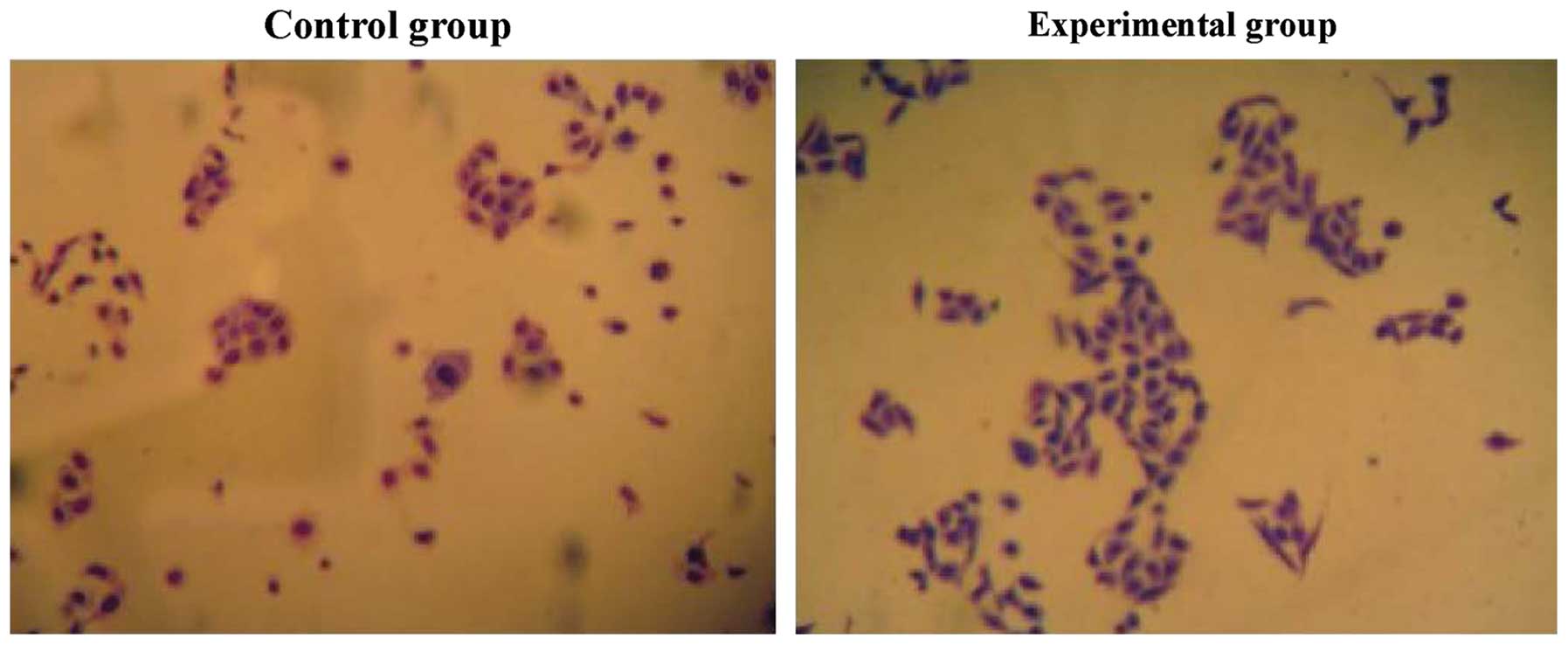
The Gonzalez-Garcia method was used to detect the number of tumor
cells (7). Scratch assay was adopted
to measure the migration ability. Immunofluorescence microscopy was
used to detect the expression of E-cadherin protein.
MTT assay
Cells were collected in the logarithmic growth phase
and seeded in wells of a 96-well culture plate at a density of
5×103 cells/well in RPMI-1640 medium containing 10%
fetal bovine serum. Cells were allowed to incubate for 24 h and
then the supernatant was removed. Twenty microliters of MTT reagent
was added to the wells every 24 h and each time point was analyzed
in triplicate. After continuous monitoring for 6 days and
cultivation for 4 h in the incubator, the supernatant was
completely removed. Then dimethyl sulfoxide (DMSO) was added to
each well and placed on a shaker for 10 min until the purple
crystals fully dissolved. Finally, the absorbance value (A490) at a
wavelength of 570 nm was measured using an ELISA plate reader.
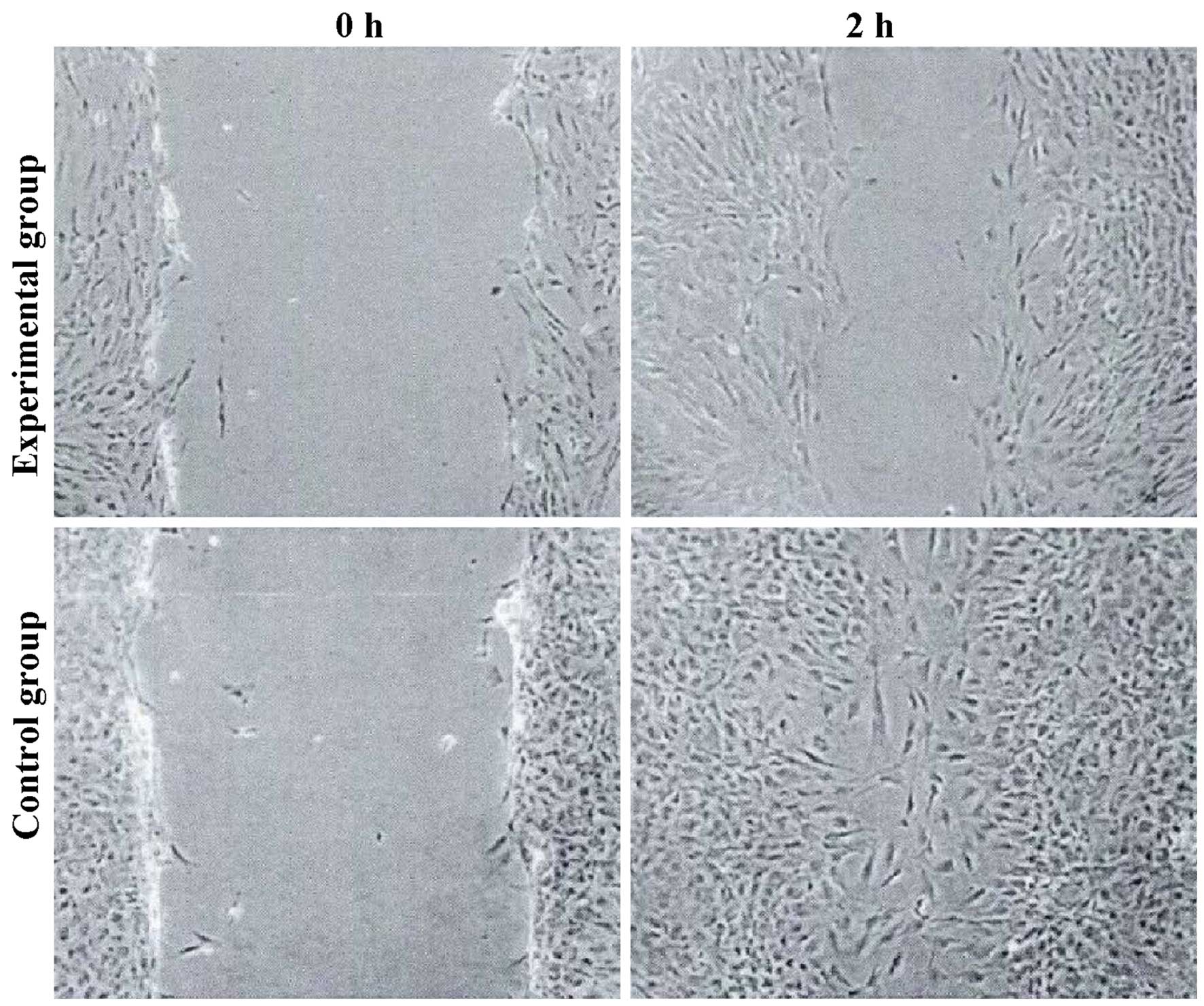
Cell scratch assay
A marker was used to draw lines across the back of
culture plates. Cells (5×105) were added to each plate
of the corresponding experimental groups. Sterile pipette tips (200
µl) were used to make a scratch in the cell monolayer across the
bottom of the well. Cells were then allowed to grow in Dulbecco's
modified Eagle's medium (DMEM) containing 5% calf serum. The cell
migration in the wounded area was observed.
Immunofluorescence assay
Sterile coverslips were placed in 6-well plates.
Cell suspension in the logarithmic growth phase was inoculated into
each well and allowed to grow under conventional conditions and
grown to an appropriate density. Cells were washed 3 times with
PBS, fixed for 20 min with pre-chilled pure acetone and then washed
with PBS again 3 times. The serum of normal non-immune animals was
used to block the cells for 10 min. The cells were washed 3 times
with PBS, and rabbit anti-human E-cadherin polyclonal antibody and
mouse anti-human β-catenin monoclonal antibody (1:200) were added
respectively, and left to incubate at 4°C overnight. Cells were
next washed with PBS 3 times for 10 min. FITC-labeled goat
anti-rabbit IgG (1:80) was added and incubation was carried out for
60 min at room temperature. Cells were washed 3 times with PBS and
5 µg/ml of propidium iodide (PI) was used to counterstain nuclei
for 30 min. The cells were washed with distilled water 3 times, and
coverslips were mounted with buffered glycerol (PBS:glycerol, 1:9).
Slides were observed and images were captured under a fluorescence
microscope (×400) as soon as possible. PBS was replaced with the
primary antibody as a blank control.
Statistical analysis
SPSS 19.0 was used to make statistical analyses.
Data are presented as the mean ± SD. Statistical significance
between the two groups was analyzed using t-test. The counting data
were presented as cases or percentage, while χ2 test was
used for comparison among groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
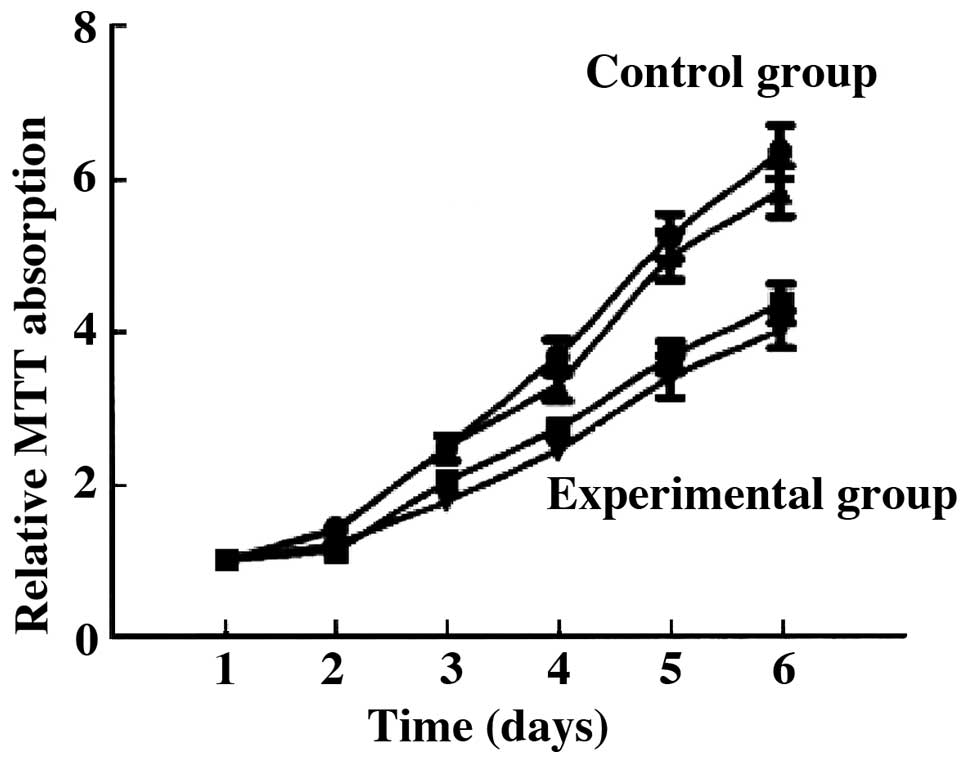
Cell proliferation
Compared with the control group, the proliferation
ability of the tumor cells in the experimental group was
significantly decreased. The number of cells was significantly
decreased compared to the control group (6.34±1.05 vs. 3.42±1.12,
t=5.624, P=0.027) (P<0.05) (Fig.
1).
Cell migration
The tumor cell migration ability of the experimental
group was decreased, and the expression levels of E-cadherin were
increased. The differences were statistically significant
(P<0.05) (Figs. 2 and 3).
Discussion
Gastric cancer is a common digestive tract cancer
with the fourth leading incidence rate and the second highest
mortality rate in the world (8). The
pathogenesis of gastric cancer is not yet fully understood.
Surgical resection is still the main method of treatment. The
average 5-year survival rate following surgery is ≤30%. Invasion
and metastasis are the main causes of death in patients with
gastric cancer (9).
Animal models of gastric cancer include (10): i) use of the carcinogen,
N-methyl-N'-nitro-N-nitrosoguanidine (MNNG); ii) use of
Helicobacter pylori (HP) infection to induce gastric
carcinoma, or combined MNNG carcinogen with HP; iii)
immunodeficient animals transplanted with gastric cancer cells; and
iv) transgenic methods using genes that regulate the formation of
gastric cancer transfected into animal embryos to form tumors. This
study used p53−/− mice to establish a mouse model of
gastric cancer through subcutaneous seeding of MFC cells. On the
one hand, p53−/− mice had steady strain and the
tumorigenesis rate was high (11); in
addition, the high mortality rate when using carcinogens was lower
(10). Previous research showed that
subcutaneous seeding of MFC cells to establish a model of gastric
cancer had a short cycle, of roughly 10 days, and the success rate
was as high as 95% (12).
Mice receiving aspirin (250 mg/kg/day) is equivalent
to the conventional oral dosage of 100 mg/day for adults. ukCAP, a
randomized double blind trial of aspirin in the UK, which aimed to
prevent the formation of colonic tumors, reported that a daily dose
of 300 mg of aspirin, supplemented with folic acid, reduced the
risk of adenomas and advanced adenomas by approximately 21 and 37%
respectively when compared to the placebo group after 3 years
(13). The mechanism by which aspirin
inhibits tumor growth and migration may involve reduction in the
expression level of COX-2, and the production of prostaglandins
(PGs) (14,15). COX-2, often overexpressed in tumor
tissues, can promote inflammation and cell proliferation. Oshima
found that the number of polyps in the colon and small intestine
decreased significantly after COX-2 gene knockout in adenomatous
polyposis coli (APC) gene knockout mouse models (16). PGE2 can inhibit the production of
lymphatic factors that have immune modulating function and can also
inhibit the proliferation of T cells and B cells (17). In addition, some studies have
indicated that aspirin can inhibit Bcl-2 activity, increase Bax
expression and inhibit the activity of NF-κB (18).
Tumor metastasis is a complex biological process
which is regulated by different genes and molecular pathways. At
present, it is believed that tumor metastasis mainly includes the
following steps: i) separation from primary lesions, in which cells
undergo epithelial-to-mesenchymal transition (EMT) in order to
detach; ii) cell invasion of surrounding tissues, in which cells
penetrate the basement membrane and reach blood vessels or
lymphatics, a process mediated by matrix metalloproteinases (MMPs)
and proteolytic enzymes; iii) survival in the vasculature or
lymphatic system; and iv) migration from the blood or lymph
vessels, adhesion in distal tissues, and formation of
micrometastatic nodes (19).
Epithelial cells express E-cadherin to achieve intercellular
adhesion, the expression of E-cadherin protein is increased, while
the occurrence of EMT is decreased (20). This study has shown that the
proliferation ability of tumor cells in the experimental group was
weakened and the number of cells and migration ability were also
decreased.
In conclusion, aspirin can inhibit the proliferation
and migration of gastric tumor cells in mice.
Acknowledgements
This study was supported by the Science and
Technology Project of Huaian, Jiangsu Province (project no.
HAS2013029).
References
|
1
|
Soon S, Chia WJ, Redekop K and Wee HL: A
cost-effectiveness analysis of aspirin in the primary prevention of
cardiovascular diseases and colorectal cancer. Value Health.
18:A4622015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wilson LF, Green AC, Kendall BJ, Jordan
SJ, Nagle CM, Bain CJ, Neale RE and Whiteman DC: Cancers prevented
in Australia in 2010 through the consumption of aspirin. Aust NZ J
Public Health. 39:414–417. 2015. View Article : Google Scholar
|
|
3
|
Wright JL, Chéry L, Holt S, Lin DW,
Luedeke M, Rinckleb AE, Maier C and Stanford JL: Aspirin and NSAID
use in association with molecular subtypes of prostate cancer
defined by TMPRSS2: ERG fusion status. Prostate Cancer Prostatic
Dis. 19:53–56. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mikami J, Kurokawa Y, Takahashi T,
Miyazaki Y, Yamasaki M, Miyata H, Nakajima K, Takiguchi S, Mori M
and Doki Y: Antitumor effect of antiplatelet agents in gastric
cancer cells: an in vivo and in vitro study. Gastric Cancer. Oct
20–2015.(Epub ahead of print).
|
|
5
|
Zhang YP, Wan YD, Sun YL, Li J and Zhu RT:
Aspirin might reduce the incidence of pancreatic cancer: a
meta-analysis of observational studies. Sci Rep. 5:154602015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wan L, Dong H, Xu H, Ma J, Zhu Y, Lu Y,
Wang J, Zhang T, Li T, Xie J, et al: Aspirin, lysine, mifepristone
and doxycycline combined can effectively and safely prevent and
treat cancer metastasis: prevent seeds from gemmating on soil.
Oncotarget. 6:35157–35172. 2015.PubMed/NCBI
|
|
7
|
Gonzalez-Gonzalez M, Garcia J, Alcazar JA,
Gutierrez ML, Gonzalez LM, Bengoechea O, Abad MM, Santos-Briz A,
Blanco O, Martin M, Rodriguez A, Fuentes M, Munoz-Bellvis L, Orfao
A and Sayagues JM: Association between the cytogenetic profile of
tumor cells and response to preoperative radiochemotherapy in
locally advanced rectal cancer. Medicine (Baltimore). 93:e1532014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fontana E, Smyth EC, Cunningham D, Rao S,
Watkins D, Allum WH, Thompson J, Waddell T, Peckitt C, Chau I, et
al: Improved survival in resected oesophageal and gastric
adenocarcinomas over a decade: the Royal Marsden experience
2001–2010. Gastric Cancer. Nov 5–2015.(Epub ahead of print).
PubMed/NCBI
|
|
9
|
Gu Q, Zhang J, Hu H, Tan YE, Shi S and
Nian Y: Clinical significance of MiR-137 expression in patients
with gastric cancer after radical gastrectomy. PLos One.
10:e01423772015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sugimura T and Fujimura S: Tumour
production in glandular stomach of rat by
N-methyl-N'-nitro-N-nitrosoguanidine. Nature. 216:943–944. 1967.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Thompson J, Epting T, Schwarzkopf G,
Singhofen A, Eades-Perner AM, van Der Putten H and Zimmermann W: A
transgenic mouse line that develops early-onset invasive gastric
carcinoma provides a model for carcinoembryonic antigen-targeted
tumor therapy. Int J Cancer. 86:863–869. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Furukawa H, Iwanaga T, Nakajima T,
Okabayashi K, Nakazato H, Hiratsuka M, Ohta K, Kito T, Yamamura Y
and Goto S: Randomized study with mitomycin C +
5-fluorouracil+cytosine arabinoside (MFC)+5-fluorouracil,
MFC+tegafur and uracil (UFT), and MF+UFT in advanced gastric
cancer: interinstitutional differences in a multicenter study in
Japan. J Surg Oncol. 60:59–64. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Logan RF, Grainge MJ, Shepherd VC,
Armitage NC and Muir KR: ukCAP Trial Group: Aspirin and folic acid
for the prevention of recurrent colorectal adenomas.
Gastroenterology. 134:29–38. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Osborn VW, Chen SC, Weiner J, Schwartz D
and Schreiber D: Impact of aspirin on clinical outcomes for African
American men with prostate cancer undergoing radiation. Tumori.
102:65–70. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Huang WK, Tu HT and See LC: Aspirin use on
incidence and mortality of gastrointestinal cancers: current state
of epidemiological evidence. Curr Pharm Des. 21:5108–5115. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Di Francesco L, López Contreras LA, Sacco
A and Patrignani P: New insights into the mechanism of action of
aspirin in the prevention of colorectal neoplasia. Curr Pharm Des.
21:5116–5126. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kraus S, Sion D and Arber N: Can we select
patients for colorectal cancer prevention with aspirin? Curr Pharm
Des. 21:5127–5134. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kastrati I, Litosh VA, Zhao S, Alvarez M,
Thatcher GR and Frasor J: A novel aspirin prodrug inhibits NFκB
activity and breast cancer stem cell properties. BMC Cancer.
15:8452015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cheng GZ, Zhang W and Wang LH: Regulation
of cancer cell survival, migration, and invasion by Twist: AKT2
comes to interplay. Cancer Res. 68:957–960. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Qin Y, Tang B, Hu CJ, Xiao YF, Xie R, Yong
X, Wu YY, Dong H and Yang SM: An hTERT/ZEB1 complex directly
regulates E-cadherin to promote epithelial-to-mesenchymal
transition (EMT) in colorectal cancer. Oncotarget. 7:351–361.
2016.PubMed/NCBI
|