Introduction
Identification of genes required for tumor
maintenance often aids therapeutic targeting in the treatment of
cancer. Philadelphia-positive (Ph+) leukemia is an
example of an oncogene-addicted disease due to the essential role
of breakpoint cluster region (BCR)-ABL in the development and
maintenance of either chronic myeloid leukemia (CML) and acute
lymphoblastic leukemia (ALL) (1–4). However,
inhibition of BCR-ABL via selective TKIs does not result in disease
eradication (5). Furthermore, the
response rates of patients with Ph+ ALL to TKIs are much
poorer than those of patients with CML, with only 50% of patients
cured by TKI plus chemotherapy and allogenic stem cell
transplantation programs (6).
Therefore, it is mandatory to identify targetable pathways that
synergize with BCR-ABL in the maintenance of Ph+
leukemias and, in particular, ALL. The sustained inactivation of
tumor suppressors is also involved in tumor maintenance. In
particular, it was previously demonstrated that restoration of p53
in murine models of different forms of cancer promotes the
induction of cancer-selective apoptosis without affecting normal
tissues (7). This supports the
concept that strategies to promote pharmacological reactivation of
p53 may be important in the eradication of cancer. Recently, it was
observed that BCR-ABL activates the deubiquitinase HAUSP to promote
phosphatase and tensin homolog (PTEN) delocalization in CML
(8). In addition to targeting PTEN
(9), HAUSP is able to regulate p53
protein stability (10,11), suggesting that the BCR-ABL/HAUSP
network may regulate p53 protein stability. Due to the requirement
of additional therapies, particularly against Ph+ ALL,
the present study investigated the p190 BCR-ABL/HAUSP network and
demonstrated that it affects p53 stability; therefore, this pathway
may be targeted by selective inhibitors.
Materials and methods
Cell culture and reagents
HEK 293T cells (ATCC, Manassas, VA, USA) were
maintained in Dulbecco's modified Eagle's medium (Euroclone S.p.A.,
Pero, Italy) supplemented with 10% heat-inactivated fetal bovine
serum (Sigma-Aldrich, St. Louis, MO, USA) and 2 mM glutamine
(Euroclone S.p.A.) at 37°C in a humidified atmosphere with 5%
CO2. The primary antibodies used were as follows:
Polyclonal rabbit anti-HAUSP (cat no. sc-30164; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA), monoclonal mouse
anti-Myc-Tag (cat no. 2276; Cell Signaling Technology, Inc.,
Danvers, MA, USA), monoclonal mouse anti-phospho-tyrosine (cat no.
sc-7020; Santa Cruz Biotechnology, Inc.), polyclonal rabbit
anti-BCR (cat no. sc-885; Santa Cruz Biotechnology, Inc.),
monoclonal mouse anti-phospho-p53 (cat no. 9286; Cell Signaling
Technology, Inc.), monoclonal mouse anti-p53 (cat no. sc-98; Santa
Cruz Biotechnology, Inc.) and polyclonal rabbit anti-heat shock
protein 90α/β (cat no. sc-7947; Santa Cruz Biotechnology, Inc.),
which was used as a loading control. Secondary goat anti-rabbit and
goat anti-mouse antibodies were were from Thermoscientific
(Waltham, MA, USA), cat nos. 31460 and 31430, respectively. Primary
and secondary antibodies were used at 1:1000 and 1:8000 dilutions,
respectively.
Plasmid construction, transfection
assay and pharmacological treatments
Myc-Tag ubiquitin-specific-processing protease 7
(HAUSP) wild-type, Myc-Tag HAUSP triple mutant and p190 BCR/ABL
plasmids were constructed as previously described (8). To perform transient transfection, the
calcium phosphate transfection method was used. Briefly, for each
10-cm dish, 10 µg DNA, 61 µl 2 M CaCl2 and enough
distilled water to bring the total volume to 0.5 ml was added
slowly to 0.5 ml HEPES buffered saline, which was aerated during
the addition. After incubating for 20 min at room temperature, the
mix was added to the plate and incubated for a further 16–24 h at
37°C in a humidified atmosphere with 5% CO2. A total of
1 or 5 µM imatinib and 4.2 µM P5091 was added for 24 h to
inactivate p190 BCR/ABL and HAUSP, respectively. The same quantity
of dimethyl sulfoxide was used for the untreated control.
Western blot and
immunoprecipitation
Total cell extraction was performed using
co-immunoprecipitation buffer [150 mM NaCl, 1 mM
ethylenediaminetetraacetic acid, 50 mM HEPES (pH, 7.5), 1% Triton
and 10% glycerol] supplemented with protease inhibitor (cat no.
036K4082; Sigma-Aldrich) and a phosphatase cocktail composed of
PMSF and Na3VO4. Following quantification by
Bio-Rad Protein assay (Bio-Rad Laboratories, Inc., Hercules, CA,
USA), 30 µg protein extract was denatured, reduced, separated by 8%
sodium dodecyl sulfate polyacrylamide gel electrophoresis and
electrophoretically transferred onto nitrocellulose membranes. The
membranes were subsequently quenched with 5% bovine serum albumin
and probed overnight with primary antibodies. Protein detection was
performed using peroxidise-conjugated secondary antibodies and
enhanced chemiluminescence reagent (cat no. 170–5060; Bio-Rad
Laboratories, Inc.), according to the manufacturer's protocol. For
immunoprecipitation experiments, 500 µg protein extracts were
precleared with agarose-conjugated normal immunoglobulin G (Santa
Cruz Biotechnology, Inc.) for 1 h at 4°C and then precipitated
overnight at 4°C with anti-Myc-Tag antibody along with
Dynabeads® protein G. The immunoprecipitate samples were
then resolved by western blot analysis. Image acquisition and
analysis was performed using ImageLab software (Bio-Rad
Laboratories, Inc.).
Statistical analysis
Two-sided Student's t-test or two way ANOVA with
Bonferroni post-test were calculated using GraphPad Prism v5.0
(GraphPad Software, Inc., La Jolla, CA, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
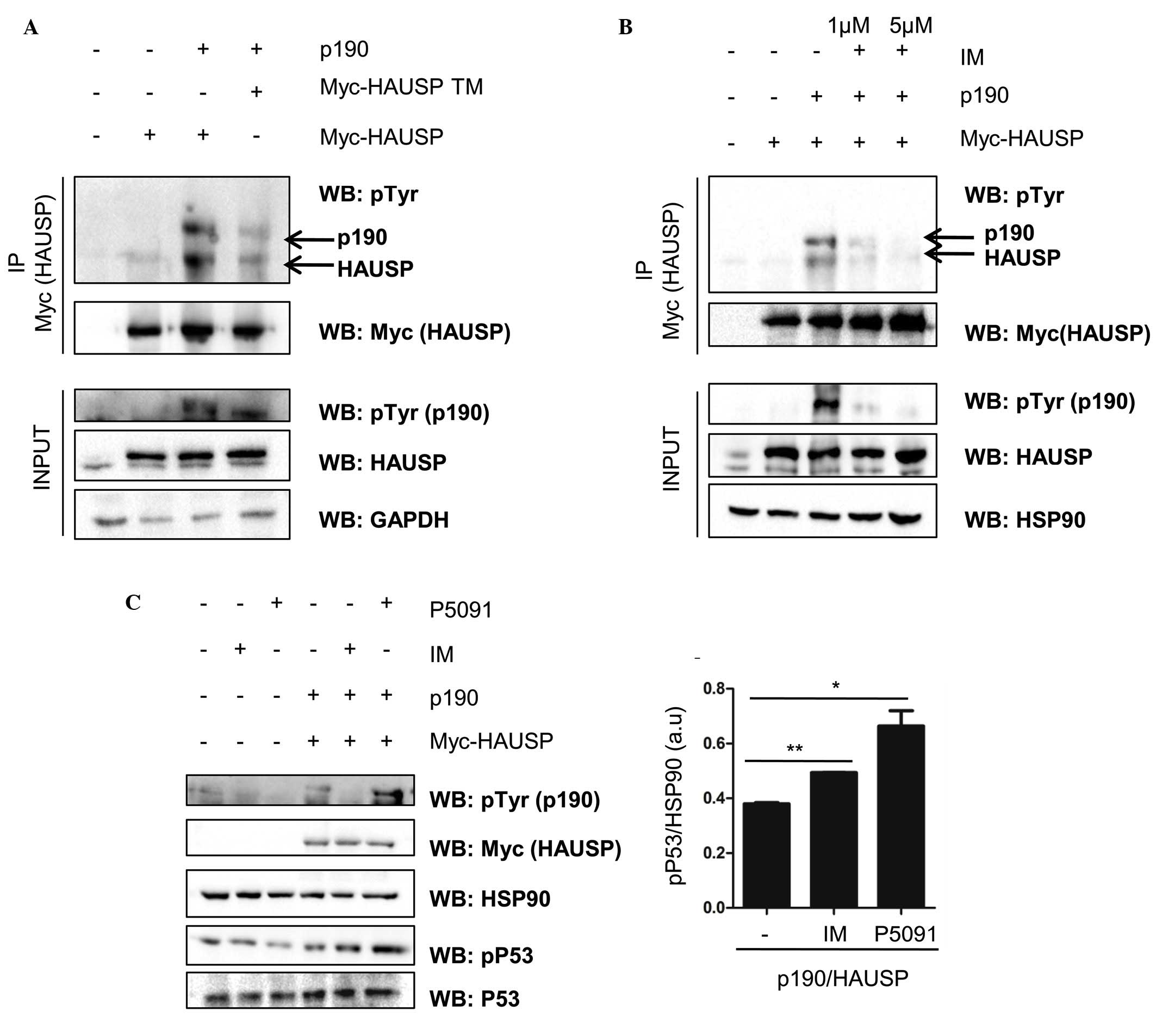
Expression of HAUSP triple mutant
reduces HAUSP phosphorylation
To investigate whether p190 BCR-ABL retains the
capability to interact with HAUSP and to promote HAUSP
phosphorylation, p190 BCR-ABL and Myc-HAUSP-expressing vectors were
transfected into HEK 293T cells. At 48 h post-transfection, the HEK
293T cells were lysed, and then immunoprecipitation with
anti-Myc-Tag antibody (HAUSP) was performed. As presented in
Fig. 1A, HAUSP was phosphorylated on
tyrosine residues by p190 BCR-ABL. Notably, expression of the HAUSP
triple mutant (Y243F/Y878F/Y947F), which bears mutants on three
tyrosine residues phosphorylated by p210 BCR/ABL (8), reduced HAUSP phosphorylation levels in
HEK 293T when compared to those of HAUSP WT, as observed with p210
BCR/ABL (8).
BCR-ABL inhibition is associated with
HAUSP dephosphorylation, and inhibition and deletion of HAUSP is
associated with p53 destabilization
To test the contribution of BCR-ABL tyrosine kinase
in the phosphorylation of HAUSP, transfected HEK 293T cells were
treated with varying concentrations of the BCR-ABL TKI, imatinib.
The results demonstrated that P190 BCR-ABL inhibition, as observed
with p210 BCR/ABL (8), was associated
with HAUSP dephosphorylation (Fig.
1B). In addition to the ability of HAUSP to deubiquitinate PTEN
and the relevance of the BCR-ABL/HAUSP/PTEN network in CML, HAUSP
is also able to target p53 (10,11). In
particular, HAUSP has been previously reported to interfere with
the mouse double minute 2 homolog (MDM2)/p53 network causing p53
destabilization (10,11). The results of the present study were
consistent with these previous findings, demonstrating that the
deletion and inhibition of HAUSP was associated with p53
upregulation (Fig. 1C).
Imatinib and P5091 promote p53
upregulation and phosphorylation
To test whether HAUSP inhibition is able to regulate
p53 protein stability downstream of BCR-ABL, p190
BCR-ABL-transfected cells were treated with imatinib and the HAUSP
inhibitor, P5091. As presented in Fig.
1C, imatinib was able to promote p53 upregulation; however, the
greatest increase in p53 protein expression and phosphorylation on
Ser15, a marker of functionally active p53, was observed following
incubation with the HAUSP inhibitor (P5091) with subsequent
apoptosis induction (data not shown).
Discussion
Identification of signaling transduction pathways
that function in the maintenance of cancer are extremely important
from a therapeutic standpoint, and targeting of these pathways may
aid the eradication of cancer. ALL therapy requires the detection
of additional targets, not only to achieve disease abolishment, but
also to achieve a more positive overall response to TKIs. The
present study identified the p190-BCR-ABL/HAUSP/p53 network as a
challenging, targetable pathway that promotes the regulation of p53
protein stability. The data obtained from the present study did not
demonstrate whether p53 upregulation is directly regulated by HAUSP
activity or is dependent on the more complex BCR/ABL/HAUSP/MDM2/p53
network, but the upregulation in p53 levels supports the assessment
of HAUSP inhibitors in the clinical setting.
Acknowledgements
The authors of the present study would like to thank
Professor Pier Paolo Pandolfi of the Beth Israel Deaconess Cancer
Center, Department of Medicine and Pathology, Beth Israel Deaconess
Medical Center, Harvard Medical School, Boston, MA, USA and
Professor Min Sup Song from the Department of Molecular and
Cellular Oncology at the MD Anderson Cancer Center, University of
Texas, Houston, TX, USA for their contributions regarding HAUSP
biology. The present study was supported by funds from the Italian
Ministero of Salute, Ricerca Finalizzata, Giovani Ricercatori
(grant no. 2011–02351167).
References
|
1
|
Pellicano F, Mukherjee L and Holyoake TL:
Concise review: Cancer cells escape from oncogene addiction:
Understanding the mechanisms behind treatment failure for more
effective targeting. Stem Cells. 32:1373–1379. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Asmussen J, Lasater EA, Tajon C,
Oses-Prieto J, Jun YW, Taylor BS, Burlingame A, Craik CS and Shah
NP: MEK-dependent negative feedback underlies BCR-ABL-mediated
oncogene addiction. Cancer Discov. 4:200–215. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sawyers CL: Shifting paradigms: The seeds
of oncogene addiction. Nat Med. 15:1158–1161. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sharma SV, Gajowniczek P, Way IP, Lee DY,
Jiang J, Yuza Y, Classon M, Haber DA and Settleman J: A common
signaling cascade may underlie ‘addiction’ to the Src, BCR-ABL, and
EGF receptor oncogenes. Cancer Cell. 10:425–435. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Morotti A, Panuzzo C, Fava C and Saglio G:
Kinase-inhibitor-insensitive cancer stem cells in chronic myeloid
leukemia. Expert Opin Biol Ther. 14:287–299. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Maino E, Sancetta R, Viero P, Imbergamo S,
Scattolin AM, Vespignani M and Bassan R: Current and future
management of Ph/BCR-ABL positive ALL. Expert Rev Anticancer Ther.
14:723–740. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ventura A, Kirsch DG, McLaughlin ME,
Tuveson DA, Grimm J, Lintault L, Newman J, Reczek EE, Weissleder R
and Jacks T: Restoration of p53 function leads to tumour regression
in vivo. Nature. 445:661–665. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Morotti A, Panuzzo C, Crivellaro S,
Pergolizzi B, Familiari U, Berger AH, Saglio G and Pandolfi PP:
BCR-ABL disrupts PTEN nuclear-cytoplasmic shuttling through
phosphorylation-dependent activation of HAUSP. Leukemia.
28:1326–1333. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Song MS, Salmena L, Carracedo A, Egia A,
Lo-Coco F, Teruya-Feldstein J and Pandolfi PP: The
deubiquitinylation and localization of PTEN are regulated by a
HAUSP-PML network. Nature. 455:813–817. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cummins JM and Vogelstein B: HAUSP is
required for p53 destabilization. Cell Cycle. 3:689–692. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cummins JM, Rago C, Kohli M, Kinzler KW,
Lengauer C and Vogelstein B: Tumour suppression: Disruption of
HAUSP gene stabilizes p53. Nature. 428:1 p following. 4862004.
View Article : Google Scholar : PubMed/NCBI
|