Introduction
Detecting carcinoma at an early stage or tackling it
at the late stage in an efficient way is always a challenge in
clinical oncology. As one of the most frequently occurring
gynecological malignancies, ovarian carcinoma is the fifth most
common cause of cancer-associated mortality in women (1). The poor clinical outcome is likely to be
caused, at least in part, by the high percentage of cases diagnosed
at an advanced stage. Tumor recurrence and chemoresistance are
common reasons for carcinoma-related mortality (2). Understanding the possible mechanisms
underlying the aggressive progression of ovarian carcinoma may,
therefore, provide better prognostic and predictive factors for
this disease.
T lymphoma invasion and metastasis protein (Tiam1)
was first identified as a gene that induces invasion and metastasis
using T lymphoma cells by proviral tagging and in vitro
selection for invasiveness (3,4). Tiam1 is
a guanine nucleotide exchange factor that mediates the specific
activation of Rac1 (5–7). Tiam1 maintains the specificity of Rac1
toward specific downstream effector pathways, whereas Rac1
regulates cell survival and cell cycle progression (8,9). Together,
Tiam1-Rac1 is a critical component in the biology of human tumors,
affecting the transformed cells themselves and the tumor
microenvironment (10–12). Tiam1 is a potent modifier of oncogenic
Ras-induced skin tumor initiation, promotion and progression
(13). Moreover, numerous studies
have demonstrated that the upregulation of Tiam1 is associated with
an aggressive phenotype and a poor clinical outcome in several
types of malignant tumors, including breast (14), colon (15), prostate (16), liver (17) and nasopharyngeal (11) tumors, and esophageal squamous cell
carcinoma (18). However, to date,
the correlation between Tiam1 expression and ovarian carcinoma has
not been adequately investigated.
The current study was undertaken to investigate the
expression of Tiam1 in human ovarian benign, borderline and
malignant tumors, and to investigate whether Tiam1 expression is
associated with the clinicopathological features of ovarian
carcinoma. Furthermore, the prognostic value of high Tiam1
expression in patients with serous ovarian carcinoma was also
assessed.
Materials and methods
Ethics statement
This study complied with the Declaration of Helsinki
and was approved by the Human Ethics and Research Ethics committees
of Yanbian University Medical College (Yanji, Jilin, China) and The
People's Hospital of Beijing University (Beijing, China). Through
the use of surgical consent forms, patients were informed that the
resected specimens would be stored by the hospital and potentially
used for scientific research, and that their privacy would be
maintained. Follow-up survival data were collected retrospectively
through medical record analyses.
Patients and tissue specimens
A total of 330 human ovarian tumor specimens,
including 182 serous carcinomas, 76 borderline serous tumors and 72
benign serous tumors, were used for this study. These tumors were
selected randomly from female patients undergoing surgery between
February 2006 and October 2010, and stored in the Tumor Tissue Bank
of Yanbian University Medical College. Pathological parameters,
including age, menopausal status, grade, metastasis and survival
data, were carefully reviewed in all 182 serous ovarian
carcinomas.
In these 182 cases, the patients ages ranged from 16
to 75 years, with a mean age of 48.3 years, and the patient age
ratio between ≥ 48 years to <48 years was 101:81. All samples
were routinely fixed in 10% buffered formalin and embedded in
paraffin, and tissue sections (4-µm) were stained with hematoxylin
and eosin (H&E). The H&E-stained slides of the different
biopsies were reviewed by two experienced pathologists and one
appropriate paraffin block was selected for this study.
Histopathological grading was performed using the World Health
Organization (Pathology and Genetics Tumors of Gynecological
System) criteria (with 72 low-grade tumors and 110 high-grade
tumors) (19). All the ovarian
carcinoma patients were clinically staged according to the
International Federation of Gynecology and Obstetrics (FIGO)
staging system [with 86 early-stage tumors (FIGO stages I and II)
and 96 late-stage tumors (FIGO stages III and IV)] (19). None of the ovarian carcinoma patients
received pre-operative radiation or chemotherapy prior to surgery.
All ovarian carcinoma patients had follow-up records of longer than
5 years.
Immunohistochemical (IHC)
analysis
IHC analysis was performed using the DAKO LSAB kit
(DAKO A/S, Glostrup, Denmark). Briefly, to eliminate endogenous
peroxidase activity, 4-µm thick tissue sections were
deparaffinized, rehydrated and incubated with 3%
H2O2 in methanol for 15 min at room
temperature (RT). The antigen was retrieved at 95°C for 20 min by
placing the slides in 0.01 M sodium citrate buffer (pH 6.0). The
slides were then incubated with the polyclonal anti-C-terminal
Tiam1 antibody (1:100 dilution; catalog no. SC-872; Santa Cruz
Biotechnology Inc., Dallas, TX, USA) at 4°C overnight. Following
incubation with the biotinylated secondary antibody (catalog no.
PV9000; ZSGB-Bio, Beijing, China) at RT for 30 min, the slides were
incubated with streptavidin-peroxidase complex at RT for 30 min.
Immunostaining was developed using 3,3′-diaminobenzidine, and
Mayer's hematoxylin was used for counterstaining (20). Rabbit immunoglobulin G (IgG) (1:2,500
dilution; catalog no. GTX35035; GeneTex Inc., Irvine, CA, USA) was
used as an isotope control. In addition, positive tissue sections
were processed while omitting the primary antibody (rabbit
anti-Tiam1) for negative controls.
As described previously (21), expression was scored by two
pathologists who did not possess knowledge of the clinical data. In
case of discrepancies, a final score was established by
reassessment on a double-headed microscope. Briefly, the
immunostaining for Tiam1 was semi-quantitatively scored as follows:
-, no or <5% positive cells; +, 5–25% positive cells; ++, 26–50%
positive cells; and +++, >50% positive cells). The cytoplasmic
and nuclear expression patterns were each considered as a positive
staining result. Tissue sections scored as ++ and +++ were
considered as strong positive results (high expression) for Tiam1.
For the survival data analysis, ++ or +++ samples were considered
as high Tiam1 expression and - or + samples were considered as low
Tiam1 expression.
Immunofluorescence (IF) staining
analysis
IF staining was used to detect the subcellular
localization of Tiam1 protein in ovarian carcinoma SK-OV-3 cells
(Korean Cell Line Bank, Seoul, Korea). All steps were performed at
RT. SK-OV-3 cells were grown to 70–80% confluence on coverslips,
then fixed with 4% paraformaldehyde for 10 min and permeabilized
with 0.5% Triton X-100 for 10 min after 24 h. Subsequent to being
blocked with 3% Albumin Bovine V (catalog no. A8020; Solarbio,
Beijing, China) for 1 h, the slides were quickly and gently washed
with phosphate-buffered saline (PBS). The cells were then incubated
with the Tiam1 antibody (1:100 dilution) at 4°C overnight, followed
by incubation with Alexa Fluor® 488 goat anti-rabbit IgG
(H+L) (catalog no. A11004; 1:1,000 dilution; Invitrogen; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) for 1 h. After washing
with PBS, the cells were counterstained with
4′,6-diamidino-2-phenylindole (catalog no. C1006; Beyotime,
Shanghai, China) and the coverslips were mounted with Antifade
Mounting Medium (catalog no. P0126; Beyotime) (21). Finally, the IF signals were visualized
and recorded using a Leica SP5II confocal microscope (Leica
Microsystems GmbH, Wetzlar, Germany).
Statistical analysis
Statistical analyses included descriptive statistics
with determination of minimal and maximal values, means and
medians, with 95% confidence intervals (CIs) for particular
variables. The χ2 test and Fisher's exact test were used
to assess correlations between clinicopathological characteristics
and the expression of the studied protein. The Kaplan-Meier method
was used to calculate the survival rates after tumor removal and
the Log-rank test was used to analyze the differences in survival
curves. Multivariate survival analysis was performed on all the
characteristics measured by univariate survival analysis (age,
menopausal status, histological grade, metastasis, FIGO stage and
Tiam1 expression) through the Cox proportional hazard regression
model. Statistical analyses were performed using the SPSS software
program for windows, version 17.0 (SPSS, Inc, Chicago, IL, USA),
and the JMP software program for Mac, version 10.0.0 (SAS Institute
Inc, Cary, NC, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Tiam1 protein is overexpressed in
serous ovarian carcinoma
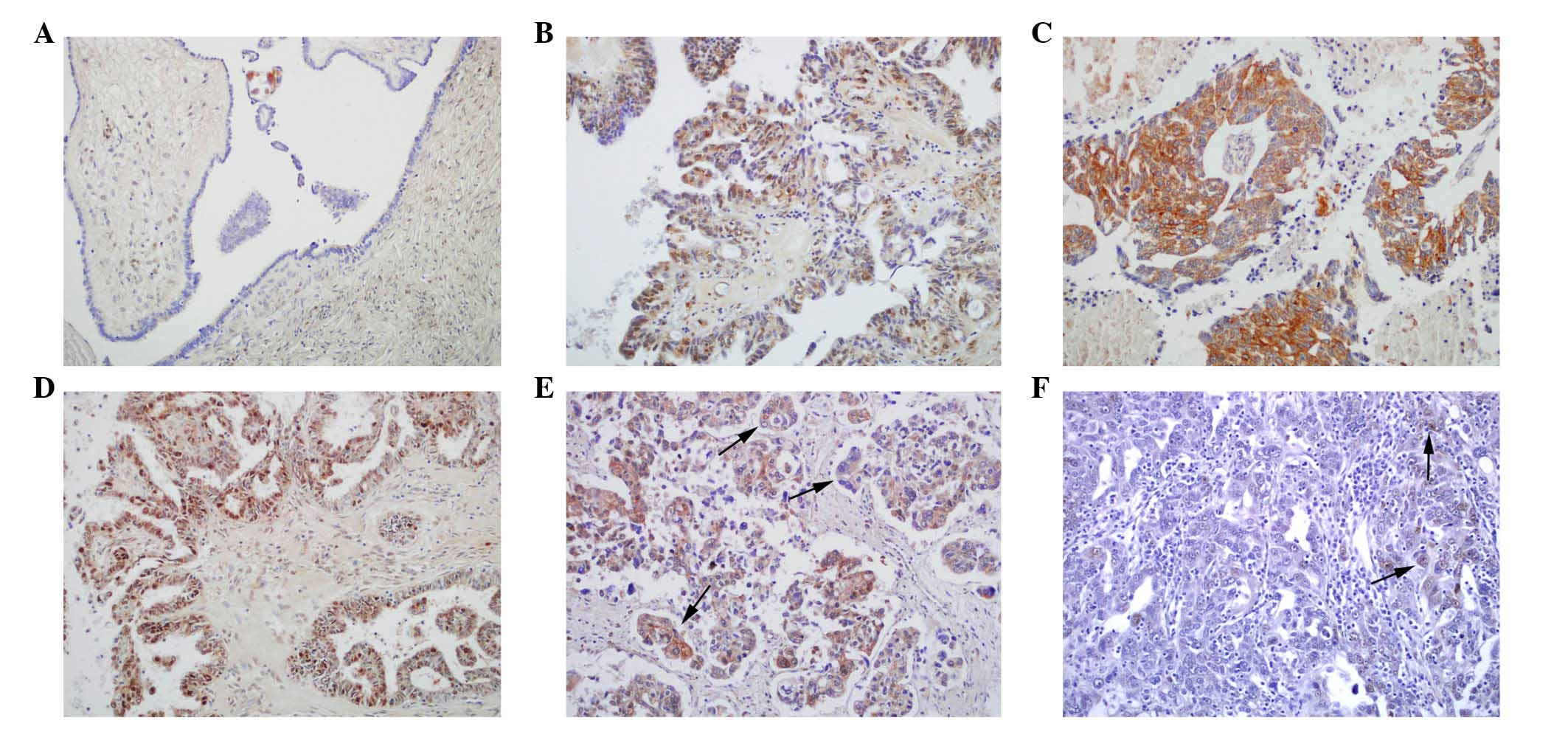
IHC staining for the Tiam1 protein was performed in
182 ovarian carcinoma tissues. The analysis of Tiam1 expression in
the ovarian carcinoma cells from the 182 patients revealed
predominantly cytoplasmic and nuclear expression patterns in the
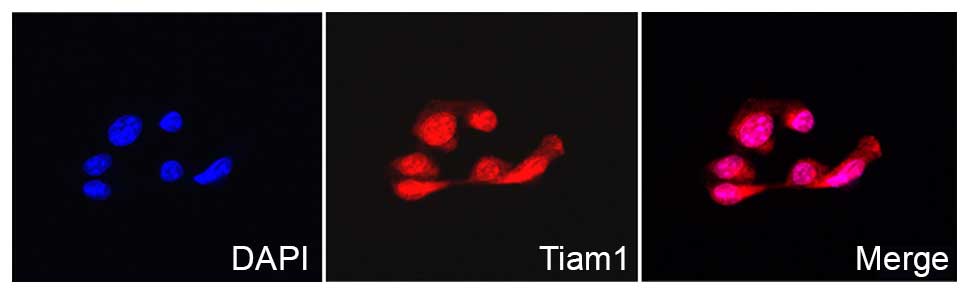
positive cases (Fig. 1). The IF
staining results also showed that Tiam1 protein was localized in
the cytoplasm and nuclei of the ovarian carcinoma SK-OV-3 cells
(Fig. 2).
IHC staining indicated that the rate of positive
Tiam1 protein expression was significantly higher in serous
carcinomas (75.8%; 138/182) than in benign serous tumors (36.1%;
26/72) (P<0.01). Similarly, the rate of strongly positive Tiam1
protein expression was significantly higher in serous carcinomas
(59.3%; 108/182) than in benign serous tumors (12.5%; 9/72)
(P<0.01). Moreover, the rates of positive (51.3%; 39/76) and
strongly-positive (31.6%; 24/76) Tiam1 protein expression in
borderline serous tumors were significantly higher than those in
benign serous tumors (P<0.05 and P<0.01, respectively)
(Table I).
 | Table I.T lymphoma invasion and metastasis 1
protein expression in ovarian carcinoma. |
Table I.
T lymphoma invasion and metastasis 1
protein expression in ovarian carcinoma.
|
|
| Positive cases,
n |
|
|
|---|
|
|
|
|
|
|
|---|
| Diagnosis | No. of cases | − | + | ++ | +++ | Positive cases,
% | Strongly-positive
cases, % |
|---|
| Serous carcinoma | 182 | 44 | 30 | 72 | 36 | 75.8a | 59.3a |
| Borderline serous
tumor | 76 | 37 | 15 | 19 | 5 | 51.3b | 31.6a |
| Benign serous
tumor | 72 | 46 | 17 | 9 | 0 | 36.1 | 12.5 |
Correlations between Tiam1 expression
status and clinicopathological features of serous ovarian
carcinoma
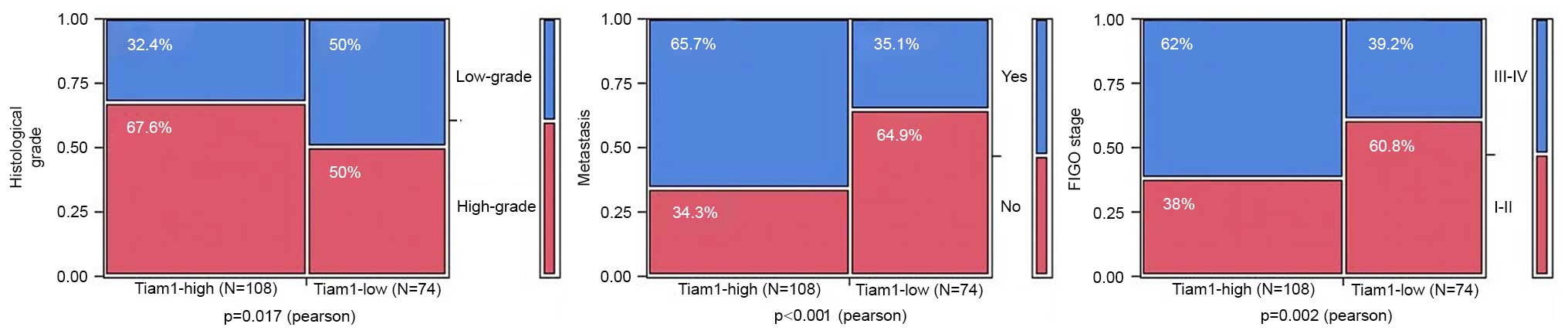
To evaluate the association between Tiam1 protein
and ovarian carcinoma progression, the correlations between high
Tiam1 expression and the clinicopathological features of ovarian
carcinomas were analyzed. The rate of strongly-positive Tiam1
protein expression was significantly higher in high-grade (66.4%;
73/110) ovarian carcinomas than in low-grade cases (48.6%; 35/72)
(P=0.017), and in ovarian carcinomas with metastasis (73.2%; 71/97)
than in cases without metastasis (43.5%; 37/85) (P<0.001).
Regarding FIGO clinical stages, the rate of strongly-positive Tiam1
expression was 69.8% (67/96) in the late-stage (IIB-IIIC) ovarian
carcinomas, but only 47.7% (41/86) in the early-stage (I–IIA) cases
(P=0.002). However, high expression of Tiam1 protein was not
associated with the age or menopausal status of the patients with
ovarian carcinoma (Fig. 3; Table II).
 | Table II.Correlations between Tiam1 protein
expression and the clinicopathological parameters of ovarian
carcinoma. |
Table II.
Correlations between Tiam1 protein
expression and the clinicopathological parameters of ovarian
carcinoma.
| Variables | No. of cases | Tiam1 strongly
positive cases, n (%) | χ2 | P-value |
|---|
| Age, years |
|
|
| 0.777 |
|
≥48 | 101 | 59 (58.4) | 0.080 |
|
|
<48 | 81 | 49 (60.5) |
|
|
| Menopausal
status |
|
|
| 0.371 |
|
Premenopausal | 86 | 54 (62.8) | 0.804 |
|
|
Postmenopausal | 96 | 54 (56.3) |
|
|
| Histological
grade |
|
|
| 0.017a |
|
Low-grade | 72 | 35 (48.6) | 5.684 |
|
|
High-grade | 110 | 73 (66.4) |
|
|
| Metastasis |
|
|
|
<0.001b |
| No | 85 | 37 (43.5) | 16.525 |
|
|
Yes | 97 | 71 (73.2) |
|
|
| FIGO stage |
|
|
| 0.002b |
|
I–II | 86 | 41 (47.7) | 9.197 |
|
|
III–IV | 96 | 67 (69.8) |
|
|
High Tiam1 expression is an
independent biomarker of a poor prognosis in patients with serous
ovarian carcinoma
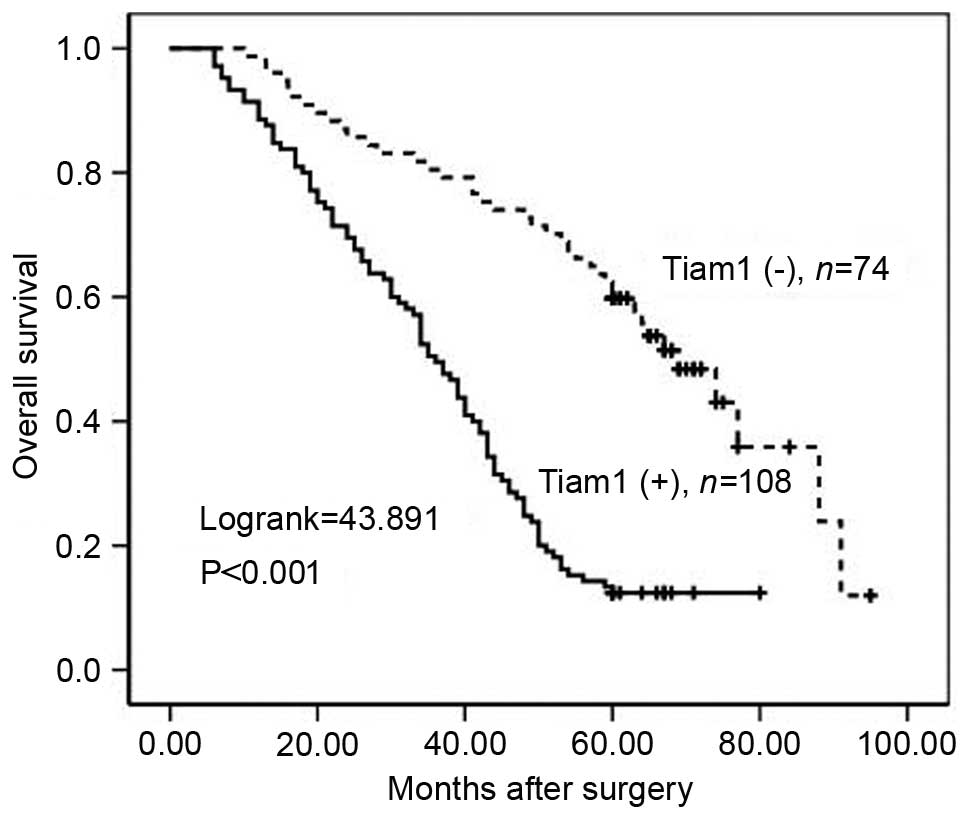
To further substantiate the importance of high Tiam1
expression in ovarian carcinoma progression, the overall survival
(OS) rate of 182 ovarian carcinoma patients was assessed using the
Kaplan-Meier method. Patients with high Tiam1 expression exhibited
a lower OS rate than those with low Tiam1 expression
(Log-rank=43.891, P<0.001) (Fig.
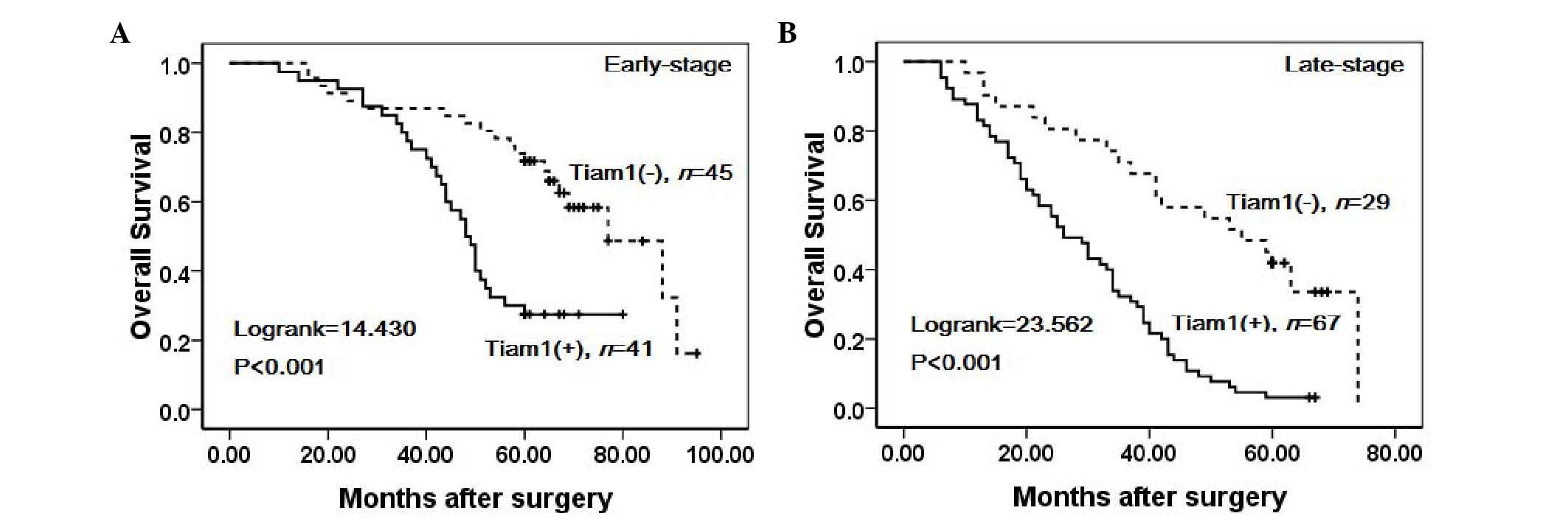
4). Similarly, ovarian carcinoma patients with high Tiam1
expression exhibited decreased OS rates compared with those with
low Tiam1 expression in early-stage cases (Log-rank=14.430,
P<0.001) and late-stage cases (Log-rank=23.562, P<0.001)
(Fig. 5). Notably, high Tiam1
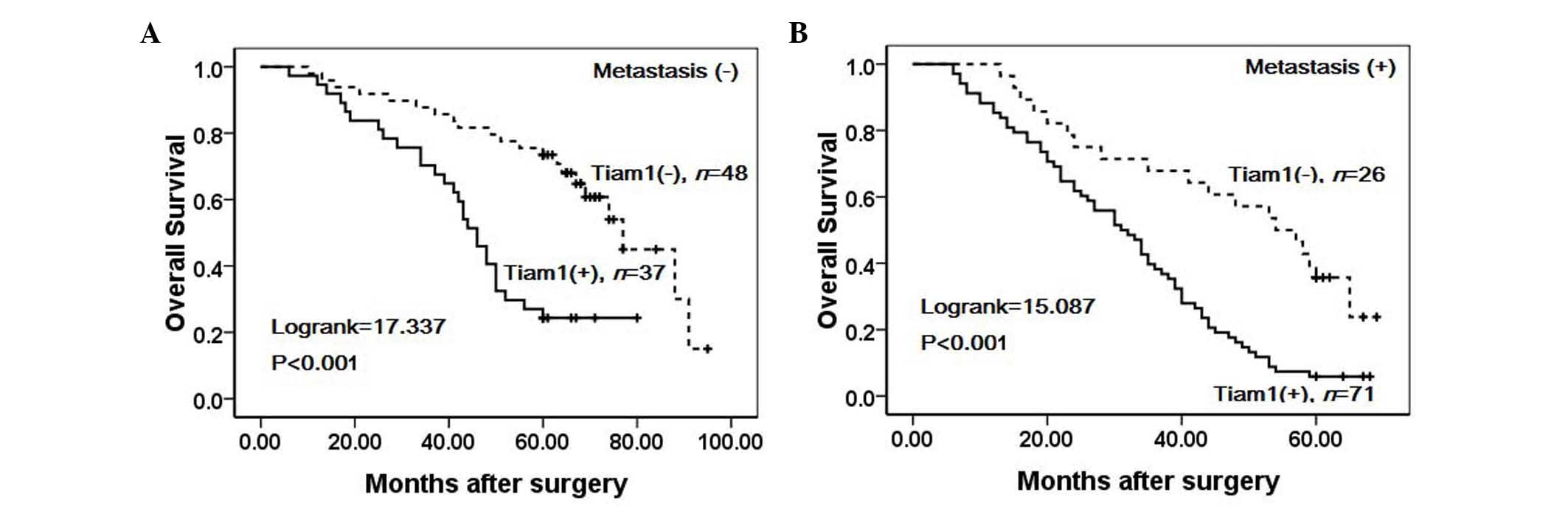
expression was associated with significantly lower OS rates in
cases with metastasis (Log-rank=15.087, P<0.001) and without
metastasis (Log-rank=17.337, P<0.001) (Fig. 6). Moreover, the survival rate of
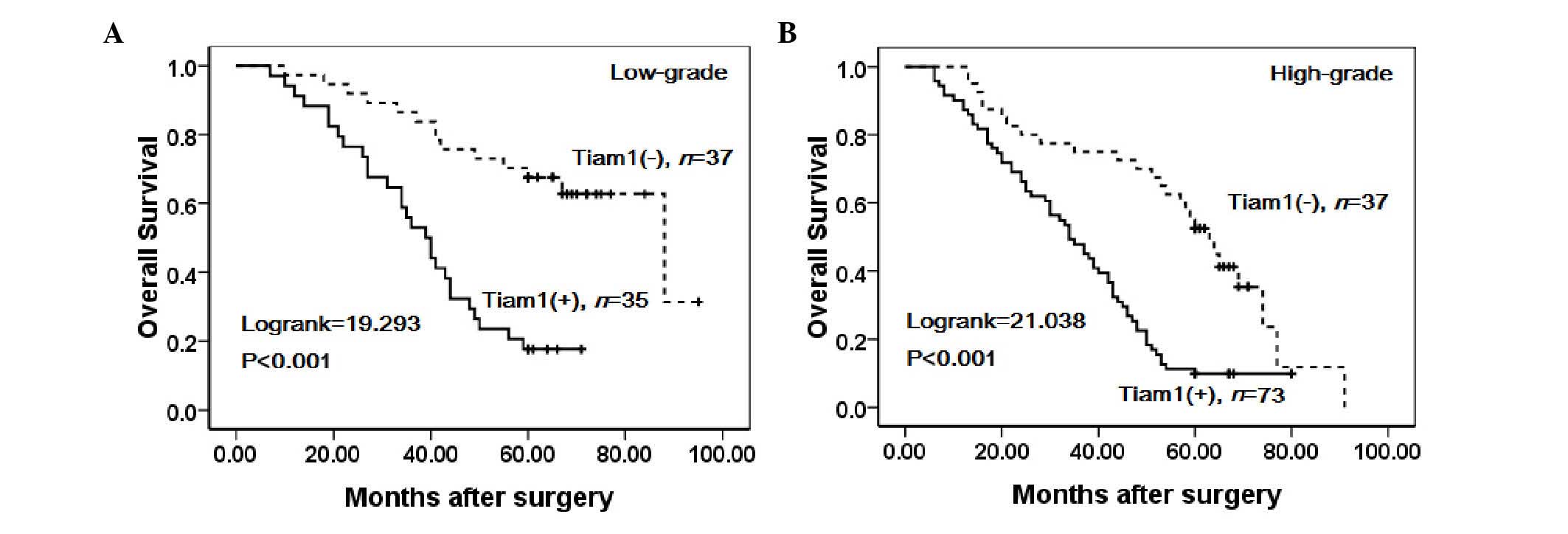
patients with low-grade (Log-rank=19.293, P<0.001) and
high-grade (Log-rank=21.038, P<0.001) ovarian carcinoma was
significantly lower in patients with tumors exhibiting high Tiam1
expression compared with those exhibiting low expression (Fig. 7).
Univariate analysis demonstrated that histological
grade (P=0.019), metastasis (P<0.001), FIGO stage (P<0.001)
and Tiam1 expression status (P<0.001) were all significantly
associated with OS rate in patients with ovarian carcinoma. These
data suggested that Tiam1 may be a valuable prognostic factor in
ovarian carcinoma. Multivariate analysis was subsequently performed
using the Cox proportional hazards model for all variables examined
in the univariate analysis. It was found that the high expression
of Tiam1 (HR, 2.559; 95% CI, 1.788–3.663; P<0.001), FIGO stage
(HR, 2.530; 95% CI, 1.835–3.488; P<0.001) and metastasis (HR,
2.088; 95% CI, 1.493–2.919; P<0.001) were significant
independent prognostic factors for survival in ovarian carcinoma
(Table III).
 | Table III.Univariate and multivariate analysis
of clinicopathological factors for the overall survival rate of 182
patients with ovarian carcinoma. |
Table III.
Univariate and multivariate analysis
of clinicopathological factors for the overall survival rate of 182
patients with ovarian carcinoma.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
|
Characteristics | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age, years | 1.079
(0.802–1.453) | 0.615 | 0.979
(0.715–1.340) | 0.894 |
| Menopausal
status | 1.093
(0.811–1.472) | 0.559 | 0.920
(0.660–1.283) | 0.624 |
| Histological
grade | 1.436
(1.062–1.942) | 0.019a | 1.147
(0.831–1.582) | 0.405 |
| Metastasis | 2.739
(1.981–3.787) |
<0.001b | 2.088
(1.493–2.919) |
<0.001b |
| FIGO stage | 2.705
(1.978–3.699) |
<0.001b | 2.530
(1.835–3.488) |
<0.001b |
| Tiam1 | 2.854
(2.075–3.927) |
<0.001b | 2.559
(1.788–3.663) |
<0.001b |
Discussion
Tiam1 exhibits a number of roles in the regulation
of cellular functions depending on the specific cell type,
substratum and other factors (17).
As a guanine nucleotide exchange factor, Tiam1 has been shown to be
essential in a range of tumor signaling pathways through the
regulation of Rho GTPase functions (22). It has been shown that Tiam1 is
involved in the rearrangement of the cytoskeleton, the migration of
cells, and the mobility of T-lymphoma cells, fibroblasts and
epithelial cells (5,6,23,24). Moreover, studies have indicated that
Tiam1 is involved in anti- and pro-apoptotic mechanisms (25). Accordingly, alterations in Tiam1
expression/function may contribute to tumorigenesis and carcinoma
progression of common types of human cancer.
Increasing evidence has focused on the regulation of
Tiam1, as well as its role in carcinoma progression and metastasis.
Liu et al found that Tiam1 expression was upregulated in
lung carcinoma compared with normal lung tissues, and analysis
further showed that Tiam1 overexpression was correlated with the
lymph node metastasis of patients with lung carcinoma (22). Yu et al demonstrated that Tiam1
was upregulated in colorectal carcinoma tissues and provided
evidence that Tiam1 was closely correlated to the metastatic
potential of colorectal carcinoma. Depletion of Tiam1 significantly
reduced the tumor growth and metastatic ability of colorectal
carcinoma cells (26). However, its
role in ovarian carcinoma remains to be elucidated. In the present
study, IHC and IF staining of Tiam1 protein was performed in
ovarian carcinoma tissues. It was found that positive staining of
Tiam1 was mainly localized in the cytoplasm and nucleus. Compared
with benign serous tumors, the positive and strongly-positive Tiam1
staining were significantly higher in serous carcinomas (both
P<0.01), which is consistent with the result of a previous study
by Li et al (27), and it was
also indicated that Tiam1 expression was markedly increased in
primary and metastatic ovarian carcinoma tissues relative to normal
ovarian tissues in a smaller group. These observations suggest that
the high expression of Tiam1 may be correlated with the potential
malignancy of ovarian carcinomas. Notably, a significant difference
was also observed in the rates of positive and strongly-positive
Tiam1 expression between borderline tumors and benign tumors
(P<0.05 and P<0.01), which may represent tumor progression.
Nonetheless, studies have shown that borderline tumors and
high-grade serous tumors have completely different etiologies
rather than being high-grade tumors arising from borderline tumors
(28). Accordingly, the present study
did not compare the expression of Tiam1 in borderline tumors with
that in serous carcinomas. Compatible with these findings, it was
also observed that the strongly-positive Tiam1 protein expression
rate was significantly higher in patients with late-stage serous
ovarian carcinomas compared with that in patients with early-stage
cancer. The analysis further showed that high Tiam1 expression
correlates with the metastasis of patients with ovarian carcinoma,
which suggests that Tiam1 may play an important role in the
progression and invasion of ovarian carcinoma. Similarly, the
strongly-positive Tiam1 protein expression rate was higher in
patients with high-grade ovarian carcinoma compared with low-grade
cases. High-grade serous ovarian carcinoma is the most lethal form
of gynecological malignant carcinoma, and the majority of patients
present with late clinical stages (FIGO stages III and IV) of
disease at the time of diagnosis. These results demonstrate that
the high expression of Tiam1 may assist in more accurate outcome
prediction in serous ovarian carcinoma.
Despite the strong association between Tiam1
expression and cancer, reports of Tiam1 expression-based outcome
analysis in tumor patients are limited. Nevertheless, several
reports have indicated that the high expression of Tiam1 is
significantly associated with the shortened survival of patients
with malignancies. For example, Engers et al reported that
high Tiam1 expression is an independent predictor of decreased
disease-free survival for patients with prostate cancer (16). Yang et al demonstrated that the
high expression of Tiam1 correlates with a poor prognosis in
hepatocellular carcinoma (29).
Recently, Du et al suggested that high Tiam1 expression is
associated with poor overall survival in patients with primary
gallbladder carcinoma (10). With
respect to survival, the present study found that ovarian carcinoma
patients exhibiting high Tiam1 expression had lower OS rates
compared with patients with low Tiam1 expression (P<0.01).
Univariate survival analysis revealed that histological grade,
metastasis, FIGO stage and Tiam1 expression status were all
significantly associated with the OS of patients with serous
ovarian carcinoma (P<0.05). Furthermore, multivariate survival
analysis revealed that Tiam1 expression was an independent
prognostic factor, as were metastasis and FIGO stage (P<0.01).
These clinical and experimental data indicate that Tiam1 may be a
useful prognostic factor and a potential therapeutic target in
patients with serous ovarian carcinoma.
In conclusion, the high expression of Tiam1 appears
to be significantly associated with ovarian carcinoma progression
and is an independent prognostic factor, along with metastasis and
clinical stage. Additional studies are warranted to further our
understanding of the role that Tiam1 plays in ovarian carcinoma
tumorigenesis.
Acknowledgements
This study was supported by grants from the Special
Research Project of the 973 National Science and Technology
Department of China (no. 2014CB560708) and the National Natural
Science Funds of China (no. 81260665).
References
|
1
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Smolle E, Taucher V, Pichler M, Petru E,
Lax S and Haybaeck J: Targeting signaling pathways in epithelial
ovarian cancer. Int J Mol Sci. 14:9536–9555. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Habets GG, Scholtes EH, Zuydgeest D, van
der Kammen RA, Stam JC, Berns A and Collard JG: Identification of
an invasion-inducing gene, Tiam-1, that encodes a protein with
homology to GDP-GTP exchangers for Rho-like proteins. Cell.
77:537–549. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Habets GG, van der Kammen RA, Stam JC,
Michiels F and Collard JG: Sequence of the human invasion-inducing
TIAM1 gene, its conservation in evolution and its expression in
tumor cell lines of different tissue origin. Oncogene.
10:1371–1376. 1995.PubMed/NCBI
|
|
5
|
Hordijk PL, ten Klooster JP, van der
Kammen RA, Michiels F, Oomen LC and Collard JG: Inhibition of
invasion of epithelial cells by Tiam1-Rac signaling. Science.
278:1464–1466. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Michiels F, Habets GG, Stam JC, van der
Kammen RA and Collard JG: A role for Rac in Tiam1-induced membrane
ruffling and invasion. Nature. 375:338–340. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mertens AE, Roovers RC and Collard JG:
Regulation of Tiam1-Rac signalling. FEBS Lett. 546:11–16. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bollag G, Crompton AM, Peverly-Mitchell D,
Habets GG and Symons M: Activation of Rac1 by human Tiam1. Methods
Enzymol. 325:51–61. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Matsuo N, Terao M, Nabeshima Y and Hoshino
M: Roles of STEF/Tiam1, guanine nucleotide exchange factors for
Rac1, in regulation of growthcone morphology. Mol Cell Neurosci.
24:69–81. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Du X, Wang S, Lu J, Wang Q, Song N, Yang
T, Dong R, Zang L, Yang Y, Wu T and Wang C: Clinical value of
Tiam1-Rac1 signaling in primary gallbladder carcinoma. Med Oncol.
29:1873–1878. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Qi Y, Huang B, Yu L, Wang Q, Lan G and
Zhang Q: Prognostic value of Tiam1 and Rac1 overexpression in
nasopharyngeal carcinoma. ORLJ Otorhinolaryngol Relat Spec.
71:163–171. 2009. View Article : Google Scholar
|
|
12
|
Xu K, Rajagopal S, Klebba I, Dong S, Ji Y,
Liu J, Kuperwasser C, Garlick JA, Naber SP and Buchsbaum RJ: The
role of fibroblast Tiam1 in tumor cell invasion and metastasis.
Oncogene. 29:6533–6542. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Malliri A, van der Kammen RA, Clark K, van
der Valk M, Michiels F and Collard JG: Mice deficient in the Rac
activator Tiam1 are resistant to Ras-induced skin tumours. Nature.
417:867–871. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Adam L, Vadlamudi RK, McCrea P and Kumar
R: Tiam1 overexpression potentiates heregulin-induced lymphoid
enhancer factor-1/beta-catenin nuclear signaling in breast cancer
cells by modulating the intercellular stability. J Biol Chem.
276:28443–28450. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Minard ME, Ellis LM and Gallick GE: Tiam1
regulates cell adhesion, migration and apoptosis in colon tumor
cells. Clin Exp Metastasis. 23:301–313. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Engers R, Mueller M, Walter A, Collard JG,
Willers R and Gabbert HE: Prognostic relevance of Tiam1 protein
expression in prostate carcinomas. Br J Cancer. 95:1081–1086. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Huang J, Ye X, Guan J, Chen B, Li Q, Zheng
X, Liu L, Wang S, Ding Y, Ding Y and Chen L: Tiam1 is associated
with hepatocellular carcinoma metastasis. Int J Cancer. 132:90–100.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu H, Shi G, Liu X, Wu H, Fan Q and Wang
X: Overexpression of Tiam1 predicts poor prognosis in patients with
esophageal squamous cell carcinoma. Oncol Rep. 25:841–848.
2011.PubMed/NCBI
|
|
19
|
Wang H, Wang H, Makki MS, Wen J, Dai Y,
Shi Q, Liu Q, Zhou X and Wang J: Overexpression of β-catenin and
cyclinD1 predicts a poor prognosis in ovarian serous carcinomas.
Int J Clin Exp Pathol. 7:264–271. 2013.PubMed/NCBI
|
|
20
|
Cui X, Li L, Yan G, Meng K, Lin Z, Nan Y,
Jin G and Li C: High expression of NQO1 is associated with poor
prognosis in serous ovarian carcinoma. BMC Cancer. 15:2442015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ma Y, Kong J, Yan G, Ren X, Jin D, Jin T,
Lin L and Lin Z: NQO1 overexpression is associated with poor
prognosis in squamous cell carcinoma of the uterine cervix. BMC
Cancer. 14:4142014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu S, Li Y, Qi W, Zhao Y, Huang A, Sheng
W, Lei B, Lin P, Zhu H, Li W and Shen H: Expression of Tiam1
predicts lymph node metastasis and poor survival of lung
adenocarcinoma patients. Diagn Pathol. 9:692014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sander EE, van Delft S, ten Klooster JP,
Reid T, van der Kammen RA, Michiels F and Collard JG:
Matrix-dependent Tiam1/Rac signaling in epithelial cells promotes
either cell-cell adhesion or cell migration and is regulated by
phosphatidylinositol 3-kinase. J Cell Biol. 143:1385–1398. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sander EE, ten Klooster JP, van Delft S,
van der Kammen RA and Collard JG: Rac downregulates Rho activity:
Reciprocal balance between both GTPases determines cellular
morphology and migratory behavior. J Cell Biol. 147:1009–1022.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Boissier P and Huynh-Do U: The guanine
nucleotide exchange factor Tiam1: A Janus-faced molecule in
cellular signaling. Cell Signal. 26:483–491. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yu LN, Zhang QL, Li X, Hua X, Cui YM,
Zhang NJ, Liao WT and Ding YQ: Tiam1 transgenic mice display
increased tumor invasive and metastatic potential of colorectal
cancer after 1,2-dimethylhydrazine treatment. PLoS One.
8:e730772013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li J, Liang S, Jin H, Xu C, Ma D and Lu X:
Tiam1, negatively regulated by miR-22, miR-183 and miR-31, is
involved in migration, invasion and viability of ovarian cancer
cells. Oncol Rep. 27:1835–1842. 2012.PubMed/NCBI
|
|
28
|
Kurman RJ and le M Shih: Pathogenesis of
ovarian cancer: Lessons from morphology and molecular biology and
their clinical implications. Int J Gynecol Pathol. 27:151–160.
2008.PubMed/NCBI
|
|
29
|
Yang W, Lv S, Liu X, Liu H, Yang W and Hu
F: Up-regulation of Tiam1 and Rac1 correlates with poor prognosis
in hepatocellular carcinoma. Jpn J Clin Oncol. 40:1053–1059. 2010.
View Article : Google Scholar : PubMed/NCBI
|