Introduction
Head and neck squamous cell carcinoma (HNSCC) is the
sixth most common cancer worldwide, with an annual incidence of
500,000 new cases and 200,000 mortalities (1,2).
Oropharyngeal squamous cell carcinoma (OSCC) is a subtype of HNSCC
arising from the oropharynx, which includes anatomically the
base-of-the tongue, the tonsils, the soft palate and the side and
back wall of the throat. The incidence rates for cancer sites in
the oropharynx, such as the tonsils and the base of the tongue, are
associated with a rise in human papilloma virus (HPV) infections,
but a decreased number of tobacco- and alcohol-related cancers
(3). In recent decades, there have
been a number of important advances in the standard treatment of
HNSCC, including surgical innovations for early-stage tumor
patients and novel multimodal treatment combinations for more
advanced-stage tumors, such as surgery followed by adjuvant
radiotherapy (RT) or chemoradiotherapy (CRT), or CRT alone
(4). However, outcomes remain poor in
advanced tumor stages due to frequent local and regional
metastases, and resistance to therapy (5,6).
Therefore, identification of specific validated biomarkers is
required to understand the underlying molecular mechanisms,
evaluate treatment efficiency and develop novel therapeutic
targets.
High mobility group box 1 (HMGB1) is a nuclear,
non-histone, chromatin-binding protein (7) that has been implicated in the activities
of various cell type, including enterocytes, cardiomyocytes,
pituicytes, macrophages and monocytes (8–11). HMGB1
is defined as one of the damage-associated molecular pattern
molecules that interact with a variety of pattern recognition
receptors in the microenvironment of damaged or necrotic tissues. A
number of studies have demonstrated the multiple functions of HMGB1
in tumorigenesis, angiogenesis, lymphangiogenesis and metastasis in
various malignancies (12–15). In the tumor microenvironment, cancer
cells and inflammatory cells have the ability to release HMGB1
(16). In turn, extracellular HMGB1
can accelerate inflammatory responses and may lead to tumor
formation and metastases (16).
Receptors for advanced glycosylation end products (RAGE) (17) and Toll-like receptor (TLR)-4 (18,19) are
also involved in HMGB1-mediated tumorigenesis. A study in HNSCC
cell lines showed that the interaction between HMGB1 and its
receptor RAGE resulted in the development of metastasis (17). Wild et al observed that HMGB1
may enhance tumor-infiltrating regulatory T cell (Treg)
immunosuppression by acting as a chemoattractant on the Tregs,
which express RAGE and TLR4 receptors (20). A correlation with a poor prognosis was
also found in laryngeal squamous cell carcinoma with a high serum
HMGB1 level (21) and in HNSCC with a
high HMGB1 protein expression level (22). To date, however, the expression
pattern of HMGB1 and its impact on survival is not known in
oropharyngeal squamous cell carcinoma (OSCC).
Another important hypothesis in generating and
maintaining malignancy and driving metastasis is concerning cancer
stem (−like) cells (CSCs) (6). As
shown in our previous studies, aldehyde dehydrogenase 1A1
(ALDH1A1)-positive CSCs exhibit characteristics (23,24) that
include self-renewal, invasion and
epithelial-mesenchymal-transition traits. The prognostic relevance
of ALDH1A1 has also been identified in patients with HNSCC
(25,26). Therefore, the identification of
ALDH1A1 expression and the assessment of its correlation with HMGB1
expression and clinicopathological parameters may aid in further
elucidating the biology of HNSCC from a CSC perspective and its
relevance for prognosis.
The present study investigated HMGB1 and ALDH1A1
expression in patients with OSCC with the aim of assessing whether
this expression was correlated with clinicopathological factors and
to investigate its association with overall survival (OS) time.
Materials and methods
Patients
A total of 59 OSCC patients with no prior history of
malignancies and treatment were included in this study. The main
clinical and pathological data were collected from the Institute of
Pathology (Charité - Medical Univerity of Berlin, Benjamin Franklin
Campus, Berlin, Germany) database and patient charts, as
illustrated in Table I. Tissue
biopsies were obtained during panendoscopy to confirm
histologically suspected HNSCC. Residual material was used for the
present study. OS time was calculated from the date of diagnosis of
OSCC to the date of mortality or last follow-up. This study was
approved by the Institutional Review Board of Charité - Medical
University of Berlin (Berlin, Germany).
 | Table I.Correlation between
clinicopathological characteristics and examined variables in 59
patients with oropharyngeal squamous cell carcinoma. |
Table I.
Correlation between
clinicopathological characteristics and examined variables in 59
patients with oropharyngeal squamous cell carcinoma.
|
|
| p16 expression, n
(%) |
| HMGB1 expression, n
(%) |
| ALDH1A1 expression, n
(%) |
|
|---|
|
|
|
|
|
|
|
|
|
|---|
| Characteristic | Total, n | + | − | P-value | + | − | P-value | + | − | P-value |
|---|
| Gender |
|
|
| 0.422 |
|
| 0.564 |
|
| 0.564 |
|
Female | 8 | 1
(12.5) | 7
(87.5) |
| 4
(50.0) | 4
(50.0) |
| 4
(50.0) | 4
(50.0) |
|
| Male | 51 | 13 (25.5) | 38 (74.5) |
| 31 (60.8) | 20 (39.2) |
| 20 (39.2) | 31 (60.8) |
|
| Age, years |
|
|
| 0.259 |
|
| 0.169 |
|
| 0.400 |
|
<60 | 33 | 6
(18.1) | 27 (81.9) |
| 17 (51.5) | 16 (48.5) |
| 15 (45.5) | 18 (54.5) |
|
|
≥60 | 26 | 8
(30.8) | 18 (69.2) |
| 18 (69.2) | 8
(30.8) |
| 9
(34.6) | 17 (65.4) |
|
| Primary tumor
site |
|
|
| 0.270 |
|
| 0.586 |
|
| 0.267 |
|
Tongue | 13 | 6
(46.1) | 7
(53.9) |
| 5
(38.5) | 8
(61.5) |
| 7
(53.9) | 6
(46.1) |
|
|
Tonsil | 39 | 7
(17.9) | 32 (82.1) |
| 27 (69.2) | 12 (30.8) |
| 16 (41.0) | 23 (59.0) |
|
|
Others | 7 | 1
(14.3) | 6
(85.7) |
| 3
(42.9) | 4
(57.1) |
| 1
(14.3) | 6
(85.7) |
|
| Smoking |
|
|
| 0.908 |
|
| 0.324 |
|
| 0.324 |
|
Never | 9 | 2
(22.2) | 7
(77.8) |
| 4
(44.4) | 5
(55.6) |
| 5
(55.6) | 4
(44.4) |
|
| Past
and present | 50 | 12 (24.0) | 38 (76.0) |
| 31 (62.0) | 19 (38.0) |
| 19 (38.0) | 31 (62.0) |
|
| Alcohol
intake-consumption |
|
|
| 0.516 |
|
| 0.056 |
|
| 0.056 |
|
Never | 21 | 6
(28.6) | 15 (71.4) |
| 9
(42.9) | 12 (57.1) |
| 12 (57.1) | 9
(42.9) |
|
| Past
and present | 38 | 8
(21.0) | 30 (79.0) |
| 26 (68.4) | 12 (31.6) |
| 12 (31.6) | 26 (68.4) |
|
| Tumor
differentiation |
|
|
| 0.917 |
|
| 0.400 |
|
| 0.018 |
|
Moderate | 33 | 8
(24.2) | 25 (75.8) |
| 18 (54.5) | 15 (45.5) |
| 9
(27.2) | 24 (72.8) |
|
|
Poor | 26 | 6
(23.0) | 20 (77.0) |
| 17 (65.4) | 9
(34.6) |
| 15 (57.7) | 11 (42.3) |
|
| Tumor stage |
|
|
| 0.942 |
|
| <0.001 |
|
| 0.691 |
|
T1-2 | 30 | 7
(23.3) | 23 (76.7) |
| 22 (73.3) | 8
(26.7) |
| 15 (50.0) | 15 (50.0) |
|
|
T3-4 | 29 | 7
(24.1) | 22 (75.9) |
| 13 (44.8) | 16 (55.2) |
| 9
(31.0) | 20 (69.0) |
|
| Lymph node
metastasis |
|
|
| 0.753 |
|
| 0.603 |
|
| 0.285 |
| N0 and
N1 | 22 | 3
(13.6) | 19 (86.4) |
| 14 (63.6) | 8
(36.4) |
| 7
(31.8) | 15 (68.2) |
|
| N2 and
N3 | 37 | 11 (29.7) | 26 (70.3) |
| 21 (56.8) | 16 (43.2) |
| 17 (45.9) | 20 (54.1) |
|
Histology and
immunohistochemistry
Sections (2-µm thick) from formalin-fixed,
paraffin-embedded specimens were collected from the Institute of
Pathology. An immunohistochemical staining method [EnVision
System-horseradish peroxidase (HRP) mouse/rabbit; Dako, Hamburg,
Germany] was used following deparaffinization in xylene and
rehydration. Primary antibodies used included mouse monoclonal
antibody specific for p16 (1:100 dilution; clone DCS-50; catalog
no. sc-65476; Neomarkers, Fremont, CA, USA), ALDH1A1 (1:100
dilution; clone 44; catalog no. 611195; BD Biosciences, San Jose,
CA, USA) and HMGB1 (1:200 dilution; catalog no. ab18256; Abcam,
Cambridge, MA, USA). Antigens were retrieved by steam heating for
20 min in a 0.01 M trisodium citrate buffer (pH 6.0). ChemMate
Peroxidase-Blocking Solution (Dako) was used to block endogenous
peroxidase activity for 10 min at room temperature. The slides were
incubated with antibodies for 2 h, followed by the addition of
HRP-labeled anti-mouse antibody at room temperature. Immunoreactive
proteins were visualized with 3.3-diaminobenzidine and
counterstained with Mayer's hematoxylin (Dako). The sections were
dehydrated and mounted. Positive and negative controls were
included in each run for the quality control of immunoreactivity.
Normal tonsillar tissue served as the positive control and an
isotype control (Dako) was used to replace the primary antibody as
a negative control.
Three experienced, independent observers (including
a pathologist) performed semiquantitative evaluation of the slides,
as described in our previous study (25). The evaluators were blinded to the
clinical data. The grading system for p16, ALDH1A1 and HMGB1 was
used, as previously published for OSCC (26,27).
Statistics
Statistical analysis was performed using SPSS 16.0
software (SPSS, Inc., Chicago, IL, USA). Categorical variables are
expressed as percentages and frequencies, and numerical variables
are represented as the mean ± standard deviation. Qualitative data
was compared using the χ2 or Fisher's exact test as
appropriate. OSCC-free survival was determined using the
Kaplan-Meier method. The Cox multivariate regression model was
applied to evaluate hazard ratios (HRs) and P-values. P<0.05 was
regarded to indicate a statistically significant difference.
Results
Patient characteristics and HPV
status
The median age of the 59 patients with OSCC was 58.0
years (range, 42–82 years), with a male to female ratio of 6.4:1.
Of the 59 lesions, 13 (22%) were located in the tongue, 39 (66%) in
the tonsil and 7 (12%) at another oropharynx site. The
tumor-node-metastasis staging of each patient was collected from
the Institute of Pathology data and clinical charts (28). At the time of the last follow-up, 30
(51%) patients had succumbed.
Due to the scarcity of material available from the
specimens, which was insufficient for HPV-DNA testing, p16 was used
as a surrogate HPV marker (29). In
total, of the 59 primary tumors, 14 specimens were p16+
(24%) (Table I). There were no
statistically significant correlations between p16 positivity and
any clinicopathological parameters.
HMGB1 and ALDH1A1 expression, and the
risk of OSCC
The study investigated the association between
HMGB1, ALDH1A1, p16 and clinicopathological variables. The
expression of HMGB1 was above the cutoff in 59% (35/59) of primary
tumors, while the expression of ALDH1A1 was above the cuttoff in
41% (24/59) (24). HMGB1 positivity
was significantly higher in patients with T1-2 stage than T3-4
stage disease (P<0.001), whereas ALDH1A1 positivity was not.
ALDH1A1+ tumors displayed significantly lower
differentiation compared with ALDH1A1− tumors (P=0.018).
There was no correlation between ALDH1A1 positivity and age,
gender, tobacco or alcohol consumption, p16 status or HMGB1
positivity (Table I). There was also
no significant correlation between HMGB1, ALDH1A1 and p16
expression (data not shown).
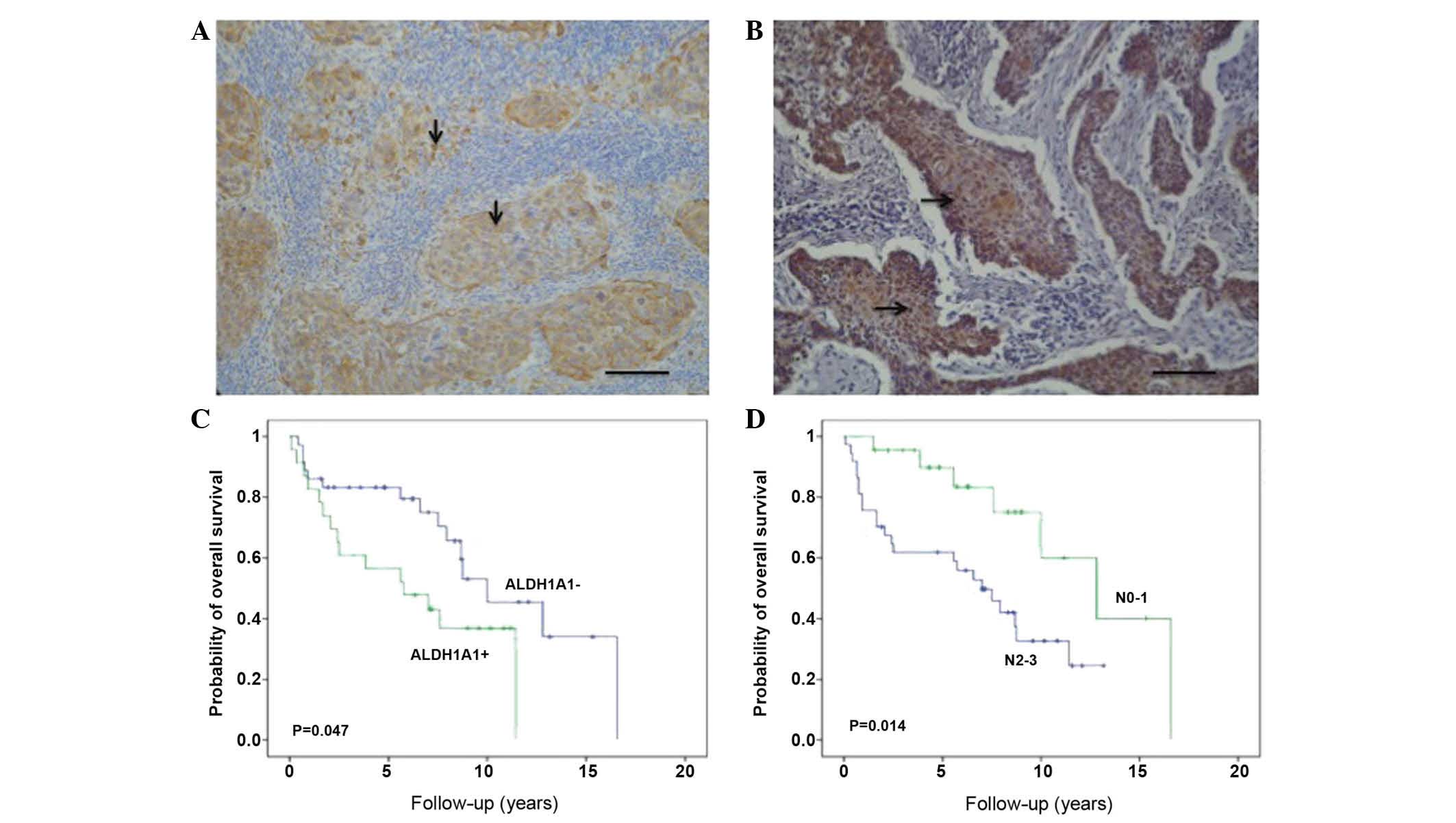
Kaplan-Meier survival estimations indicated that
patients with ALDH1A1 positivity in the primary tumor experienced
significantly reduced OS times (P=0.047) (Fig. 1). Patients with N0-1 stage disease
experienced better survival than patients with N2-3 stage (P=0.014)
(Fig. 1). However, HMGB1 positivity
and a negative HPV status were not significantly associated with
outcome in the patient cohort studied (data not shown). In
addition, the clinicopathological parameters, and ALDH1A1, HMGB1
and p16 positivity were analyzed by the Cox proportional hazards
model (Table II). On univariate and
multivariate analysis, ALDH1A1 positivity and N2-3 stage exhibited
a significant effect on OS. These data suggest that ALDH1A1
positivity and nodal status are independent prognostic factors in
OSCC. No other parameters were associated with outcome.
 | Table II.Univariate and multivariate analysis
for factors possibly influencing overall survival in 59 patients
with oropharyngeal squamous cell carcinoma. |
Table II.
Univariate and multivariate analysis
for factors possibly influencing overall survival in 59 patients
with oropharyngeal squamous cell carcinoma.
|
| Univariate | Multivariate |
|---|
|
|
|
|
|---|
| Factor | Hazard ratio | P-value | Hazard ratio | P-value |
|---|
| History of smoking
(yes vs. no) | 1.867 | 0.260 | 1.710 | 0.398 |
| Alcohol intake (yes
vs. no) | 1.411 | 0.380 | 1.781 | 0.216 |
| Tumor
differentiation (high-grade vs. intermediate-grade) | 1.519 | 0.286 | 0.887 | 0.781 |
| Tumor stage (pT3-4
vs. pT1-2) | 0.658 | 0.280 | 0.580 | 0.230 |
| Lymph node
metastasis (pN2-3 vs. pN0-1) | 1.354 | 0.026 | 1.346 | 0.036 |
| p16 (positive vs.
negative) | 0.535 | 0.149 | 0.619 | 0.316 |
| ALDH1A1 (positive
vs. negative) | 1.516 | 0.039 | 1.650 | 0.041 |
| HMGB1 (positive vs.
negative) | 0.337 | 0.051 | 0.348 | 0.052 |
Discussion
Increasing evidence has demonstrated the multiple
functions of HMGB1 in cancer progression, including apoptosis,
angiogenesis, inflammatory process, invasion and metastasis,
indicating the significance of HMGB1 as a potential therapeutic
target in human malignancies. The present study investigated the
correlation of HMGB1 expression with clinicopathological
characteristics and prognosis, as assessed by immunohistochemical
assay in patients with OSCC. It was demonstrated that the
expression of HMGB1 in OSCC patients with stage T1-2 was higher
than that in those with stage T3-4. This observation is different
to the observation reported by Liu et al, which showed a
stronger expression of HMGB1 in patients with stage 3–4 in a study
cohort of patients with HNSCC mainly located in the supraglottic
and glottic regions (22). In
addition to the report of expression on the tissue level, another
study found that the serum HMGB1 level in laryngeal squamous cell
carcinoma patients was significantly increased in patients with
T3-4 stage disease when compared with patients with T1-2 stage
(21). However, a recent case control
study in patients staged N0 with early tongue carcinoma (T1/2N0M0)
showed that HMGB1 expression cannot predict occult neck metastasis
(27). The present study found that
there was also no correlation between HMGB1 expression and the
presence of nodal metastasis. These clinicopathological
observations from the aforementioned studies presents a more
complicated pattern of HMGB1 expression in tumors derived from
different head and neck regions, and disease states, suggesting
currently unknown reasons for the distinct biological behavior
other than anatomical reasons. In the present analysis, no
correlation between the expression of p16 and HMGB1 was evident,
indicating mechanisms independent of HPV/p16-induced disease. In
addition to the role of HMGB1 in promoting carcinogenesis, recent
studies in cancer cell lines and animal models have suggested an
antitumor role of intracellular HMGB1. Zuo et al described
an anti-metastatic effect of HMGB1 in a cell line derived from
human lung cancer (A549 cells) and tumor models (30). After knockdown of HMGB1, β-actin
polymerization, cellular skeleton formation, cancer cell migration
and invasion were significantly increased. HMGB1 has also been
described with functions as a tumor suppressor and radiosensitizer
in breast cancer (31). Therefore,
further studies investigating this aspect of the underlying role of
HMGB1 in tumor biology would be noteworthy.
CSCs take part in the initiation and progression of
HNSCC, as well as in predicting prognosis. In the current study
cohort, it was found that the overexpression of ALDH1A1 was
correlated with poorly-differentiated tumor tissue and reduced
survival time in patients with OSCC. This finding was consistent
with a previously studied cohort of OSCC obtained from a different
region in Germany (26). A recent
meta analysis concluded that, in the head and neck region, ALDH1A1
expression was highly correlated with tumor differentiation and
lymph node metastasis, but not significantly with T stage (32). A correlation of higher ALDH1A1
expression with decreased OS and disease-free survival time was
also presented. Taken together, these data suggested that ALDH1A1
could potentially be used as a prognostic biomarker and predictor
for evaluating the risk of OSCC, depending on ALDH1A1 expression.
The correlation of ALDH1A1 and HMGB1 expression is also of concern
since HMGB1 may also be involved in the progression of HNSCC.
Further statistical analyses were therefore performed in the
present study, however, no correlation between ALDH1A1 and HMGB1
expression (data not shown) was found. Moreover, the overexpression
of HMGB1 did not predict OS time. Therefore, the ability of HMGB1
expression as a biomarker to evaluate the disease state and predict
prognosis in OSCC remains under debate. Due to the double-edged
function of intracellular and extracellular HMGB1 in
carcinogenesis, it will be of future note to study the interaction
of HMGB1 with cultured CSCs and bulk tumor cells in the laboratory.
As recently reviewed by our group, CSCs are able to escape
immunoreaction by inhibiting T-cell proliferation and activation,
triggering T-cell apoptosis and inducing regulatory T cells (Treg),
and the investigation of this regarding the involvement of HMGB1
will be noteworthy (33). Wild et
al found an elevated HMGB1 level in the sera of HNSCC
(laryngeal, pharyngeal SCC and oral cavity) patients and in tumor
tissues (20). These increased HMGB1
levels could act, as it was hypothesized, as a chemoattractant for
Treg, promoting the survival of Treg, and promoting the suppressive
capacity of Treg in a dose-dependent manner. HMGB1 was monitored
for its regulation of tumor growth by increasing microRNA (miR)-21
expression to mediate the enhanced activity of matrix
metalloproteinases (MMPs) through MMP inhibitors (34). In our recent study, it was found that
upregulated miR-21 could stimulate cancer cell proliferation in
HNSCC lines and it was observed that miR-21 expression may act as a
marker of progression, with prognostic value in patients with HNSCC
(35). Therefore, the biological
effects of tumor-derived HMGB1 with a CSC population within the
tumor microenvironment on regulating tumor immune responses leaves
more questions open than answered.
One important therapeutic strategy for head and neck
cancer, particularly OSCC, is the better prognosis of patient
subgroups with HPV infection and no history of tobacco abuse
(36). In the present study, p16
expression was employed as a surrogate marker in determining HPV
status. No significant correlations between the expression of
ALDH1A1 or HMGB1 and p16 were found. Furthermore, p16 did not
predict survival time in this study population of patients with
OSCC, which had also been recently shown for European populations
(37), but was in contrast with
studies from the United States of America (38). This is probably due to less cessation
of tobacco abuse in European regions.
In summary, the current study results present and
confirm the prognostic value of ALDH1A1 expression as a CSC marker
in patients with OSCC. HMGB1 may be involved in the pathogenesis of
OSCC, but did not predict survival in the studied patient cohort.
Cervical lymph node metastasis is also presented as an important
prognostic factor in OSCC. Future expansion and inclusion of
multiple approaches in understanding the pathogenesis of OSCC would
provide opportunities for system-level monitoring of disease and
development of individualized cancer therapies.
Acknowledgements
The study was partly supported by a grant from the
Opening Project of Zhejiang Provincial Top Key Discipline of
Clinical Medicine (grant no. LKFJ008).
References
|
1
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J,
Murray T and Thun MJ: Cancer statistics, 2008. CA Cancer J Clin.
58:71–96. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gibson MK and Forastiere AA:
Multidisciplinary approaches in the management of advanced head and
neck tumors: State of the art. Curr Opin Oncol. 16:220–224. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Leemans CR, Braakhuis BJ and Brakenhoff
RH: The molecular biology of head and neck cancer. Nat Rev Cancer.
11:9–22. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Albers AE, Chen C, Köberle B, Qian X,
Klussmann JP, Wollenberg B and Kaufmann AM: Stem cells in squamous
head and neck cancer. Crit Rev Oncol Hematol. 81:224–240. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bustin M, Lehn DA and Landsman D:
Structural features of the HMG chromosomal proteins and their
genes. Biochim Biophys Acta. 1049:231–243. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kang R, Chen R, Zhang Q, Hou W, Wu S, Cao
L, Huang J, Yu Y, Fan XG, Yan Z, et al: HMGB1 in health and
disease. Mol Aspects Med. 40:1–116. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang WK, Lu QH, Zhang JN, Wang B, Liu XJ,
An FS, Qin WD, Chen XY, Dong WQ, Zhang C, et al: HMGB1 mediates
hyperglycaemia-induced cardiomyocyte apoptosis via ERK/Ets-1
signalling pathway. J Cell Mol Med. 18:2311–2320. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu S, Stolz DB, Sappington PL, Macias CA,
Killeen ME, Tenhunen JJ, Delude RL and Fink MP: HMGB1 is secreted
by immunostimulated enterocytes and contributes to cytomix-induced
hyperpermeability of Caco-2 monolayers. Am J Physiol Cell Physiol.
290:C990–C999. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang H, Vishnubhakat JM, Bloom O, Zhang M,
Ombrellino M, Sama A and Tracey KJ: Proinflammatory cytokines
(tumor necrosis factor and interleukin 1) stimulate release of high
mobility group protein-1 by pituicytes. Surgery. 126:389–392. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim NG, Choi YR, Baek MJ, Kim YH, Kang H,
Kim NK, Min JS and Kim H: Frameshift mutations at coding
mononucleotide repeats of the hRAD50 gene in gastrointestinal
carcinomas with microsatellite instability. Cancer Res. 61:36–38.
2001. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang S, Xu L, Yang T and Wang F:
High-mobility group box-1 and its role in angiogenesis. J Leukoc
Biol. 95:563–574. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kang R, Tang D, Schapiro NE, Loux T,
Livesey KM, Billiar TR, Wang H, Van Houten B, Lotze MT and Zeh HJ:
The HMGB1/RAGE inflammatory pathway promotes pancreatic tumor
growth by regulating mitochondrial bioenergetics. Oncogene.
33:567–577. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Qiu Y, Chen Y, Fu X, Zhang L, Tian J and
Hao Q: HMGB1 promotes lymphangiogenesis of human lymphatic
endothelial cells in vitro. Med Oncol. 29:358–363. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sims GP, Rowe DC, Rietdijk ST, Herbst R
and Coyle AJ: HMGB1 and RAGE in inflammation and cancer. Annu Rev
Immunol. 28:367–388. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Choi J, Lee MK, Oh KH, Kim YS, Choi HY,
Baek SK, Jung KY, Woo JS, Lee SH and Kwon SY: Interaction effect
between the receptor for advanced glycation end products (RAGE) and
high-mobility group box-1 (HMGB-1) for the migration of a squamous
cell carcinoma cell line. Tumori. 97:196–202. 2011.PubMed/NCBI
|
|
18
|
Mittal D, Saccheri F, Venereau E, Pusterla
T, Bianchi ME and Rescigno M: TLR4-mediated skin carcinogenesis is
dependent on immune and radioresistant cells. EMBO J. 29:2242–2252.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yu LX, Yan L, Yang W, Wu FQ, Ling Y, Chen
SZ, Tang L, Tan YX, Cao D, Wu MC, et al: Platelets promote tumour
metastasis via interaction between TLR4 and tumour cell-released
high-mobility group box 1 protein. Nat Commun. 5:52562014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wild CA, Brandau S, Lotfi R, Mattheis S,
Gu X, Lang S and Bergmann C: HMGB1 is overexpressed in tumor cells
and promotes activity of regulatory T cells in patients with head
and neck cancer. Oral Oncol. 48:409–416. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Qiu G, Li Y, Liu Z, Wang M, Ge J and Bai
X: Clinical value of serum HMGB1 in diagnosis and prognosis of
laryngeal squamous cell carcinoma. Med Oncol. 31:3162014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu Y, Xie C, Zhang X, Huang D, Zhou X,
Tan P, Qi L, Hu G, Tian Y and Qiu Y: Elevated expression of HMGB1
in squamous-cell carcinoma of the head and neck and its clinical
significance. Eur J Cancer. 46:3007–3015. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen C, Wei Y, Hummel M, Hoffmann TK,
Gross M, Kaufmann AM and Albers AE: Evidence for
epithelial-mesenchymal transition in cancer stem cells of head and
neck squamous cell carcinoma. PloS One. 6:e164662011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liao T, Kaufmann AM, Qian X, Sangvatanakul
V, Chen C, Kube T, Zhang G and Albers AE: Susceptibility to
cytotoxic T cell lysis of cancer stem cells derived from cervical
and head and neck tumor cell lines. J Cancer Res Clin Oncol.
139:159–170. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Qian X, Wagner S, Ma C, Coordes A, Gekeler
J, Klussmann JP, Hummel M, Kaufmann AM and Albers AE: Prognostic
significance of ALDH1A1-positive cancer stem cells in patients with
locally advanced, metastasized head and neck squamous cell
carcinoma. J Cancer Res Clin Oncol. 140:1151–1158. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Qian X, Wagner S, Ma C, Klussmann JP,
Hummel M, Kaufmann AM and Albers AE: ALDH1-positive cancer
stem-like cells are enriched in nodal metastases of oropharyngeal
squamous cell carcinoma independent of HPV status. Oncol Rep.
29:1777–1784. 2013.PubMed/NCBI
|
|
27
|
Hanakawa H, Orita Y, Sato Y, Takeuchi M,
Takao S, Ohno K, Kohno T, Iwaki N, Marunaka H, Tamamura R, et al:
Does HMGB1 predict occult neck lymph node metastasis in early
tongue carcinoma? A case-control study of 26 patients. J Laryngol
Otol. 128:926–931. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cardesa A and Slootweg PJ: Pathology of
the Head and Neck. 1st. Springer-Verlag; Heidelberg: pp. 142006
|
|
29
|
Rainsbury JW, Ahmed W, Williams HK,
Roberts S, Paleri V and Mehanna H: Prognostic biomarkers of
survival in oropharyngeal squamous cell carcinoma: Systematic
review and meta-analysis. Head Neck. 35:1048–1055. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zuo Z, Che X, Wang Y, Li B, Li J, Dai W,
Lin CP and Huang C: High mobility group Box-1 inhibits cancer cell
motility and metastasis by suppressing activation of transcription
factor CREB and nWASP expression. Oncotarget. 5:7458–7470. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jiao Y, Wang HC and Fan SJ: Growth
suppression and radiosensitivity increase by HMGB1 in breast
cancer. Acta Pharmacol Sin. 28:1957–1967. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhou C and Sun B: The prognostic role of
the cancer stem cell marker aldehyde dehydrogenase 1 in head and
neck squamous cell carcinomas: A meta-analysis. Oral Oncol.
50:1144–1148. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Qian X, Ma C, Nie X, Lu J, Lenarz M,
Kaufmann AM and Albers AE: Biology and immunology of cancer stem
(−like) cells in head and neck cancer. Crit Rev Oncol Hematol.
95:337–345. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chen M, Liu Y, Varley P, Chang Y, He XX,
Huang H, Tang D, Lotze MT, Lin J and Tsung A: High-mobility group
box 1 promotes hepatocellular carcinoma progression through
miR-21-mediated matrix metalloproteinase activity. Cancer Res.
75:1645–1656. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sun Z, Li S, Kaufmann AM and Albers AE:
miR-21 increases the programmed cell death 4 gene-regulated cell
proliferation in head and neck squamous carcinoma cell lines. Oncol
Rep. 32:2283–2289. 2014.PubMed/NCBI
|
|
36
|
Hammarstedt L, Lindquist D, Dahlstrand H,
Romanitan M, Dahlgren LO, Joneberg J, Creson N, Lindholm J, Ye W,
Dalianis T and Munck-Wikland E: Human papillomavirus as a risk
factor for the increase in incidence of tonsillar cancer. Int J
Cancer. 119:2620–2623. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tinhofer I, Jöhrens K, Keilholz U,
Kaufmann A, Lehmann A, Weichert W, Stenzinger A, Stromberger C,
Klinghammer K, Becker ET, et al: Contribution of human papilloma
virus to the incidence of squamous cell carcinoma of the head and
neck in a European population with high smoking prevalence. Eur J
Cancer. 51:514–521. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ang KK, Harris J, Wheeler R, Weber R,
Rosenthal DI, Nguyen-Tân PF, Westra WH, Chung CH, Jordan RC, Lu C,
et al: Human papillomavirus and survival of patients with
oropharyngeal cancer. N Engl J Med. 363:24–35. 2010. View Article : Google Scholar : PubMed/NCBI
|