Introduction
Gene therapy for liver cancer remains a rapidly
progressing field (1,2). For example, the p53 gene plays a pivotal
role in gene therapy for liver cancer. Irrespective of whether the
p53 gene was mutated, wild-type or heterozygous in the cells of
interest, tumor cell growth was suppressed subsequent to
introducing an exogenous wild-type p53 gene (3,4). It was
subsequently found that increased p53 protein expression levels
could markedly suppress tumor cell growth. In addition, suicide
genes have been extensively utilized in the setting of liver cancer
gene therapy (5).
Previously, a large number of targeted genes have
been selected for cancer gene therapy, and gene transfer methods
have also been advanced during this time (6). Newcastle disease virus (NDV) is a member
of the Paramyxoviridae family whose genome is a
non-segmented single-stranded negative-sense RNA (7). The virus genome encodes 6 genes in the
following order (3′-5′): Nucleoprotein (NP); phosphoprotein (P);
matrix protein (M); fusion protein (F); hemagglutinin neuraminidase
(HN); and large polymerase protein (L) (3′-NP-P-M-F-HN-L-5′)
(8,9).
It was previously shown that the HN protein plays a critical role
in the anti-tumor effects of NDV (10). HN hydrolyzes the surface sialic acid
of the host cell, exposes biological recognition sites, and induces
tumor necrosis factor-associated apoptosis-inducing ligand (TRAIL)
expression at the surface of mononuclear cells in the host
peripheral blood, and yet does so independently of viral
replication (11,12). In addition, HN positioning in the
tumor cell membrane also led to formation of identical recognition
sites, which in turn improved the cytotoxic effects of the host
immune system against tumor cells (13).
Apoptin is a small protein that is derived from
chicken anemia virus. It can only induce apoptosis in transformed
or tumorigenic cells, rather than primary cells (14). Apoptin-induced apoptosis of tumor
cells is independent of functional p53 expression, and occurs in
the presence of high Bcl-2 expression levels. A DNA vaccine
containing the VP3 gene can activate specific cytotoxic T
lymphocytes (CTLs) and T-helper cells, and humoral immune responses
(15).
Interleukin (IL)-18 has similar biological
activities as those reported for IL-12, yet is more powerful than
IL-12 at inducing interferon (IFN)-γ (16). Thus, it is also termed IFN-γ inducing
factor. IL-18 is a cytokine with pleiotropic biological activities
in terms of promoting dendritic cell maturation, stimulating NK and
CTL cell-mediated cytotoxicity, inducing secretion of cytokines
such as IFN, IL-2 and granulocyte-macrophage colony-stimulating
factor (GM-CSF), and subsequent expression of major
histocompatibility complex class I molecules (16). Therefore, IL-18 can be used as an
adjuvant to enhance the immunological effects of specific candidate
vaccines.
In the present study, with respect to the identical
positioning of the NDV HN protein at the membrane surface
subsequent to expression in tumor cells, NDV HN was employed as a
tumor-specific antigen in the form of a DNA vaccine to improve host
immunity, thereby strengthening the immunological clearance of
tumor cells carrying this protein. As an IFN-γ inducer, IL-18
promotes anti-tumor effects by enhancing the activity of NK and T
cells. In this context, a recombinant DNA plasmid was constructed
to co-express HN, VP3 and IL-18, and the mechanisms responsible for
the anti-tumor effects was investigated both in vitro and
in vivo.
Materials and methods
Ethics
All procedures involving animals were performed in
accordance with protocols that were approved by the Committee for
Animal Research of Xiamen University (Xiamen, Fujian, China) and
complied with the Guide for the Care and Use of Laboratory Animals
(17).
Plasmids, cell culture and
transfection
The recombinant plasmids pIRESneo (Invitrogen;
Thermo Fisher Scientific, Inc., Waltham, MA, USA), pIRHN, PIRVP3
and pIRVP3IL-18HN were constructed and validated at the Department
of Gastroenterology, Zhongshan Hospital Affiliated to Xiamen
University (Xiamen, China). Briefly, the pIRESneo plasmid was
spliced by SmaI and XbaI restriction enzymes (Takara
Bio, Inc., Otsu, Japan) and transfected with HN, VP3 and VP3IL-18HN
gene fragments (BGI, Shenzhen, China) using T4 DNA ligase (Takara
Bio, Inc.) to obtain pIRHN, PIRVP3 and pIRVP3IL-18HN plasmids,
respectively. Mouse H22 hepatoma cells (Experimental Animal Centre
of Jilin University, Changchun, Jilin, China) were cultured in
Dulbecco's modified Eagle medium containing 10% fetal bovine serum,
100 U/l penicillin, and 100 U/l streptomycin (Gibco; Thermo Fisher
Scientific, Inc.) at 37°C in an atmosphere of CO2. Cell
lines that stably expressed HN, VP3 and IL-18 were established by
transducing H22 cells with the pIRVP3IL-18HN lentiviral vector.
Western blot analysis
H22 cells were transfected with pIRVP3IL-18HN and
pIRESneo using Lipofectamine 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) for 24 h. Subsequently, 2×105
transfected H22 cells were lysed in radioimmunoprecipitation assay
buffer consisting of 50 mmol/l Tris (pH 8.0), 0.1% sodium dodecyl
sulfate, 0.5% sodium deoxycholate, 1% NP-40, 150 mmol/l NaCl, and 1
tablet of complete mini protease inhibitor/10 ml of buffer (Roche
Diagnostics GmbH, Penzberg, Germany). Equal amounts of total cell
lysate were electrophoresed and transferred to
FluoroTrans® W membranes (Wako Pure Chemical Industries
Ltd., Wako, Japan). The membranes were then incubated with
monoclonal goat anti-mouse Flag antibody (cat. no. 66008-2-Ig;
1:1,000; Proteintech Group, Inc., Rosemont, IL, USA). Subsequent to
washing, the membrane was incubated with horseradish
peroxidase-conjugated goat anti-rabbit IgG secondary antibody (cat.
no. SA00001-2; 1:5,000; Proteintech Group, Inc.) and visualized by
an enhanced chemiluminescence detection system (ECL Advance; GE
Healthcare Life Sciences, Chalfont, UK).
Flow cytometry
H22 cells were transfected with pIRVP3IL-18HN and
pIRESneo for 24 h, following which 5×105 cells were
stained using fluorescein isothiocyanate (FITC)-labeled monoclonal
goat anti-mouse HLA-A, B and C antibodies (cat. no. ab23840; 1:100;
Abcam, Cambridge, MA, USA) and Rhodamine 123 at a final
concentration of 25 mg/ml. Following antibody incubation for 1 h at
4°C in the dark, the cells were washed and treated with
fluorescence-activated cell sorting lysing solution, according to
the manufacturer's protocol (Becton Dickinson, Franklin Lakes, NJ,
USA). In each sample, 104 cells were counted by
multi-parameter flow cytometry (FACScan; Becton Dickinson) and
analyzed by CellQuest 5.1 software (Becton Dickinson).
2,7-Dichlorofluorescin diacetate
(DCFA) analyses
DCFA analyses were performed to assess reactive
oxygen species activation. H22 cells were transfected with pIRESneo
and pIRVP3IL 18HN. After incubation for 72 h, a total of
2×104 HCC cells were harvested and stained with 5 µmol/l
DCFA at 37°C for 30 min in the dark. Cells were then washed twice
with phosphate-buffered saline (PBS)and counted by multi parameter
flow cytometry (FACScan; Becton Dickinson) and analyzed by
CellQuest 5.1 software as described above.
Proliferation assay
H22 cells were transfected with the pIRVP3IL-18HN
and pIRESneo plasmids for 24 h. The cells were seeded at a density
of 3,000 cells per well into 96-well plates and incubated at 37°C
overnight. The next day, the media was aspirated and the cells were
washed twice in PBS and then starved for 24 h in a serum-free
medium containing 0.1% bovine serum albumin. Methyl thiazolyl
tetrazolium reagent (Sigma-Aldrich; Merck Millipore, Darmstadt,
Germany) was added to each well, cells were lysed with dimethyl
sulfoxide and then quantified by measuring the absorbance at A570
nm using an enzyme-linked immunosorbent assay plate reader (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). Each treatment was
conducted in triplicate, and all experiments were conducted
independently at least three times.
Acridine orange (AO)/ethidium bromide
(EB) staining
In total, 25 µl of a suspension of cells
(0.5–2.0×106) were mixed gently with 1 µl of AO/EB
solution, which was a mixture of 100 µg/ml AO in PBS and 100 µg/ml
EB in PBS. Subsequently, 10 µl of stained cell suspension was
loaded onto a slide and ≥300 cells were counted under a
fluorescence microscope that was equipped with a fluorescence
filter and an objective lens of ×40 magnification.
Caspase-3 activity analysis
In total, ~5×106 cells were resuspended
in 200 µl lysis buffer (HD Biosciences Co., Ltd., Shanghai, China)
consisting of 20 mmol/l piperazine-N,N'-bis(2-ethanesulfonic acid)
(pH 7.2), 100 mmol/l NaCl, 1 mmol/l ethylenediaminetetraacetic acid
(EDTA; HD Biosciences Co., Ltd.), 10 mmol/l dithiothreitol (DTT)
(Solarbio Science & Technology Co., Ltd., Beijing, China), 0.1%
3-[(3-cholamidopropyl) dimethylammonio]-1-propanesulfonate (HD
Biosciences Co., Ltd.) and 10% sucrose (HD Biosciences Co., Ltd.)
and incubated on ice prior to centrifugation at 3,000 × g
for 5 min. The supernatant was harvested for protein quantification
and caspase-3 activity was measured using a caspase-3 assay kit
(Abcam) according to the manufacturer's instructions, at an
absorbance of 405 nm using a microplate analyzer.
Extraction of cytochrome c
Following transfection with the pIRVP3IL-18HN
recombinant plasmid for 72 h, 2×106 H22 tumor cells were
lysed in 300 µl of buffer A, consisting of 10 mM
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid-KOH (pH 7.4),
0.25 M sucrose, 1 mM ethylene glycol-bis(β-aminoethyl
ether)-N,N,N',N'-tetraacetic acid, 1 mM EDTA, 1 mM DTT, 10 mM
MgCl2 and 1 mM phenylmethylsulfonyl fluoride (all
obtained from Solarbio Science & Technology Co., Ltd.) and
homogenized in a Dounce homogenizer (IKA, Guangzhou, China) for 10
min. The supernatant was mixed with TNC buffer, consisting of 10 mM
Tris-acetate (pH 8.0) (HD Biosciences Co., Ltd.), 0.5% Nonidet P-40
(HD Biosciences Co., Ltd.) and 5 mM CaCl2 (Solarbio
Science & Technology Co., Ltd.), and the precipitate was
dissolved in buffer A to obtain the cytosol, which together with
the mitochondria were stored at −80°C for subsequent western blot
analysis.
Establishment of H22 hepatoma-bearing
C57BL/6 mouse model
Six-week-old C57BL/6 male mice (n=15) were obtained
from the Experimental Animal Center of the Academy of Military
Medical Sciences of the Chinese People's Liberation Army (license
number: SCXK-[Army] 2002-001; Beijing, China). The mice were used
to establish the H22-bearing mouse model. H22 cells in the
logarithmic growth phase were adoptively transferred subcutaneously
into the right hind foot of mice at a dose of 0.1 ml each. When the
tumors had grown to >5 mm in diameter, the tumor-bearing mice
were randomly divided into 5 groups as follows: PBS control group;
blank plasmid pIRESneo-treated group; pIRHN-treated group;
pIRVP3-treated group; and pIRVP3IL-18HN-treated group. An extra
group of wild-type mice were included as normal controls. All
groups were inoculated once every 7 days, a total of 3 times, at a
dose of 100 µl/mouse with PBS or plasmids that were diluted in PBS
to a concentration of 50 µg/ml (5 µg plasmids/mouse). All mice were
sacrificed on day 7 subsequent to the last injection and were
evaluated for various markers.
Scanning electron microscopy
analysis
Animals were sacrificed on day 7 subsequent to the
last injection and the tumors were removed and quickly fixed in
ice-cold glutaraldehyde (Sigma-Aldrich; Thermo Fisher Scientific,
Inc.). Subsequently, the tumor was sectioned into 1 mm3
blocks, and then fixed for another 12 h in cold glutaraldehyde at
4°C. Following three washes in 0.13 M phosphate buffer, the tissue
blocks were fixed with ice-cold 1% osmium tetroxide (Sinopharm
Chemical Reagent Co., Ltd., Shanghai, China) for 1.5–2 h, followed
by sequential 15 min incubations in 50, 70 and 80% ethanol at 4°C.
Subsequently, room temperature fixing steps in 90 and 100% ethanol
and a final acetone step at room temperature were performed. The
blocks were gradually permeabilized in a series of buffers that
consisted of dehydrating agents mixed with epoxy resin at a ratio
of 2:1, 1:1 and 1:3, followed by 100% resin, for 0.5–1 h each
incubation. The blocks were then placed into labeled capsules
containing embedding medium and polymerized at 35, 45 and 60°C for
12, 12 and 24 h, respectively, in an incubator. The polymerized
tissue blocks were shaped and dissected into slices at a thickness
of 600–700 Å. The slices were placed on a wax plate covered by a
copper mesh and stained with uranyl acetate dye and lead citrate
dye for 15–20 and 5–10 min, respectively, followed by distilled
water washes and examination by electron microscopy.
Immunohistochemistry
The mice were sacrificed and the tumors were
instantly removed and incubated in 10% neutral formalin for 24–72 h
for fixing. The formalin was discarded and the tumor tissue was
treated sequentially with 60, 70, 80, 95 and 100% ethanol, 50%
ethanol plus 50% xylene, and xylene. Subsequently, the tissue was
embedded in a paraffin block and sectioned into slices at 4–6 µm
thickness, which were then incubated in a 45°C water bath and
transferred onto glass slides, and then covered with protein
glycerin prior to dehydrating in an oven at 60°C for 2 h.
Subsequent to routine hematoxylin-eosin staining, the slices were
mounted with mounting medium and examined under a standard light
microscope (×10 and ×40 magnifcation).
Statistical analysis
Data were expressed as the mean ± standard
deviation. Groups were compared using Student's t-test, and values
of P<0.05 were considered to indicate a statistically
significant difference.
Results
Inhibition of liver cancer cell growth
via pro-apoptotic pathways in vitro by a recombinant DNA vaccine
containing the NDV HN gene
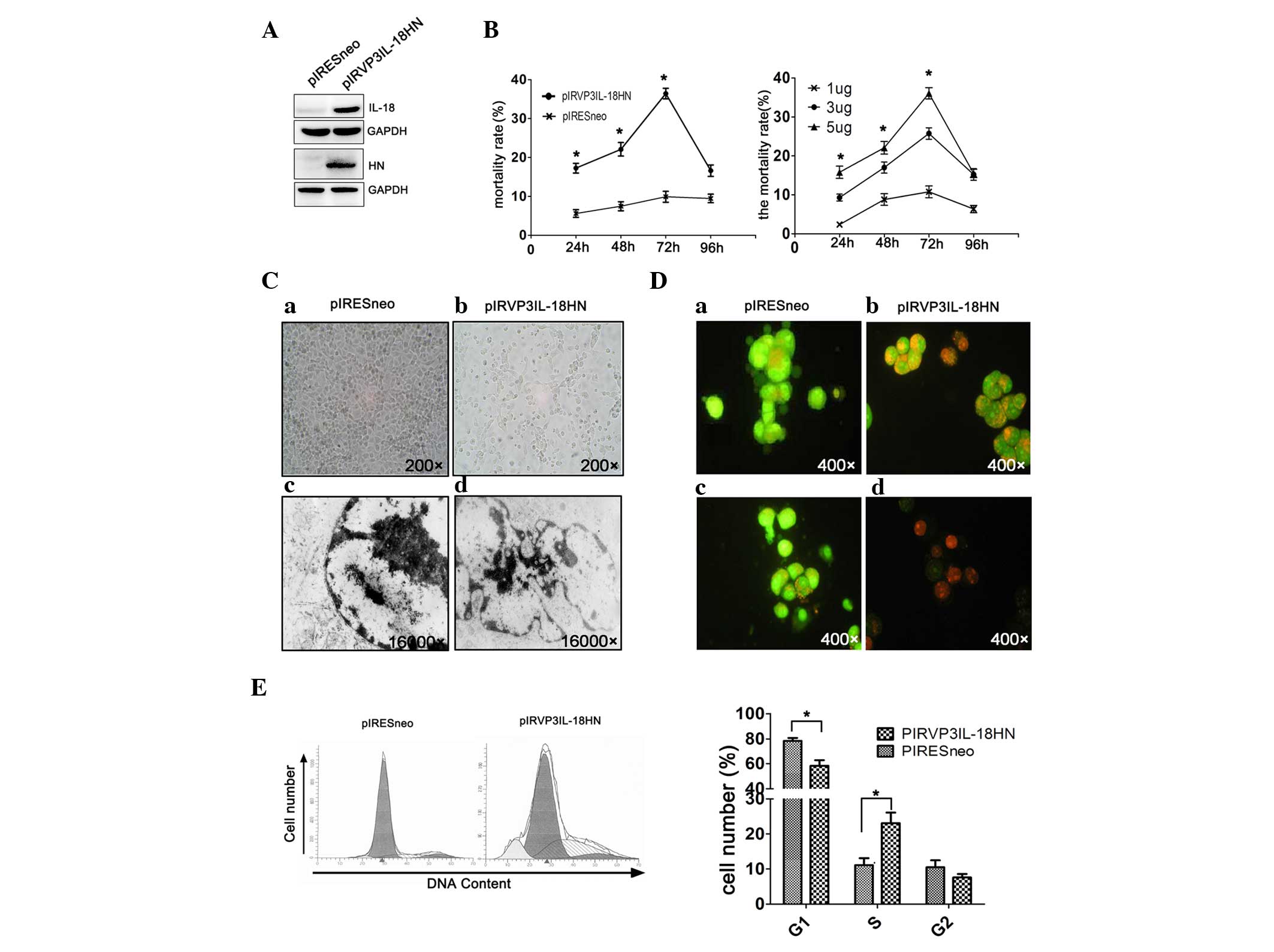
H22 hepatoma cells were transfected with the newly
constructed DNA vaccine pIRVP3IL-18HN, to validate its function
against liver cancer (Fig. 1A). It
was found that the cytotoxic effects of pIRVP3IL-18HN on H22
hepatoma cells were positively associated with time and DNA
concentrations, and ultimately peaked 72 h subsequent to
transfection (Fig. 1B). As observed
under optical microscopy, H22 hepatoma cells that were transfected
with pIRVP3IL-18HN detached from the plate after 72 h, which was in
contrast to those transfected with the control plasmid pIRESneo
(Fig. 1C). Additional examination by
electron microscopy visualized chromatin margination and
condensation in transfected H22 cells, which were typical apoptotic
features, and known as pyknotic nuclei. By contrast, tumor cells
transfected with the control plasmid exhibited relatively normal
morphology and nuclear structures (Fig.
1C). Upon AO/EB staining, the control cells were presented as
ubiquitously fluorescent green cells.
However, at 72 h subsequent to transfection with
pIRVP3IL-18HN in vitro, certain H22 tumor cells were
exhibited as bright orange cells with chromatin condensation. In
addition, certain cells were fluorescent red following a general
loss of cell membrane integrity in combination with reduced sizes
of the nuclei, and particularly so in the later stage of apoptosis
(Fig. D). In addition, cell cycle changes were observed in H22
cells, as detected by propidium iodide staining followed by flow
cytometry. An apoptotic peak appeared in the G1 phase of the cell
cycle with increasing numbers of H22 cells that were arrested at
the S phase of the cell cycle, which led to an apoptosis rate of
11% (Fig. 1E).
Apoptosis of tumor cells was possibly
induced via the mitochondrial pathway by the recombinant DNA
vaccine containing the NDV HN gene
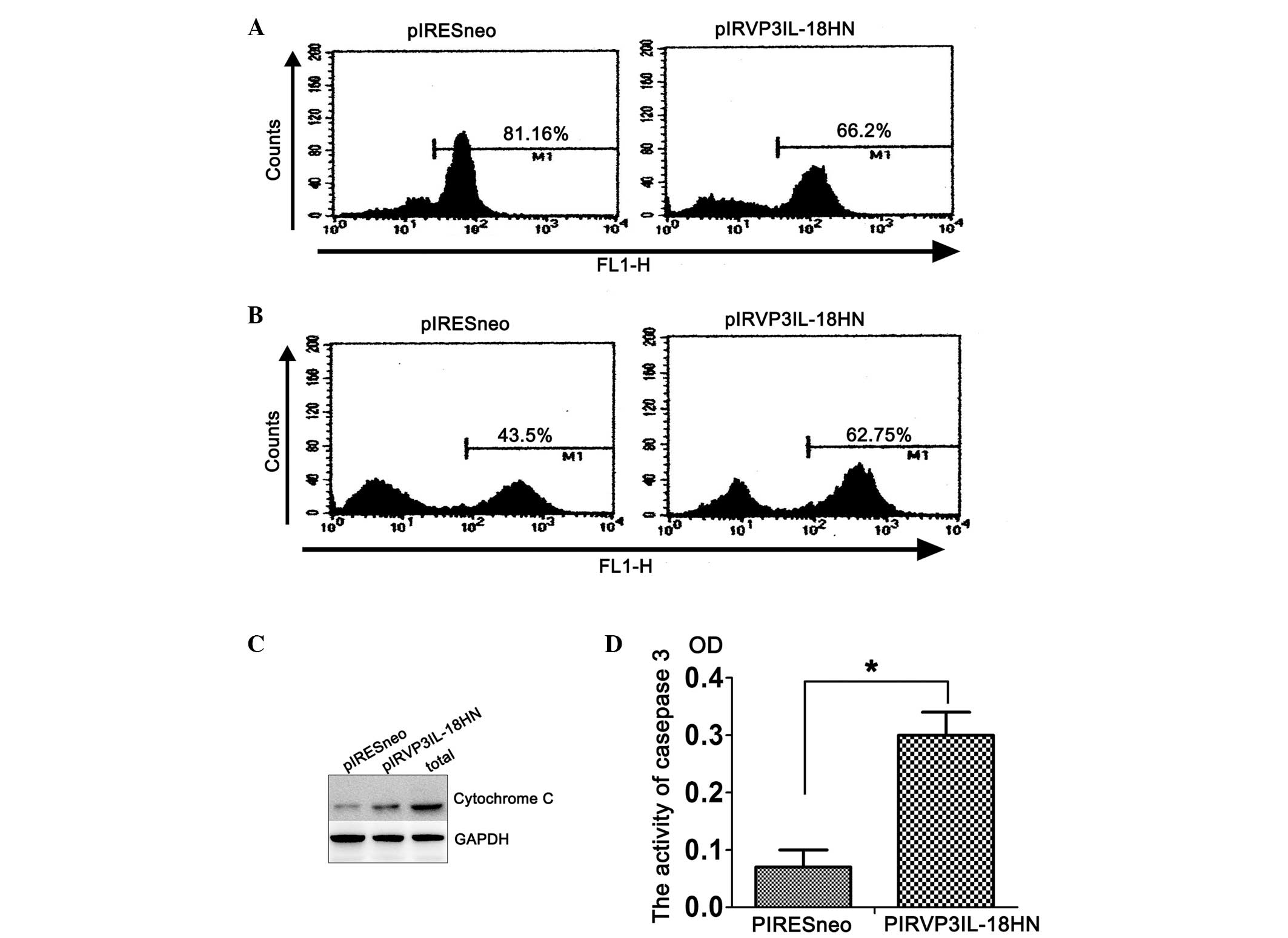
The present study found that, compared with cells
carrying the control empty plasmid, mitochondrial uptake of
Rhodamine 123 was substantially decreased in H22 tumor cells that
had been transfected with pIRVP3IL-18HN in vitro, leading to
a peak shift from right to left in flow cytometric analysis, which
indicates a change in cell number. This result indicates that
pIRVP3IL-18HN could downregulate the mitochondrial membrane
potential in H22 cells, as an early event of apoptosis (Fig. 2A). Additional analysis by DCFA
combined with flow cytometry revealed that intracellular levels of
reactive oxygen species (ROS) were elevated in H22 cells following
transfection with pIRVP3IL-18HN compared with the control cells, as
indicated by a peak shift to the right (Fig. 2B). Enhanced ROS production led to
increased apoptosis of tumor cells. Consequently, there were
markedly increased levels of cytochrome c detected in
pIRVP3IL-18HN-transfected H22 cells compared with control cells
(Fig. 2C). The caspase proteases play
a critical role in proteolysis and activation of proteins to
coordinate the central events that drive apoptosis. The present
study analyzed caspase-3 activity in H22 cells that were
transfected with pIRESneo and pIRVP3IL-18HN plasmids. According to
the optical density values, caspase-3 was activated in H22 cells at
72 h after transfection with the pIRVP3IL-18HN plasmid, but not in
cells transfected with the control plasmid.
Inhibitory effects of the NDV HN
recombinant DNA vaccine on liver cancer growth in vivo
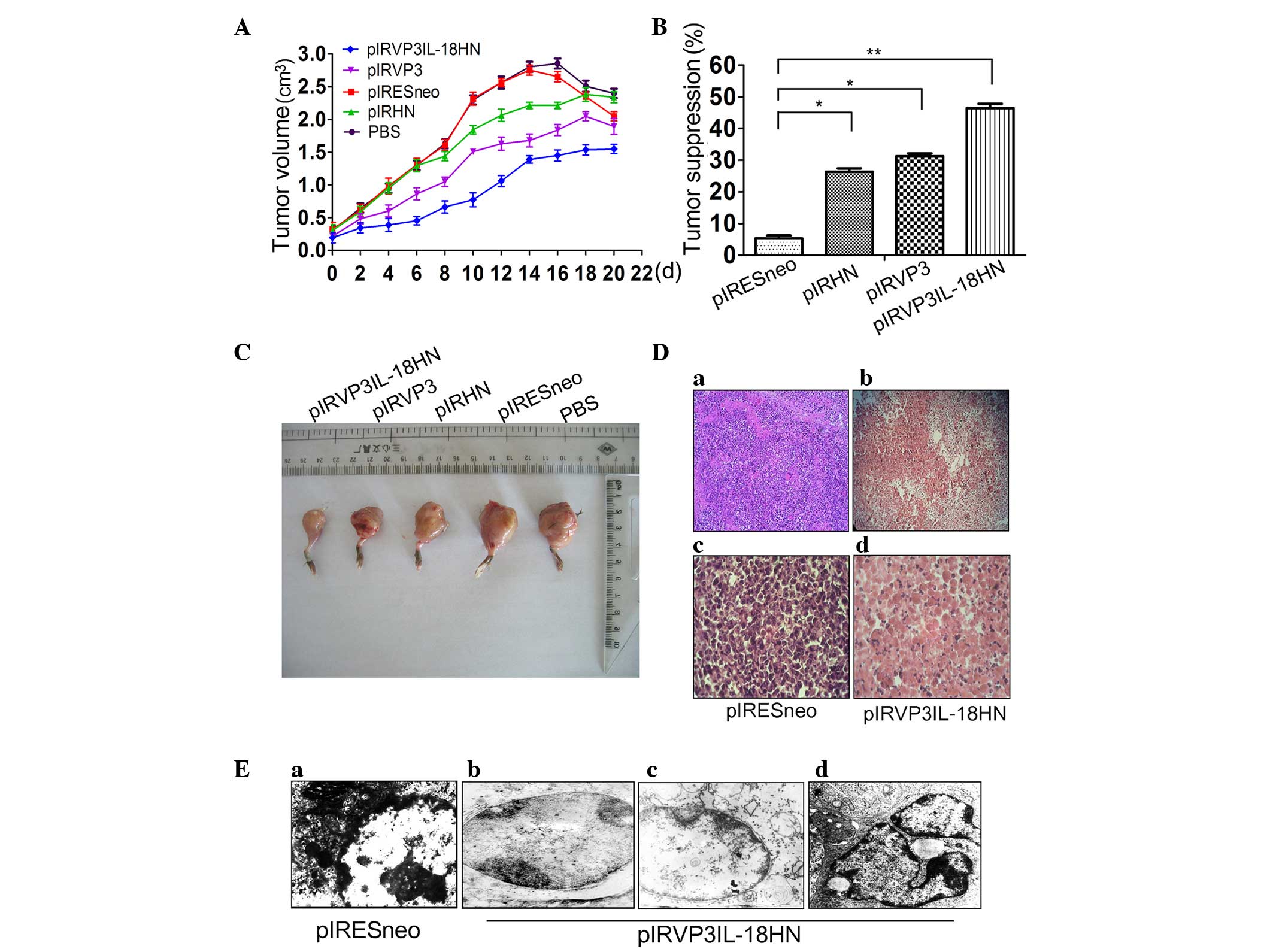
A tumor-bearing C57BL/6 mouse model was established
using H22 cells (Fig. 3). Compared to
the PBS group and the empty plasmid treatment group, treatment with
the recombinant plasmid containing individual pIRHN or pIRVP3 genes
inhibited H22 tumor growth. Co-expression of the HN, VP3 and IL-18
genes in the pIRVP3IL-18HN plasmid markedly increased the
inhibitory effects on H22 tumor volume compared with plasmids
containing the single genes, and resulted in the lowest growth rate
of the tumor (Fig. 3A and C).
Compared with the empty plasmid treatment group, the pIRVP3IL-18HN
recombinant plasmid carrying the HN, VP3 and IL-18 genes had a
tumor inhibition rate of 46.28%, whereas the pIRHN and pIRVP3
groups had inhibition rates of 26.57 and 31.36%, respectively
(Fig. 3B). Histopathological analysis
was performed on biopsy specimens that were obtained from
tumor-bearing C57BL/6 mice. Normal cancer cell morphology was
observed, which also showed vigorous proliferation (Fig. 3Da and c). By contrast, in
pIRVP3IL-18HN-transfected tumors, a vast majority of the cancer
cells exhibited vacuoles and only in certain areas were there
non-viable cells (Fig. 3Da and d).
Additional ultrastructural analysis of tumor tissues revealed
normal structure of the tumorigenic nuclei, with evenly distributed
chromatin in the PBS group (Fig.
3Ea). However, in the pIRVP3IL-18HN group, nuclear shrinkage
with chromatin margination (Fig.
3Eb), mitochondrial swelling, disappearance of the
mitochondrial crista and lighter coloring by electron microscopic
examination (Fig. 3Ec), as well as
formation of typical apoptotic bodies (Fig. 3Ed), were observed.
Discussion
Combined gene therapy refers to strategies that
utilize two or more genes simultaneously in the treatment of cancer
(18). It combines complementary
advantages of differential gene expression to potentiate the
therapeutic effects. Certain studies have integrated 4 target
genes, including B7-1, GM-CSF, p53 and
IL-2, into a single adenoviral vector, which was imported
into liver tumor cells and subsequently achieved satisfactory
efficacy (19,20). Su et al (21) also evaluated the synergistic effect of
the HSV-tk and IL-2 genes in a liver cancer mouse
model and confirmed that joint application of the HSV-tk and
IL-2 genes had an improved therapeutic efficacy over gene
therapy using any of the genes alone. In the present study, a
recombinant DNA vaccine co-expressing the NDV HN gene,
chicken anemia virus VP3 gene and IL-18 was
constructed. The overall objective was to exploit the tumoricidal
effects of the DNA vaccine and the synergistic function between
expression of all three genes to reinforce tumor cell
inhibition.
The development and progression of tumors is a
highly complicated process involving comprehensive mechanisms, such
as the mutation of the tumor supressor genes p53 and BRCA1
(22). Tumorigenesis is associated
with abnormal proliferation, arrested differentiation and
imbalanced apoptosis of tumor cells. Apoptosis in mammals engages
the interplay of intricate mechanisms that are affected by multiple
factors. There are three genes, consisting of p53,
Bcl-2 and c-myc, that are considered the major
apoptosis-associated genes (23–25). The
occurrence and progression of tumors may be controlled when the
tumor cells are induced to undergo apoptosis. Following a viral
infection, the body will activate self-controlled genes to trigger
programmed-cell death or apoptosis in order to maintain normal
physiological activities and to minimize the damage caused by the
virus, which ultimately leads to cell death (26,27).
In addition, apoptosis may be regulated at the
genetic level due to the expression of certain viral proteins or
the triggering of novel genes (28,29). In
recent years, specifically infecting tumor cells with viruses to
induce apoptosis became an important aspect of cancer biotherapy,
thus further manipulating tumor development (18,30).
Apoptosis is the process of naturally occurring cell death under
genetic regulation that also plays pivotal roles in the development
of multicellular animals in terms of cytotoxicity (31), anti-viral activity, immune regulation,
transcriptional regulation and other biological activities
(32). The death of virus-infected
cells would substantially limit viral replication and viral protein
expression, thus ultimately eliminating the spread of the virus
within the host. With additional research in cancer biology and
comprehensive understanding of the complex associations between the
tumor and the host, the rationale for cancer gene therapy may be
expanded with an increasing number of potential targeted genes. The
findings of the present study suggest that recombinant DNA vaccines
containing the VP3, IL-18 and HN genes may be applied as a
potential treatment for liver cancer.
Acknowledgements
The present study was supported by National Natural
Science Foundation of China (grant nos. 81225025 and 91229201) and
Ministry of Health Foundation for State Key Clinical Department,
863 and 973 programs, in China (grant nos. 2012AA02A201 and
2013CB944903).
References
|
1
|
Brand K, Löser P, Arnold W, Bartels T and
Strauss M: Tumor cell-specific transgene expression prevents liver
toxicity of the adeno-HSVtk/GCV approach. Gene Ther. 5:1363–1371.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Baqué P, Pierrefite-Carle V, Gavelli A,
Brossette N, Benchimol D, Bourgeon A, Staccini P, Saint-Paul MC and
Rossi B: Naked DNA injection for liver metastases treatment in
rats. Hepatology. 35:1144–1152. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li Y, Li B, Li CJ and Li LJ: Key points of
basic theories and clinical practice in rAd-p53 (Gendicine™) gene
therapy for solid malignant tumors. Expert Opin Biol Ther.
15:437–454. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Baliaka A, Zarogoulidis P, Domvri K,
Hohenforst-Schmidt W, Sakkas A, Huang H, Le Pivert P, Koliakos G,
Koliakou E, Kouzi-Koliakos K, et al: Intratumoral gene therapy
versus intravenous gene therapy for distant metastasis control with
2-diethylaminoethyl-dextran methyl methacrylate copolymer non-viral
vector-p53. Gene Ther. 21:158–167. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Niu HX, Du T, Xu ZF, Zhang XK and Wang RG:
Role of wild type p53 and double suicide genes in interventional
therapy of liver cancer in rabbits. Acta Cir Bras. 27:522–528.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fargnoli AS, Katz MG, Williams RD,
Margulies KB and Bridges CR: A needleless liquid jet injection
delivery method for cardiac gene therapy: A comparative evaluation
versus standard routes of delivery reveals enhanced therapeutic
retention and cardiac specific gene expression. J Cardiovasc Transl
Res. 7:756–767. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang J, Wang C, Feng N, Wang H, Zheng X,
Yang S, Gao Y, Xia X, Yin R, Liu X, et al: Development of a reverse
genetics system based on RNA polymerase II for Newcastle disease
virus genotype VII. Virus Genes. 50:152–155. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Alexander DJ: Newcastle disease and other
avian paramyxoviruses. Rev Sci Tech. 19:443–462. 2000.PubMed/NCBI
|
|
9
|
Zeng J, Fournier P and Schirrmacher V:
Induction of interferon-alpha and tumor necrosis factor-related
apoptosis-inducing ligand in human blood mononuclear cells by
hemagglutinin-neuraminidase but not F protein of Newcastle disease
virus. Virology. 297:19–30. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sui H, Bai Y, Wang K, Li X, Song C, Fu F,
Zhang Y and Li L: The anti-tumor effect of Newcastle disease virus
HN protein is influenced by differential subcellular targeting.
Cancer Immunol Immunother. 59:989–999. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rajmani RS, Singh PK, Kumar G Ravi, Saxena
S, Singh LV, Kumar R, Sahoo AP, Gupta SK, Chaturvedi U and Tiwari
AK: In-vitro characterization and evaluation of apoptotic potential
of bicistronic plasmid encoding HN gene of Newcastle disease virus
and human TNF-α. Anim Biotechnol. 26:112–119. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Brenu EW, van Driel ML, Staines DR, Ashton
KJ, Ramos SB, Keane J, Klimas NG and Marshall-Gradisnik SM:
Immunological abnormalities as potential biomarkers in chronic
fatigue syndrome/myalgic encephalomyelitis. J Transl Med. 9:812011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dong D, Gao J, Sun Y, Long Y, Li M, Zhang
D, Gong J, Xu L, Li L, Qin S, et al: Adenovirus-mediated
co-expression of the TRAIL and HN genes inhibits growth and induces
apoptosis in Marek's disease tumor cell line MSB-1. Cancer Cell
Int. 15:202015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Guan G, Jin N, Li X, Sun L, Jin C, Lou W,
Shi P and Hao Y: Anti-tumor effects on human laryngeal carcinoma
Hep-2 of recombinant fowlpox virus expressing chicken anemia virus
Apoptin gene. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi.
23:264–266. 2009.(In Chinese). PubMed/NCBI
|
|
15
|
Natesan S, Kataria JM, Dhama K, Bhardwaj N
and Sylvester A: Anti-neoplastic effect of chicken anemia virus VP3
protein (apoptin) in Rous sarcoma virus-induced tumours in chicken.
J Gen Virol. 87:2933–2940. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Du G, Ye L, Zhang G, Dong Q, Liu K and
Tian J: Human IL18-IL2 fusion protein as a potential antitumor
reagent by enhancing NK cell cytotoxicity and IFN-γ production. J
Cancer Res Clin Oncol. 138:1727–1736. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
National Research Council (US) Institute
for Laboratory Animal Research, . Guidance for the Description of
Animal Research in Scientific Publications. National Academies
Press; Washington DC: 2011
|
|
18
|
Witlox MA, Lamfers ML, Wuisman PI, Curiel
DT and Siegal GP: Evolving gene therapy approaches for osteosarcoma
using viral vectors: Review. Bone. 40:797–812. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pan D, Wei X, Liu M, Feng S, Tian X, Feng
X and Zhang X: Adenovirus mediated transfer of p53, GM-CSF and B7-1
suppresses growth and enhances immunogenicity of glioma cells.
Neurol Res. 32:502–509. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shimizu T, Shimada H, Ochiai T and Hamada
H: Enhanced growth suppression in esophageal carcinoma cells using
adenovirus-mediated fusion gene transfer (uracil phosphoribosyl
transferase and herpes simplex virus thymidne kinase). Cancer Gene
Ther. 8:512–521. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Su H, Lu R, Ding R and Kan YW:
Adeno-associated viral-mediated gene transfer to hepatoma:
Thymidine kinase/interleukin 2 is more effective in tumor killing
in non-ganciclovir (GCV)-treated than in GCV-treated animals. Mol
Ther. 1:509–515. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cai BH, Chen JY, Lu MH, Chang LT, Lin HC,
Chang YM and Chao CF: Functional four-base A/T gap core sequence
CATTAG of P53 response elements specifically bound tetrameric P53
differently than two-base A/T gap core sequence CATG bound both
dimeric and tetrameric P53. Nucleic Acids Res. 37:1984–1990. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Knoll S, Fürst K, Thomas S, Baselga S
Villanueva, Stoll A, Schaefer S and Pützer BM: Dissection of cell
context-dependent interactions between HBx and p53 family members
in regulation of apoptosis: A role for HBV-induced HCC. Cell Cycle.
10:3554–3565. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Barneda-Zahonero B, Collazo O, Azagra A,
Fernández-Duran I, Serra-Musach J, Islam AB, Vega-García N,
Malatesta R, Camós M, Gómez A, et al: The transcriptional repressor
HDAC7 promotes apoptosis and c-Myc downregulation in particular
types of leukemia and lymphoma. Cell Death Dis. 6:e16352015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cao Y, Yang G, Hunter ZR, Liu X, Xu L,
Chen J, Tsakmaklis N, Hatjiharissi E, Kanan S, Davids MS, et al:
The BCL2 antagonist ABT-199 triggers apoptosis, and augments
ibrutinib and idelalisib mediated cytotoxicity in CXCR4 Wild-type
and CXCR4 WHIM mutated Waldenstrom macroglobulinaemia cells. Br J
Haematol. 170:134–138. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Moon J, Koh SS, Malilas W, Cho IR,
Kaewpiboon C, Kaowinn S, Lee K, Jhun BH, Choi YW and Chung YH:
Acetylshikonin induces apoptosis of hepatitis B virus X
protein-expressing human hepatocellular carcinoma cells via
endoplasmic reticulum stress. Eur J Pharmacol. 735:132–140. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yang JJ, Zhang Y, Deng XZ, Xu K, Wang ZC,
Wang J, Feng L and Ding WL: Influence of F protein of hepatitis C
virus subtype 1b inhibits on human hepatocellular carcinoma HepG2
cell apoptosis. Zhonghua Liu Xing Bing Xue Za Zhi. 30:388–392.
2009.PubMed/NCBI
|
|
28
|
Follstaedt SC, Barber SA and Zink MC:
Mechanisms of minocycline-induced suppression of simian
immunodeficiency virus encephalitis: Inhibition of apoptosis
signal-regulating kinase 1. J Neurovirol. 14:376–388. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Son KN and Lipton HL: Inhibition of
Theiler's virus-induced apoptosis in infected murine macrophages
results in necroptosis. Virus Res. 195:177–182. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kim ES, Khuri FR and Herbst RS: Epidermal
growth factor receptor biology (IMC-C225). Curr Opin Oncol.
13:506–513. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lee SH, Kim MJ, Kim DW, Kang CD and Kim
SH: Amurensin G enhances the susceptibility to tumor necrosis
factor-related apoptosis-inducing ligand-mediated cytotoxicity of
cancer stem-like cells of HCT-15 cells. Cancer Sci. 104:1632–1639.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Youn YS, Shin MJ, Chae SY, Jin CH, Kim TH
and Lee KC: Biological and physicochemical evaluation of the
conformational stability of tumor necrosis factor-related
apoptosis-inducing ligand (TRAIL). Biotechnol Lett. 29:713–721.
2007. View Article : Google Scholar : PubMed/NCBI
|