Introduction
Acute lymphoblastic leukemia (ALL) is an aggressive
hematological tumor characterized by the overproduction of
lymphoblasts (immature lymphoid cells) in the peripheral blood,
bone marrow and other organs, including the lymph nodes and spleen
(1). T-cell ALL (T-ALL) is a subtype
of ALL based on immunophenotype (1,2). It
accounts for ~12% of cases of ALL in children, whereas it
constitutes ~25% of cases in adult patients with ALL (2,3). Generally
speaking, patients with T-ALL have a poorer prognosis than other
ALL patients, with an increased risk of experiencing early relapse,
induction failure and isolated central nervous system relapse
(4). Therefore, it is essential to
identify efficient markers and novel clinical treatment targets of
ALL to enhance the efficacy of existing regimens while reducing
their toxic side effects in order to improve the prognosis of
patients with ALL.
MicroRNAs (miRNAs or miRs) are single-stranded
noncoding regulatory RNA molecules (5) that regulate gene expression by binding
to the 3′-untranslated regions (UTR) of messenger RNA (mRNA)
transcripts and repressing the translation or degrading the target
mRNA (6). miRNAs regulate cell
differentiation, proliferation and apoptosis, and in this way they
influence the occurrence and development of inflammation, endocrine
diseases and tumors (5). It has been
demonstrated that specific miRNAs act as oncogenes or
anti-oncogenes by regulating cellular pathways (7). Furthermore, previous studies have
suggested that miR-21 is an oncogenic miRNA that is significantly
upregulated in tumor tissue, peripheral blood and particular body
fluids (such as the cerebrospinal fluid) of patients with
malignancies, including lung, hepatic and breast cancer, as well as
B-cell and central nervous system lymphoma (8–11). miR-21
binds to the 3′-UTR region of target genes, including programmed
cell death 4 (PDCD4), phosphatase and tensin homolog (PTEN) and
reversion inducing cysteine rich protein with kazal motifs (RECK),
thus promoting proliferation and invasion whilst inhibiting
apoptosis (12–14). However, the expression and biological
function of miR-21 in T-ALL remains poorly understood. In the
present study, levels of miR-21 expression were measured in a T-ALL
cell line (Jurkat) to investigate the mechanism of miR-21 oncogenic
function. Jurkat cells were infected with recombinant adenovirus
adv-miR-21 or adv-anti-miR-21 and the effects of miR-21 expression
and knockout on cell proliferation, invasion and apoptosis were
explored.
Signal transducer and activator of transcription
(STAT) 3 is involved in a number of cellular functions. It
regulates genes associated with cell growth, movement and
apoptosis, and is overactive in various types of cancer, including
breast, pancreatic and prostate cancer, as well as leukemia and
lymphoma (15). Evidence suggests
that miRNAs are closely associated with the STAT3 signaling
pathway. It has been demonstrated that miR-21 is involved in
STAT3-mediated tumorigenesis, and that the STAT3 protein regulates
the expression of miR-21 by binding to the STAT3-binding site
within the promoter of the miR-21 gene on chromosome 17q23.2
(16,17). Furthermore, Fujita et al
(18) identified a feedback mechanism
between nuclear factor (NF)-IB and miR-21. Previous studies using
TargetScan and PicTar (16,17) suggested that STAT3 may be a target of
miR-21. Therefore, the present study investigated whether a
feedback loop between STAT3 and miR-21 exists. To verify this
hypothesis, a luciferase reporter assay, reverse
transcription-quantitative polymerase chain reaction (RT-qPCR) and
western blotting were performed.
Materials and methods
Cell culture
Cells from the human Jurkat cell line (obtained from
the Committee on Type Culture Collection of the Chinese Academy of
Sciences, Shanghai, China) were cultured in RPMI 1640 medium
(Thermo Fisher Scientific, Inc., Waltham, MA, USA) containing 10%
fetal bovine serum (FBS; Biological Industries, Beit-Haemek,
Israel). Human kidney (HEK) 293A and 293T cell lines (both obtained
from the Committee on Type Culture Collection of the Chinese
Academy of Sciences) were cultured in Dulbecco's modified Eagle's
medium (DMEM; Thermo Fisher Scientific, Inc.) containing 10% FBS.
Cells from the Jurkat, HEK 293A and 293T cell lines were kept in
the laboratory and incubated in cell culture incubators (Sanyo
Electric, Co., Ltd., Osaka, Japan) at 37°C with 5%
CO2.
Construction of recombinant
adenovirus
High expression of miRNA-21 by recombinant vector
pAV-MCMV-EGFP-3FLAG-miRNA-21 (Shanghai Sunbio Technology Co., Ltd,
Shanghai, China) and knockdown of miR-21 by recombinant vector
pAVsiRNA1.1-anti-miR-21 (Shanghai Sunbio Technology Co., Ltd.) were
successfully completed. The adenovirus expression plasmid and the
adenovirus backbone plasmid underwent co-transfection in 293A cells
using Trans-EZ (Shanghai Sunbio Technology Co., Ltd.) to package
adv-miR-21, adv-miR-21-control, adv-anti-miR-21 and
adv-anti-miR-21-control. Virus titer was determined by Titer-EZ
adenoviral titer detection reagent (Shanghai Sunbio Technology Co.,
Ltd.).
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
To compare the expression of miR-21 in Jurkat cells
with normal lymphocytes, peripheral blood samples were obtained
from patients at the First Affiliated Hospital of Zhengzhou
University (Zhengzhou, China) from July 2013 to October 2013, once
written informed consent was obtained, in accordance with the
Declaration of Helsinki. Experiments conformed to regulatory
standards and were approved by the Zhengzhou University Life
Science Ethics Review Committee (Zhengzhou, China). Lymphocytes
from normal human peripheral blood were isolated using the
Ficoll-Paque® Plus medium (GE Healthcare Life Sciences,
Chalfont, UK). Jurkat cells were infected with recombinant
adenovirus, adv-miR-21, adv-miR-21-control, adv-anti-miR-21 or
adv-anti-miR-21-control, and collected 48 h after infection. Total
RNA was extracted from Jurkat cells and normal lymphocytes using
TRIzol (Invitrogen; Thermo Fisher Scientific, Inc.), and RT-qPCR
was performed as described previously (8). The sequences of the primers (synthesized
by Genewiz, Inc., South Plainfield, NJ, USA) are presented in
Table I. U6 (with a stem-loop primer)
was used as reference gene. Each sample was analyzed in triplicate.
The ΔΔCq values were calculated following normalization to
reference U6 RNA and quantified using the 2−ΔΔCq method
(9).
 | Table I.Primers for miR-21. |
Table I.
Primers for miR-21.
| Primers | Sequence (5′→3′) |
|---|
| miR21-RT |
CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGTCAACATC |
| miR21-qPCR | Forward:
TAGCTTATCAGACTGA |
|
| Reverse:
TGGCAGGGTCCGAGGT |
| U6-RT |
TCGTATCCATGGCAGGGTCCGAGGTATTCGCCATGGATACGACACAAAAATATGGAACGCTT |
| U6-qPCR | Forward:
GTGCTCGCTTCGGCAGCACA |
|
| Reverse:
TGGCAGGGTCCGAGGT |
Cell proliferation assay
Cell proliferation in vitro was measured
using a CCK-8 Cell Counting kit (Vazyme, Piscataway, NJ, USA).
Jurkat cells were infected with recombinant adenovirus for 24, 48,
72 and 96 h in a 96-well plate. A total of 10 µl CCK-8 was added to
each well, and samples were incubated for a further 4 h in a cell
culture incubator. Optical density (OD) values were measured using
a microplate reader (Bio-Rad Laboratories, Inc., Hercules, CA, USA)
at 450 nm. Each experiment was performed in triplicate. Cell
viability was calculated by the following formula: Cell viability =
(dosing OD-blank OD / control cells OD-blank OD) ×100%.
In vitro invasion assay
For the in vitro invasion assay, Jurkat cells
were infected with recombinant adenovirus. Cells were seeded on a
Matrigel™-coated membrane matrix (BD Biosciences, Franklin Lakes,
NJ, USA) in the insert of a 24-well Transwell culture plate fitted
with polycarbonate filters (8-µm pore size; Costar; Corning
Incorporated, Corning, NY, USA) 72 h following infection. Cells in
the upper chamber were cultured in RPMI 1640 medium without FBS,
while 10% FBS was added to the lower chamber. After 24 h, migrating
cells located in the lower chamber were counted directly under a
microscope (Olympus Corporation, Tokyo, Japan). The invasive
ability of Jurkat cells was determined by the number of cells
observed migrating into the lower chamber.
Flow cytometric analysis
A PE Annexin V Apoptosis Detection kit I (BD
Biosciences) was used to detect the apoptosis rate of Jurkat cells.
Jurkat cells were infected with recombinant adenovirus for 96 h.
Following the collection and resuspension of 1×105 cells
using 100 µl 1X Binding Buffer, 5 µl PE Annexin V and 5 µl
7-aminoactinomycin D were added to each sample for 15 min at room
temperature and a further 400 µl of 1X Binding Buffer was added to
each system. Stained samples were analyzed within 1 h by flow
cytometry (LSR II; BD Biosciences).
Prediction of target genes of
miR-21
TargetScan (www.targetscan.org) and PicTar (pictar.mdc-berlin.de)
were used to identify possible target genes of miR-21. Among the
same target genes that the two prediction software identified, the
target genes of different scores were filtered according to the
conservation and the integrated score of the base distribution of
the 3′-UTR of the target genes. Finally, STAT3 was selected as the
target gene of miR-21 that was associated with T-ALL (Fig. 1).
Dual luciferase reporter assay
A dual luciferase reporter assay was performed to
validate that STAT3 was the target gene of miR-21. The 3′-UTR
fragment, which contained the binding site of miR-21 to STAT3 mRNA,
was cloned, as well as a mutant fragment that lacked the binding
site. Then, either the 3′-UTR fragment or the mutant fragment was
recombined with the pmir-GLO Vector (Promega Corporation, Madison,
WI, USA), consisting of the Renilla luciferase (hRluc-neo)
gene and a firefly luciferase gene in the XhoI site, using
the ClonExpress® MultiS One Step Cloning kit (Vazyme).
For the reporter assays, HEK 293T cells were seeded onto 24-well
plates. The recombinant pmir-GLO-3′-UTR vector or the
pmir-GLO-mutant-3′-UTR vector were transfected into HEK 293T cells
using Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.). Subsequently, 6 h following transfection, either
adv-miR-21 or adv-miR-21-control were added to the HEK 293T cells.
Following 48 h of cultivation, firefly and Renilla
luciferase activities were detected using the
Dual-Glo®Luciferase Assay System (Promega Corporation)
and a MicroLumatPlus LB96V luminometer (Berthold Technologies GmbH
& Co. KG, Bad Wildbad, Germany). Firefly luciferase values were
normalized to Renilla luciferase activity, and the ratio of
firefly/Renilla luciferase activity was presented. Primers
for STAT3-3′-UTR-wild type or mutant fragment are presented in
Table II.
 | Table II.Primers for STAT3-3′-UTR-wild type or
mutant fragments. |
Table II.
Primers for STAT3-3′-UTR-wild type or
mutant fragments.
| Primer | Sequence
(5′→3′) |
|---|
| STAT3-3′-UTR
fragment | F:
TTGGCTGGCTAGCTCGCCTCT |
|
| R:
CTTATAGGTAGGTAAGCAACC |
| STAT3-3′-UTR-wild
type | F:
GTTGTTTAAACGAGCTCGTGGCAACTCAAAACCACC |
|
| R:
GGTCGACTCTAGACTCGAGGGGCTCAGCTCCTCTCAG |
|
STAT3-3′-UTR-mutation |
|
Fragment 1 | F:
GTTGTTTAAACGAGCTCGTGGCAACTCAAAACCACC |
|
| R:
TTCTGTTTATCAGTTATATGTACTGAAGAGT |
|
Fragment 2 | F:
ACTCTTCAGTACATATAACTGATAAACAGAA |
|
| R:
GGTCGACTCTAGACTCGAGGGGCTCAGCTCCTCTCAG |
STAT3 mRNA quantification by
RT-qPCR
Normal lymphocytes, Jurkat cells and Jurkat cells
infected with adv-miR-21-control or adv-miR-21 were collected 48 h
following infection. Total RNA was extracted with TRIzol and
subjected to RT. In the RT step, TransScript First-Strand cDNA
Synthesis Supermix (Beijing Transgen Biotech Co., Ltd., Beijing,
China) was used to synthesize the complementary DNA of STAT3, and
the cycling conditions were as follows: 5 min at 25°C, 60 min at
42°C, 5 min at 85°C, and hold at 4°C. STAT3 mRNA qPCR was performed
as described previously (19). The
expression of STAT3 mRNA was normalized to the glyceraldehyde
3-phosphate dehydrogenase (GAPDH) mRNA. The sequences of the
primers (synthesized by Genewiz, Inc.) are presented in Table III.
 | Table III.Primers for STAT3 messenger RNA
quantification by qPCR. |
Table III.
Primers for STAT3 messenger RNA
quantification by qPCR.
| Primers | Sequence
(5′→3′) |
|---|
| STAT3-qPCR | F:
GGAGGCATTCGGAAAGTATTGTCG |
|
| R:
ATGGTATTGCTGCAGGTCGTTGGT |
| GAPDH-qPCR | F:
AGAAGGCTGGGGCTCATTTG |
|
| R:
AGGGGCCATCCACAGTCTTC |
Western blot assay
Firstly, Jurkat cells were harvested following
infection with adv-miR-21-control or adv-miR-21 for 72 h. Jurkat
cells and normal lymphocytes were subsequently lysed with
radioimmunoprecipitation assay lysis buffer (Beijing Dingguo
Changsheng, Biotechnology Co., Ltd., Beijing, China), and total
protein was extracted. Protein concentrations were measured by a
BCA Protein Assay kit (Beijing Dingguo Changsheng, Biotechnology
Co., Ltd.), according to a standardized curve. Equal protein
amounts were separated on 10% Tris-glycine sodium dodecyl
sulfate-polyacrylamide gel electrophoresis and then transferred
onto polyvinylidene difluoride membranes (EMD Millipore, Billerica,
MA, USA). Membranes were blocked with 5% nonfat dried milk for 2 h
at room temperature and incubated with antibodies against STAT3
(1:2,000; catalogue number 12640; Cell Signaling Technology, Inc.,
Danvers, MA, USA) and GAPDH (1:3,000; catalogue number sc-47724;
Santa Cruz Biotechnology, Inc., Dallas, TX, USA) overnight at 4°C.
The quantification of western blotting was performed using image
processing and analysis software (Fusion SL; Vilber Lourmat
Deutschland GmbH, Eberhardzell, Germany), and the relative
expression of STAT3 protein was normalized to GAPDH.
Statistical analysis
All experiments were performed three times. Data
were processed using SPSS 17.0 software (SPSS, Inc., Chicago, IL,
USA) and presented as the mean ± standard deviation. Statistical
analysis was conducted using Student's t-test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Expression of miR-21 in Jurkat cells,
normal lymphocytes and Jurkat cells infected with recombinant
adenovirus
RT-qPCR was performed to detect miR-21 levels in
normal lymphocytes, Jurkat cells, and Jurkat cells infected with
adv-miR-21-control, adv-miR-21, adv-anti-miR-21-control or
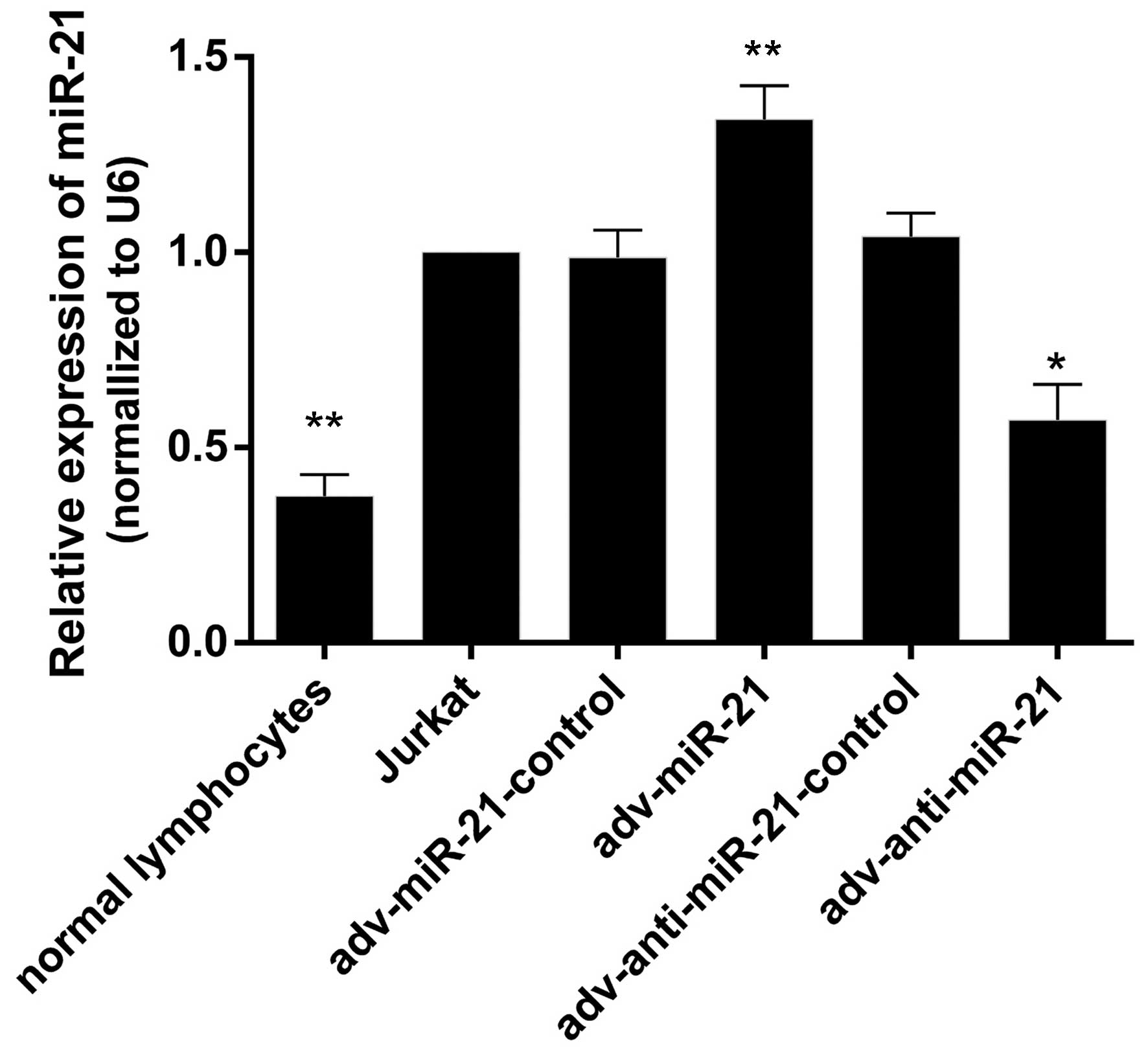
adv-anti-miR-21. The results indicated that the expression levels
of miR-21 were 0.37±0.03, 1.00±0.00, 0.98±0.07, 1.34±0.86,
1.04±0.06 and 0.57±0.09 in normal lymphocytes, Jurkat cells, and
Jurkat cells infected with adv-miR-21-control, adv-miR-21,
adv-anti-miR-21-control and adv-anti-miR-21, respectively. The
expression of miR-21 in Jurkat cells was significantly higher than
in normal lymphocytes (P<0.01), and miR-21 levels were
significantly upregulated or downregulated in Jurkat cells infected
with adv-miR-21 (P<0.01) or adv-anti-miR-21 (P<0.05),
respectively, compared with normal Jurkat cells (Fig. 2). These results indicated that high
expression of miR-21 and knockdown of miR-21 by recombinant
adenovirus successfully occurred.
Upregulation of miR-21 in Jurkat cells
promotes proliferation, while knockdown of miR-21 in Jurkat cells
inhibits proliferation
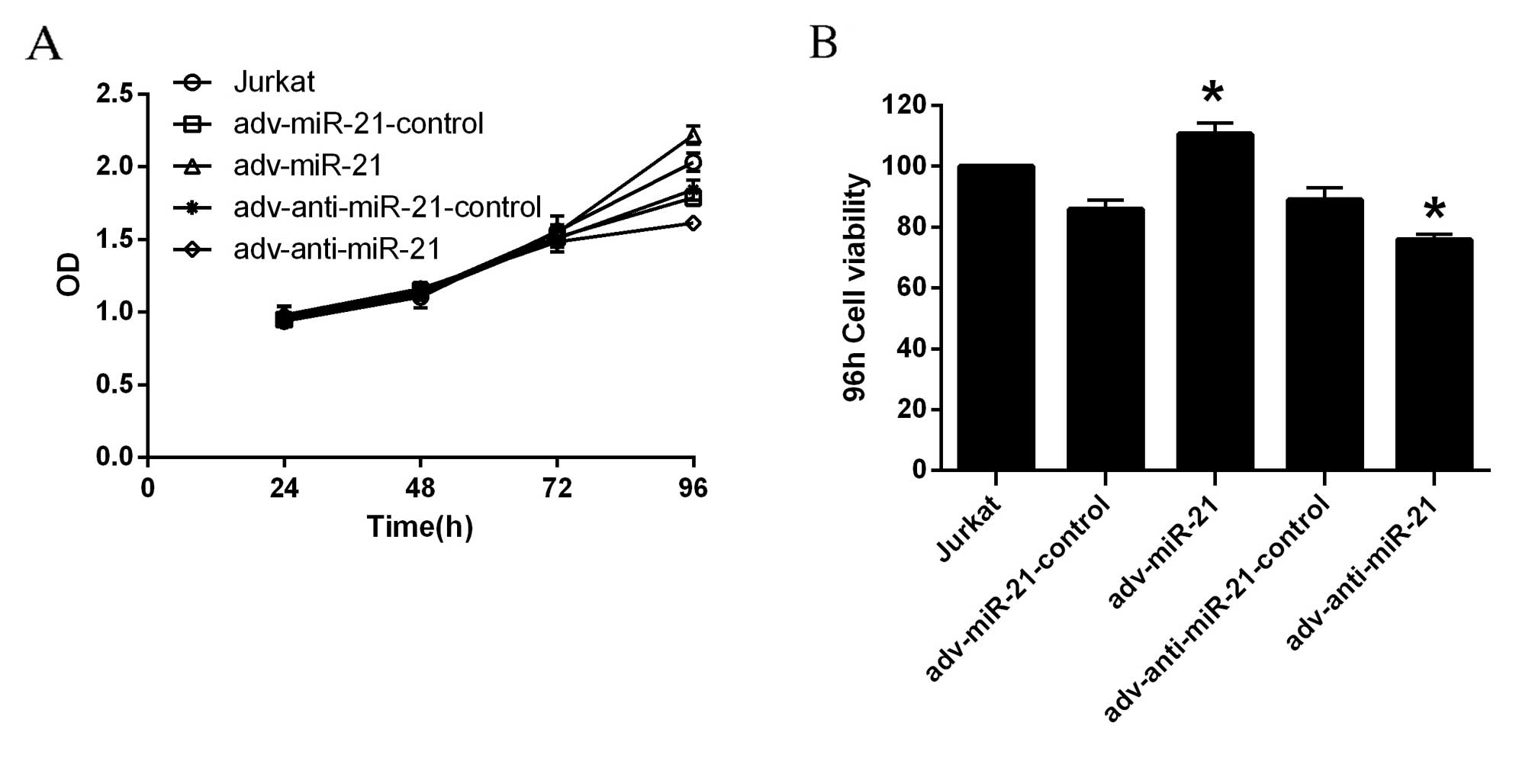
According to the OD values obtained, the
proliferation of Jurkat cells infected with adv-miR-21 or
adv-anti-miR-21 was significantly different to that observed in the
relevant control group 96 h after infection, while the differences
were not significant at 24, 48 or 72 h (Fig. 3A). The viability of Jurkat cells was
calculated by the formula: Cell viability=(dosing OD-blank
OD/control cells OD-blank OD)x100%. As demonstrated in Fig. 3B, the viability of Jurkat cells
infected with adv-miR-21 significantly increased to 128.80% after
96 h compared with ~90% viability in the adv-miR-21 control.
However, the viability of Jurkat cells infected with
adv-anti-miR-21 was significantly inhibited to 85.33% compared with
~90% viability in adv-anti-miR-21-control after 96 h. Therefore,
upregulation of miR-21 in Jurkat cells promoted proliferation,
while knockdown of miR-21 in Jurkat cells inhibited
proliferation.
In vitro invasion assay
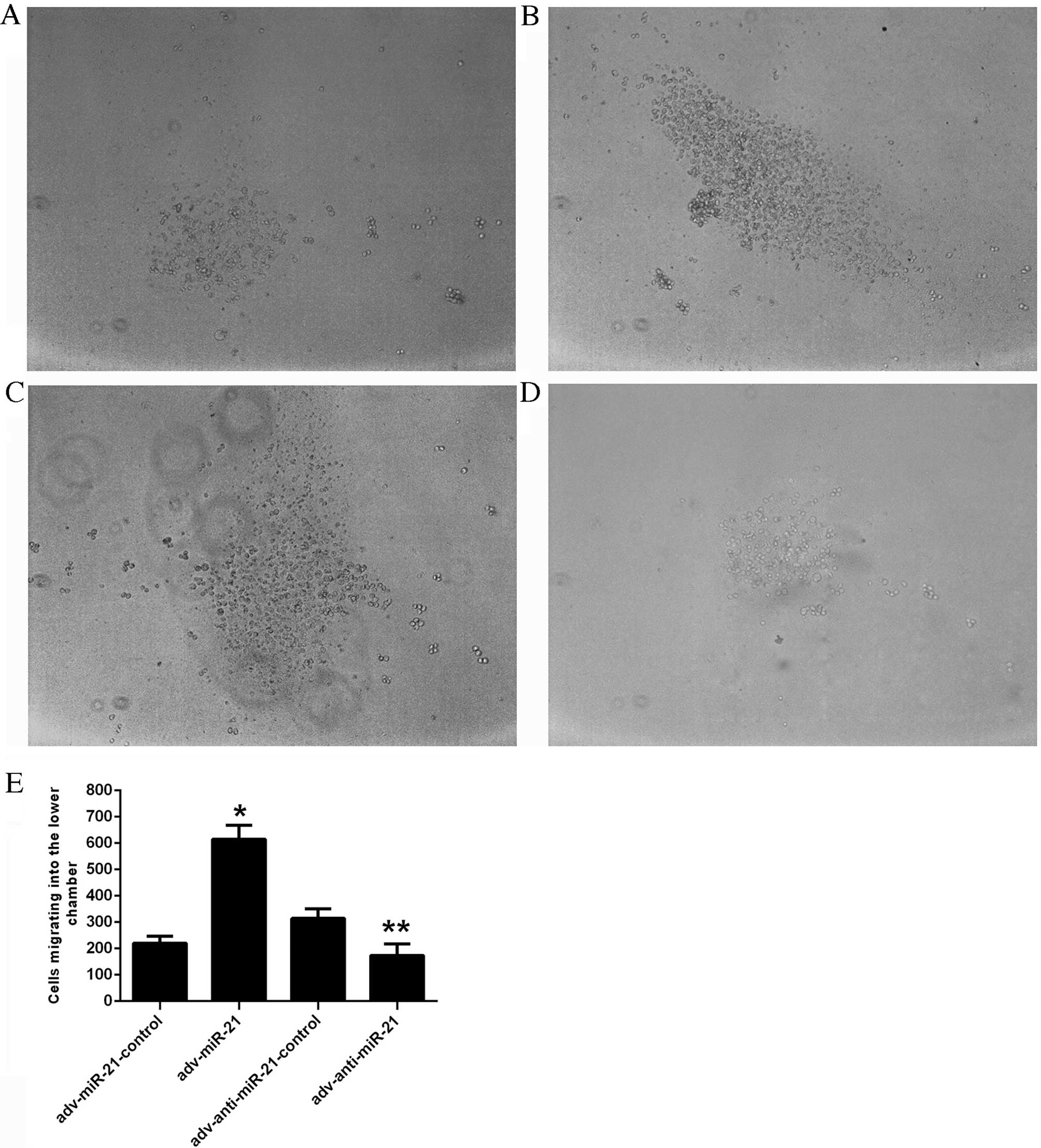
An in vitro Matrigel Transwell analysis was
applied to determine the influence of miR-21 on the invasive
ability of Jurkat cells. Due to the characteristics of suspension
cells, Jurkat cells migrating into the lower chamber floated in the
culture medium rather than attaching to the membrane of the
Transwell. The number of cells migrating into the lower chamber was
220.11±26.78 in the adv-miR-21-control group, 615.33±52.20 in the
adv-miR-21 group, 314.00±36.70 in the adv-anti-miR-21-control group
and 173.89±43.50 in the adv-anti-miR-21 group (Fig. 4E). A higher proportion of Jurkat cells
infected with adv-miR-21 migrated into the lower chamber than the
adv-miR-21-control group (P<0.001), while fewer Jurkat cells
infected with adv-anti-miR-21 migrated into the lower chamber
compared with the adv-anti-miR-21-control group (P<0.01;
Fig. 4). These results indicated that
upregulating miR-21 expression in Jurkat cells significantly
promoted invasive ability, whereas downregulating miR-21 expression
significantly inhibited invasive ability.
miR-21 influences the apoptosis of
Jurkat cells
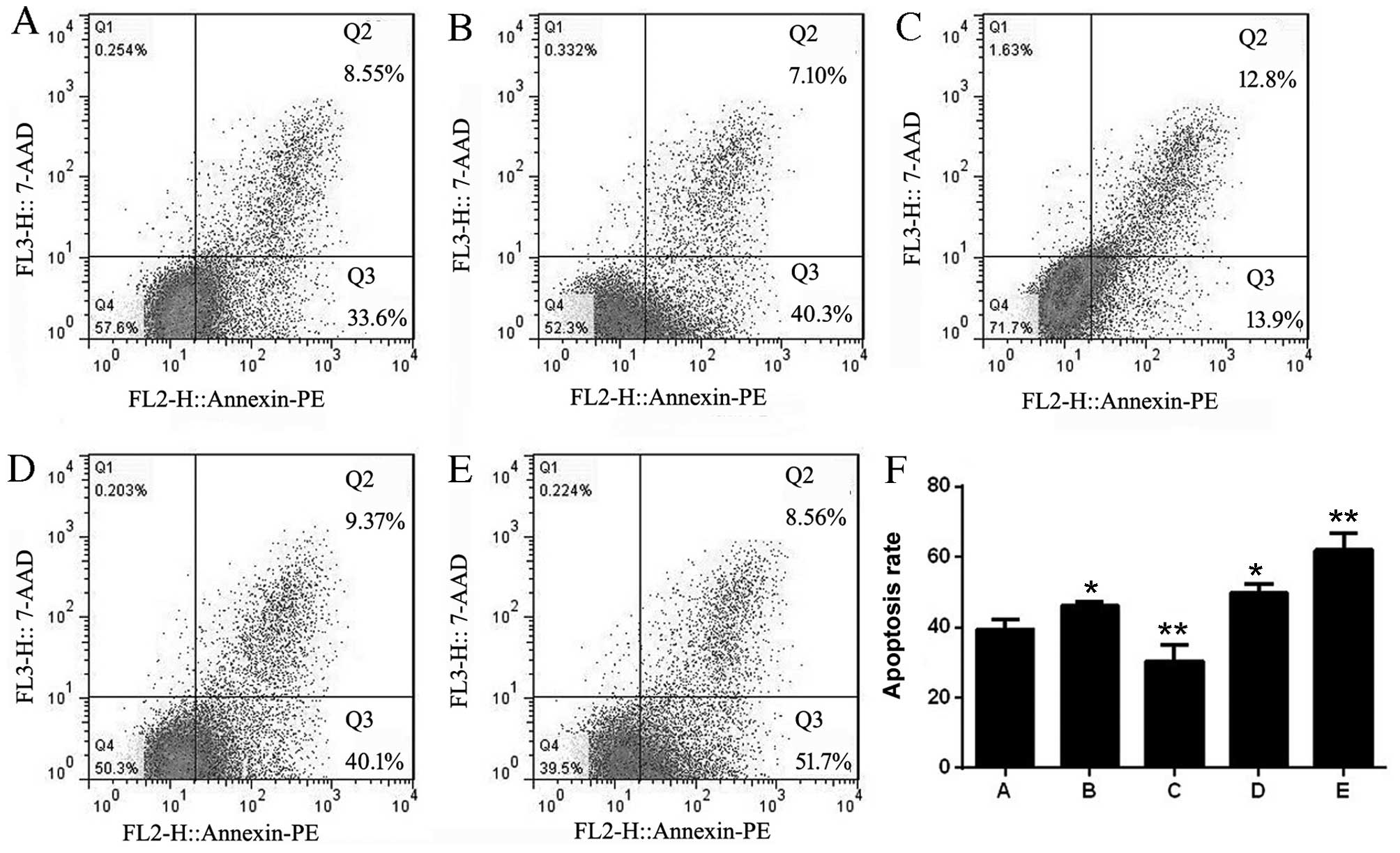
The apoptosis rates of each group were 39.42±2.90
(non-infected Jurkat cells), 46.20±1.18 (adv-miR-21-control),
30.36±4.71 (adv-miR-21), 49.62±2.77 (adv-anti-miR-21-control) and
61.96±4.75 (adv-anti-miR-21). The results of flow cytometry
analysis indicated that the apoptosis rates of Jurkat cells
infected with adv-miR-21-control or adv-anti-miR-21-control were
upregulated compared with control Jurkat cells, indicating that the
adenovirus affected the apoptosis of Jurkat cells. However,
compared with the adv-miR-21-control group, there was a significant
decrease in the apoptosis rate of Jurkat cells infected with
adv-miR-21. In addition, a significant increased rate of apoptosis
was also detected in Jurkat cells infected with adv-anti-miR-21
compared with the adv-anti-miR-21-control group (Fig. 5). These results demonstrated that
upregulating miR-21 expression in Jurkat cells inhibited apoptosis,
while downregulating miR-21 promoted apoptosis.
STAT3 is a direct target gene of
miR-21
STAT3 was selected as the predicted target gene of
miR-21 associated with T-ALL. The results of the dual luciferase
reporter assay (Fig. 6A) indicated
that the relative luciferase activity of the
pmir-GLO-STAT3-3′-UTR-wild-type (STAT3-wt) reporter was
significantly inhibited in the adv-miR-21 group (0.61±0.12)
compared with the adv-miR-21-control group (1.31±0.28). However,
the relative luciferase activity of the
pmir-GLO-STAT3-3′-UTR-mutant (STAT3-mt) reporter (1.22±0.37) was
almost the same as the STAT3-mt-adv-miR-21-control group
(1.23±0.37) and the STAT3-mt-adv-miR-21 group (1.16±0.22). These
results suggested that miR-21 targets STAT3 by binding to a
specific region of the 3′-UTR.
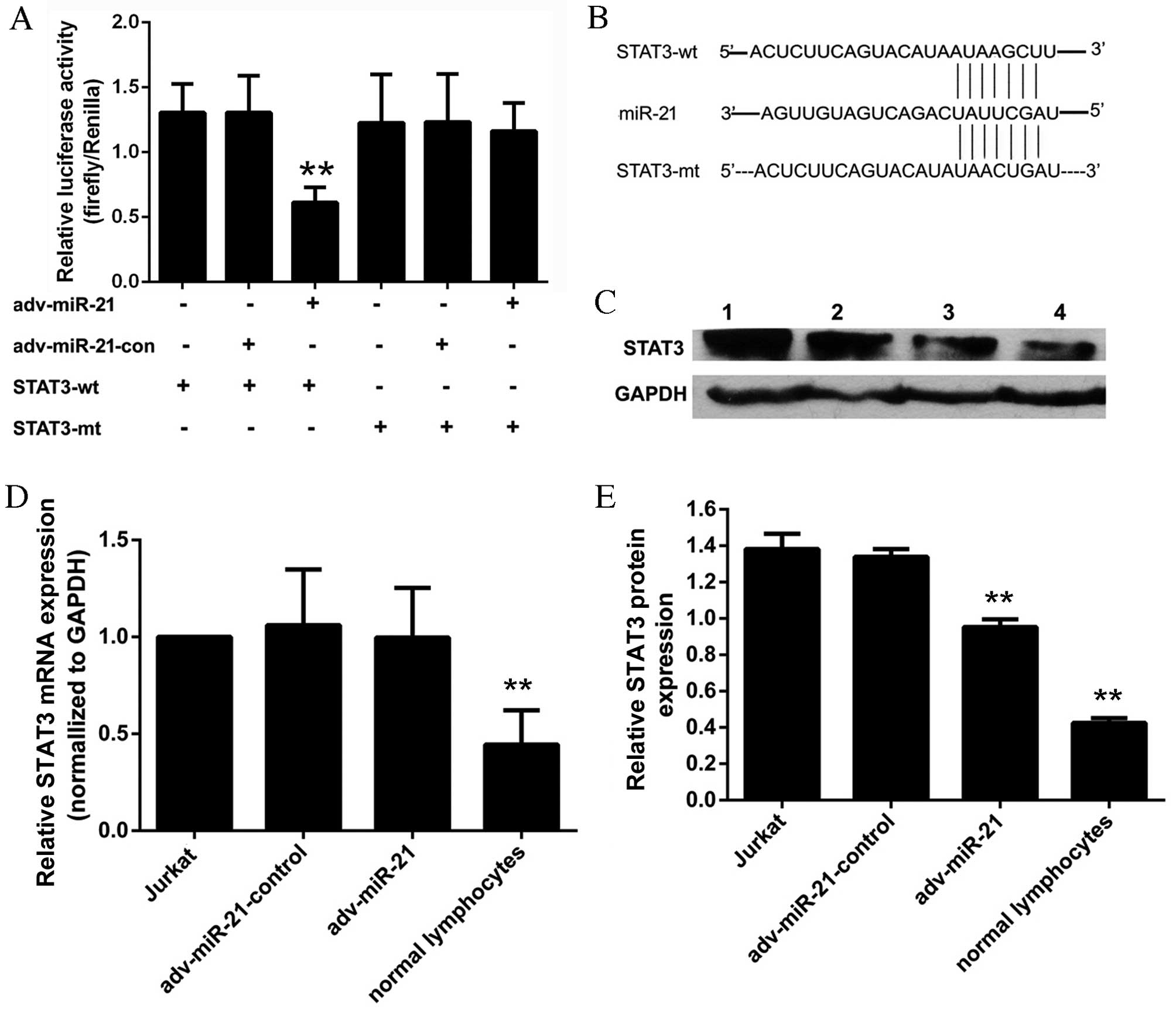 | Figure 6.Effect of miR-21 on STAT3 in Jurkat
cells. (A) The relative luciferase activity of the STAT3-wt
reporter was significantly inhibited in the adv-miR-21 group
compared with the control group (**P<0.01). However, the
relative luciferase activity of the STAT3-mt reporter was almost
the same as the control group (P=0.690). (B) Sequence of the 3′-UTR
fragment that contained the binding site to miR-21 of STAT3 mRNA
and a mutant 3′-UTR fragment that lacked the binding site to
miR-21. (C) Lanes 1–4 represent the results from a western blot
analysis of Jurkat cells, adv-miR-21-control, adv-miR-21 and normal
lymphocytes, respectively. The relative STAT3 protein expression
normalized to GAPDH protein in normal lymphocytes was significantly
lower than that in Jurkat cells (**P<0.01). (D) The relative
STAT3 mRNA expression normalized to GAPDH mRNA in normal
lymphocytes was significantly lower than that in Jurkat cells
(**P<0.01); however, there was no significant difference in
relative STAT3 mRNA levels between the adv-miR-21 group and the
adv-miR-21-control group (P=0.79). (E) The relative STAT3 protein
expression in Jurkat cells infected with adv-miR-21 was
significantly lower than that in the adv-miR-21-control group
(**P<0.01). Wt, wild type; mt, mutant; miR-21, microRNA-21; con,
control; adv, adenovirus; STAT3, signal transducer and activator of
transcription; UTR, untranslated region; GAPDH,
glyceraldehyde-3-phosphate dehydrogenase; mRNA, messenger RNA. |
miR-21 regulates STAT3 by inhibiting
translation
Western blot analysis and qPCR were applied to
evaluate the effects of miR-21 on STAT3. The relative STAT3 mRNA
expression of the Jurkat cell, adv-miR-21-control, adv-miR-21 and
normal lymphocyte groups were 1.00±0.00, 1.06±0.29, 0.99±0.27 and
0.44±0.18, respectively (Fig. 6D).
The results of western blot analysis demonstrated that the relative
grayscale rates of the Jurkat cells, adv-miR-21-control, adv-miR-21
and normal lymphocytes groups were 1.38±0.08, 1.33±0.04, 0.95±0.04
and 0.42±0.02, respectively (Fig.
6E). Furthermore, the results indicated that the levels of
STAT3 mRNA (Fig. 6D) and STAT3
protein (Fig. 6C and E) in normal
lymphocytes were significantly lower than those in Jurkat cells.
Following infection with adv-miR-21, endogenous STAT3 protein
levels were significantly decreased compared with the control group
(Fig. 6E), however STAT3 mRNA levels
were approximately the same as the control group (Fig. 6D). These results suggest that miR-21
targets STAT3 by inhibiting translation rather than degrading the
target mRNA.
Discussion
Aberrant expression of miR-21 has been observed in
various types of malignancies, and it serves an important role in
tumorigenesis and other oncogenic processes by controlling the
expression of target genes (8–11). The
current study demonstrated that miR-21 expression in Jurkat cells
was significantly upregulated compared with normal lymphocytes,
indicating that miR-21 may be a novel diagnostic marker for
T-ALL.
To further investigate the effects of miR-21 on
T-ALL, Jurkat cells were infected with miR-21 recombinant
adenovirus, which induced either increased expression or knockdown
of miR-21. The present study identified that upregulating miR-21
increased the proliferation and invasive ability of Jurkat cells
and decreased their rate of apoptosis. Conversely, knockdown of
miR-21 suppressed the proliferation and invasive ability of Jurkat
cells and promoted their apoptosis. These results suggest that
miR-21 may serve an oncogenic role in T-ALL by regulating multiple
biological functions, including proliferation, apoptosis or
invasion of T-ALL cells.
Previous studies have demonstrated that miR-21 may
be oncogenic, as it regulates target genes, including PTEN, PDCD4
and B-cell lymphoma (Bcl)-2, which are associated with cell
proliferation, apoptosis and invasion (10,12,13). The
effects of miR-21 on a specific target are context and sequence
specific (20). A perfect match of ≥6
nucleotides (nucleotides 2–7) is required to bind miR-21 to the
3′-UTR of the target gene and form the RNA induced silencing
complex, which is responsible for regulating the translation of
target genes (21,22). Degradation of miR-21 target mRNA may
be the predominant mechanism in plants, whereas inefficient
translation of the target gene may be a more common regulation
mechanism in animals (22).
TargetScan and PicTar are examples of common computational target
prediction softwares used to predict miRNA target sites conserved
among orthologous 3′-UTRs of protein-coding genes (23). In the present study, TargetScan and
PicTar were used to predict the target genes of miR-21 and the
mechanism of action of miR-21 in Jurkat cells. Among all the
predicted target genes, PDCD4, PTEN, Bcl-2 and RECK had previously
been demonstrated to be target genes of miR-21 (12–14). STAT3
was another predicted target gene of miR-21, and previous studies
have determined that the STAT3 protein is able to regulate miR-21
expression by binding to the miR-21 gene promoter (16,17,24).
Therefore, it was hypothesized that a feedback loop may exist
between miR-21 and STAT3.
STAT3 is a member of the STATs family, and serves an
important role in malignant transformation and oncogenesis by
regulating cell proliferation, differentiation and invasion
(25). Members of the miR-17 cluster
family regulate STAT3, which in turn regulates certain
cancer-associated miRNAs, including miR-155 and miR-21, by binding
to the promoters of these genes (26). It has previously been demonstrated the
existence of regulatory feedback loops between the STAT3 pathway
and miRNAs in different cancer contexts, including interleukin-6
(IL-6) receptor-STAT3-NF-κB-Lin-28-let-7a and
IL-6-STAT3-miR-24/miR-629-hepatocyte nuclear factor 4α-miR-124
(27).
The present study established that STAT3 expression
in Jurkat cells was significantly higher than in normal
lymphocytes. The results of the luciferase reporter assay
demonstrated that miR-21 could bind to a specific region of the
STAT3 3′-UTR. Furthermore, the results of western blot assay
indicated that upregulating the expression of miR-21 in Jurkat
cells resulted in decreased levels of STAT3 protein, whereas there
were no significant differences between the levels of STAT3 mRNA
among any of the groups. Therefore, the current study determined
that STAT3 was a target gene of miR-21 and, as STAT3 protein is
able to regulate miR-21 expression by binding to the miR-21 gene
promoter, the existence of a regulatory feedback loop between
miR-21 and STAT3 was suggested. This feedback loop may balance
miR-21 and STAT3 expression in Jurkat cells, in order to precisely
regulate the biological function of Jurkat cells.
In conclusion, miR-21 affects T-ALL by promoting the
proliferation and invasion and inhibiting the apoptosis of Jurkat
cells. In addition, miR-21 targets STAT3 by inhibiting translation
rather than degrading the target mRNA. The regulatory mechanism of
miR-21 in Jurkat cells is subtle and complicated. Future
investigations are necessary to improve the understanding of the
interactions between miR-21 and STAT3 and to facilitate the
development of novel therapeutic strategies to treat T-ALL, for
which the current study may provide valuable evidence.
Acknowledgements
The present study was supported by grants from the
Youth Foundation of the First Affiliated Hospital of Zhengzhou
University (Zhengzhou, China; grant no. 13Y0165) the Key Science
and Technology Research Project of the Education Department of
Henan province (Zhengzhou, China; grant no. 13A320413) and the
General Program of the National Natural Science Foundation of China
(Beijing, China; grant no. 81470364).
References
|
1
|
Jabbour EJ, Faderl S and Kantarjian HM:
Adult acute lymphoblastic leukemia. Mayo Clin Proc. 80:1517–1527.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Coustan-Smith E, Mullighan CG, Onciu M,
Behm FG, Raimondi SC, Pei D, Cheng C, Su X, Rubnitz JE, Basso G, et
al: Early T-cell precursor leukaemia: A subtype of very high-risk
acute lymphoblastic leukaemia. Lancet Oncol. 10:147–156. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Armstrong SA and Look AT: Molecular
genetics of acute lymphoblastic leukemia. J Clin Oncol.
23:6306–6315. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Goldberg JM, Silverman LB, Levy DE, Dalton
VK, Gelber RD, Lehmann L, Cohen HJ, Sallan SE and Asselin BL:
Childhood T-cell acute lymphoblastic leukemia: The Dana-Farber
cancer institute acute lymphoblastic leukemia consortium
experience. J Clin Oncol. 21:3616–3622. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Winter J, Jung S, Keller S, Gregory RI and
Diederichs S: Many roads to maturity: MicroRNA biogenesis pathways
and their regulation. Nat Cell Biol. 11:228–234. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yendamuri S and Calin GA: The role of
microRNA in human leukemia: A review. Leukemia. 23:1257–1263. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kumar MS, Lu J, Mercer KL, Golub TR and
Jacks T: Impaired microRNA processing enhances cellular
transformation and tumorigenesis. Nat Genet. 39:673–677. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Baraniskin A, Kuhnhenn J, Schlegel U, Chan
A, Deckert M, Gold R, Maghnouj A, Zöllner H, Reinacher-Schick A,
Schmiegel W, et al: Identification of microRNAs in the
cerebrospinal fluid as marker for primary diffuse large B-cell
lymphoma of the central nervous system. Blood. 117:3140–3146. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gu L, Song G, Chen L, Nie Z, He B, Pan Y,
Xu Y, Li R, Gao T, Cho WC and Wang S: Inhibition of miR-21 induces
biological and behavioral alterations in diffuse large B-cell
lymphoma. Acta Haematol. 130:87–94. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu C, Yu J, Yu S, Lavker RM, Cai L, Liu
W, Yang K, He X and Chen S: MicroRNA-21 acts as an oncomir through
multiple targets in human hepatocellular carcinoma. J Hepatol.
53:98–107. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wu R, Jiang Y, Wu Q, Li Q, Cheng D, Xu L,
Zhang C, Zhang M and Ye L: Diagnostic value of microRNA-21 in the
diagnosis of lung cancer: Evidence from a meta-analysis involving
11 studies. Tumour Biol. 35:8829–8836. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xu LF, Wu ZP, Chen Y, Zhu QS, Hamidi S and
Navab R: MicroRNA-21 (miR-21) regulates cellular proliferation,
invasion, migration, and apoptosis by targeting PTEN, RECK and
Bcl-2 in lung squamous carcinoma, Gejiu City, China. PLoS One.
9:e1036982014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Frankel LB, Christoffersen NR, Jacobsen A,
Lindow M, Krogh A and Lund AH: Programmed cell death 4 (PDCD4) is
an important functional target of the microRNA miR-21 in breast
cancer cells. J Biol Chem. 283:1026–1033. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wu MF, Yang J, Xiang T, Shi YY and Liu LJ:
miR-21 targets Fas ligand-mediated apoptosis in breast cancer cell
line MCF-7. J Huazhong Univ Sci Technolog Med Sci. 34:190–194.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Frank DA: STAT3 as a central mediator of
neoplastic cellular transformation. Cancer Lett. 251:199–210. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Löffler D, Brocke-Heidrich K, Pfeifer G,
Stocsits C, Hackermüller J, Kretzschmar AK, Burger R, Gramatzki M,
Blumert C, Bauer K, et al: Interleukin-6 dependent survival of
multiple myeloma cells involves the Stat3-mediated induction of
microRNA-21 through a highly conserved enhancer. Blood.
110:1330–1333. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
van der Fits L, van Kester MS, Qin Y,
Out-Luiting JJ, Smit F, Zoutman WH, Willemze R, Tensen CP and
Vermeer MH: MicroRNA-21 expression in CD4+ T cells is regulated by
STAT3 and is pathologically involved in Sezary syndrome. J Invest
Dermatol. 131:762–768. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fujita S, Ito T, Mizutani T, Minoguchi S,
Yamamichi N, Sakurai K and Iba H: miR-21 gene expression triggered
by AP-1 is sustained through a double-negative feedback mechanism.
J Mol Biol. 378:492–504. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ou H, Li Y and Kang M: Activation of
miR-21 by STAT3 Induces Proliferation and Suppresses apoptosis in
nasopharyngeal carcinoma by targeting PTEN gene. PLoS One.
9:e1099292014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Croce CM: Causes and consequences of
microRNA dysregulation in cancer. Nat Rev Genet. 10:704–714. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sontheimer EJ: Assembly and function of
RNA silencing complexes. Nat Rev Mol Cell Biol. 6:127–138. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lewis BP, Burge CB and Bartel DP:
Conserved seed pairing, often flanked by adenosines, indicates that
thousands of human genes are microRNA targets. Cell. 120:15–20.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lewis BP, Shih IH, Jones-Rhoades MW,
Bartel DP and Burge CB: Prediction of mammalian microRNA targets.
Cell. 115:787–798. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bourguignon LY, Earle C, Wong G, Spevak CC
and Krueger K: Stem cell marker (Nanog) and Stat-3 signaling
promote MicroRNA-21 expression and chemoresistance in
hyaluronan/CD44-activated head and neck squamous cell carcinoma
cells. Oncogene. 31:149–160. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kohanbash G and Okada H: MicroRNAs and
STAT interplay. Semin Cancer Biol. 22:70–75. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Carraro G, El-Hashash A, Guidolin D,
Tiozzo C, Turcatel G, Young BM, De Langhe SP, Bellusci S, Shi W,
Parnigotto PP and Warburton D: miR-17 family of microRNAs controls
FGF10-mediated embryonic lung epithelial branching morphogenesis
through MAPK14 and STAT3 regulation of E-Cadherin distribution. Dev
Biol. 333:238–250. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cao Q, Li YY, He WF, Zhang ZZ, Zhou Q, Liu
X, Shen Y and Huang TT: Interplay between microRNAs and the STAT3
signaling pathway in human cancers. Physiol Genomics. 45:1206–1214.
2013. View Article : Google Scholar : PubMed/NCBI
|