Introduction
In a previous study, low concentrations of
9-aminoacridine (9-AAA) were observed to effectively decrease the
threshold of the direct current electric field strength required
for cell electroporation, but did not affect normal and cancer cell
viability when applied for 2–3 h (1).
Nevertheless, when normal and cancer cells were left to grow in the
presence of 9-AAA for 2–5 days, the rat prostate cancer cells and
human melanoma cells died, whereas normal human skin fibroblasts
(HSFs) continued to grow (1).
The drug 9-AAA and its derivatives have been studied
since the 1960′s, and have been shown to exhibit a broad spectrum
of biological activity. At the beginning of the 20th century, these
compounds were applied in medicine against protozoan infections and
diseases caused by bacteria and yeasts (2–4). In the
second half of the century, studies recognized the mutagenic
activity of 9-AAA and its derivatives, in particular in bacteria
and yeasts, and have since been extensively studied (5–8). The
biological activity of the compounds was previously observed to be
associated, among other effects, with their capacity to intercalate
into DNA (9). 9-AAA has also been
found to be useful in research concerning ion channels in
biological membranes (10–12) and has been shown to improve the
banding patterns of human and plant chromosomes for image analysis
(13). Furthermore, 9-AAA has been
applied for analysis of the surface electric potential on surfaces
of cells, protoplasts and liposomes (14–17).
A previous study reported that 9-AAA inhibits the
growth of animal cells and demonstrates anticancer activity in
vivo (18). In 1969, Mendecki
et al reported that 9-AAA inhibited the synthesis of RNA in
regenerating rat liver cells grown in vitro (19). In general, 9-AAA is applied for a
short time at relatively high concentrations (>10 µM), and the
effects are observed for a short time (usually <48 h). In the
majority of modern molecular studies concerning the effects of
9-AAA on cancer cells, the effects of 9-AAA were followed for 1–2
days (19–25).
Certain studies have shown that cell responses to
external factors are often delayed and become visible after a few
days (26,27). In contrast to the majority of reported
research, the present study therefore examined the effects of 9-AAA
on the growth of 3 cancer cell lines (2 prostate cancer cell lines
differing in malignancy and 1 human malignant melanoma) and on
normal HSFs in cell culture. The tested 9-AAA was present
continuously in the cell culture medium at concentrations that did
not significantly affect the viability of cells during the first 8
h of its application. The effects of 9-AAA were compared with the
5-fluorouracil (5-FU), a known anticancer drug that is commonly
used for cancer therapy in clinics (28,29).
Materials and methods
Cell cultures
All experiments were performed with normal HSFs and
3 cancer cell lines, including human melanoma A375 cells and 2 rat
prostate cancer cell lines from the Dunning R-3327 system: Highly
malignant Mat-LyLu and moderately malignant AT-2 (1,30,31). The cells were plated in 6-well Falcon
culture plates at a density of 20 000 cells per well, 24 h prior to
the addition of 9-AAA or 5-FU (Sigma-Aldrich, St. Louis, MO, USA).
HSFs and A375 cells were grown in Dulbecco's modified Eagle's
medium (DMEM) with a high glucose concentration (4,500 mg/l;
Sigma-Aldrich), and rat prostate adenocarcinoma AT-2 and Mat-LyLu
cell lines were grown in RPMI-1640 medium (Lonza Group, Basel,
Switzerland). The two media were supplemented with 10% heat
inactivated fetal calf serum (FCS; Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) and with a 1% antibiotics solution at final
concentration of 100 international units penicillin, 100 µg
streptomycin and 0.25 µg amphotericin per ml (Gibco; Thermo Fisher
Scientific, Inc.). The cells were propagated at 37°C in humid air
with 5% CO2. The tested inhibitors, 9-AAA and 5-FU, were
dissolved in cell culture medium and applied in concentrations in
the range 1–30 µM, as shown in the Results section. Cells cultured
in DMEM alone were used as the control. To estimate the effect of
the tested compounds on cell growth, the cells were harvested after
24, 48, 72 and 96 h by trypsinization, washed in phosphate-buffered
saline (PBS) by centrifugation at 400 × g and counted using a
Bürker haemocytometer.
Cell motile activity
Cell motile activity was examined by recording the
movements of individual cells and analyzing cell trajectories, as
described in detail in previous studies (30–32). The
results are presented in the form of circular diagrams, correlation
diagrams and the results of calculations.
Cell viability
Cell viability was tested using trypan blue
exclusion tests as described previously (32), and in each sample at least 300 cells
were analyzed. The type of cell death, apoptosis or necrosis, was
examined with two complementary methods.
An analysis of the apoptotic/necrotic cell death of
AT-2 cells, Mat-LyLu cells and HSFs growing in the control media or
in the presence of 1, 5 or 10 µM 9-AAA or 5, 10 or 15 µM 5-FU was
performed following 24, 48 or 72 h of cell culture using two
methods. For the first method, the cells were harvested by
trypsinization, washed in PBS and stained with propidium iodide (50
µg/ml; Sigma-Aldrich). The cells were analyzed with a FlowSight
image flow cytometer and Ideas 5.0 software (Amnis Corporation,
Seattle, WA, USA). For each sample, images of 10,000 single cells
were analyzed at 488 and 785 nm light wavelength using three
channels, as described previously (33). For the second method, the cells were
fixed in 3.5% formaldehyde for 20 min. The cells were then stained
in 0.5 ml Hoechst 33342 in PBS (1 µg/ml), prior to direct
observation under a fluorescence microscope (Leica DMI6000B, type
AF7000; Leica Microsystems GmbH, Wetzlar, Germany) at a light
excitation wavelength of 352 nm. For each sample, >250 cells
were observed and analyzed. The number of apoptotic cells with
fragmented nuclei was counted.
Statistical analysis
All experiments were conducted in triplicate, giving
similar results, and data are presented as the mean values. For
analysis of cell motility, a Mann-Whitney test was used and results
were considered significant if P<0.05 (n=50). For other
analyses, a two-sample independent Student's t-test was used and
P<0.05 was considered to indicate a statistically significant
difference.
Results
Cell type-specific effects of 5-FU and
9-AAA upon growth of cancer and normal cells in vitro
In the first series of experiments, the effect of
the continuous presence of 9-AAA in cell culture medium, at
concentrations of 1–20 µM, on cell proliferation was examined. The
effect of 9-AAA on cell proliferation was compared with the effect
of 5-FU, a known and commonly used cytostatic drug in clinical
oncology, which was applied at concentrations of 5–30 µM. In
preliminary experiments (data not shown), 9-AAA and 5-FU, in the
examined range of concentrations, did not show acute toxicity, and
>90% of all tested cell types survived for 4 and 8 h of
incubation in their presence, as tested with the trypan blue
exclusion test. The response to these two substances was tested on
HSFs, as an example of normal cells, and on 3 neoplastic,
established cell lines, including the human melanoma A375, rat
prostate highly metastatic adenocarcinama Mat-LyLu and moderately
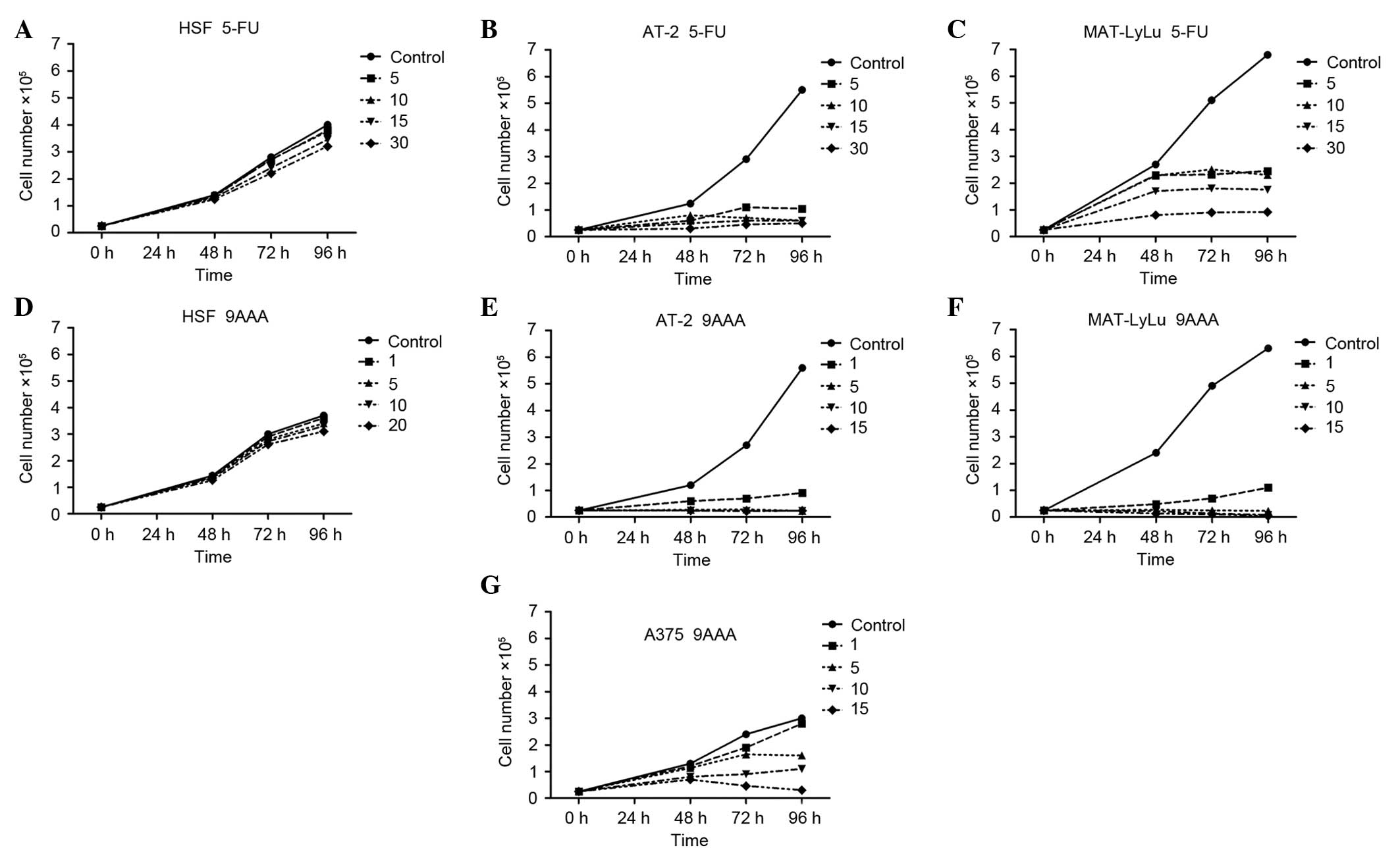
malignant rat prostate AT-2 cell lines (Fig. 1). The highly cell type-specific
effects on cell proliferation were recorded.
The examined substances, 5-FU (Fig. 1A-C) and 9-AAA (Fig. 1D-G), in the highest tested
concentrations (30 and 20 µM, respectively) had no statistically
significant effect upon growth of HSFs. At the concentrations of 5,
10, 15 and 30 µM, 5-FU inhibited proliferation in the two tested
rat prostate adenocarcinoma cell lines (Fig. 1B and C; AT-2 P=0.017, P=0.016,
P=0.016, P=0.015; MAT-LyLu P=0.031, P=0.029, P=0.027, P=0.025,
respectively). At the concentrations of 1, 5, 10 and 15 µM, 9-AAA
inhibited cell growth in the tested two rat prostate adenocarcinoma
cell lines differing in malignancy (Fig.
1E and F; AT-2 P=0.016, P=0.01, P=0.01, P=0.01; MAT-LyLu
P=0.018, P=0.01, P=0.01, P=0.01, respectively). At the lowest
tested concentration, 1 µM, 9-AAA fully inhibited the growth of
both rat prostate cancer cell lines (P=0.01), but not human
melanoma cells. In the A375 melanoma cell culture, 9-AAA inhibited
the growth of cells with a delay, when applied at the following
concentrations: 5, 10 or 15 µM (Fig.
1G; P=0.034, P=0.031, P=0.019). Notably, the inhibition of
neoplastic cell growth caused by 9-AAA and 5-FU was not immediate.
Independently, whether the substances were added at the start of
the culture or on the second day, the growth inhibition only became
marked after 24–48 h incubation of cells in the presence of 5-FU or
9-AAA.
Pro-apoptotic activity of 5-FU and
9-AAA in cancer cells, but not in normal HSFs
The subsequent experiments aimed to examine whether
the growth inhibition of the tested cancer cells by 9-AAA is
associated with the specific killing of cells by apoptosis or by
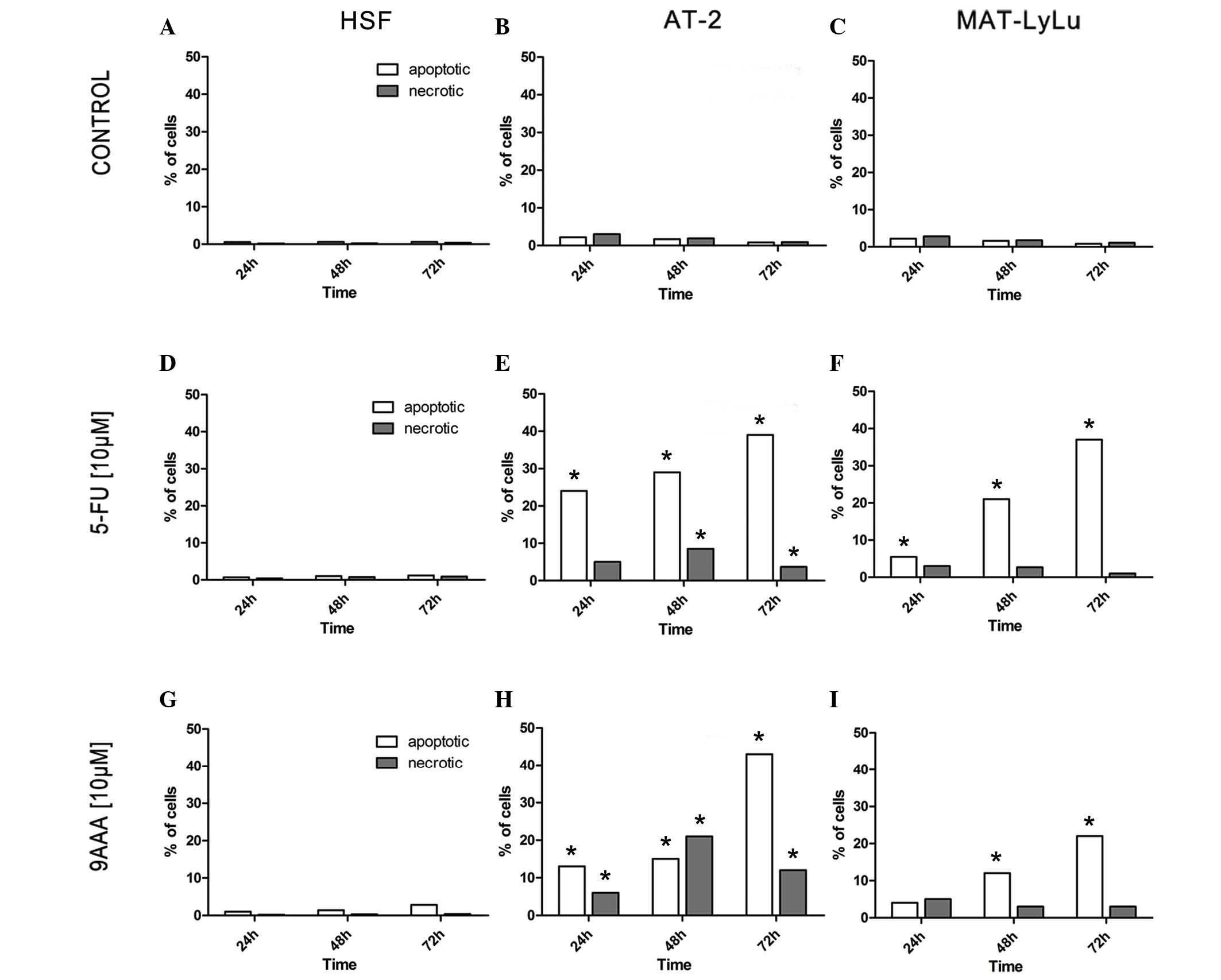
necrosis. Two methods were applied: i) Cells stained with Hoechst
33342 were directly counted under a microscope to observe the
morphology of nuclei in apoptotic cells and necrotic cells. This
method permits observation of individual single cells, and
therefore is limited to observation of several hundred cells in a
sample. ii) In parallel, the samples from the same cell culture
were analyzed with the modern method using FlowSight, which
analyzes thousands of cells (33).
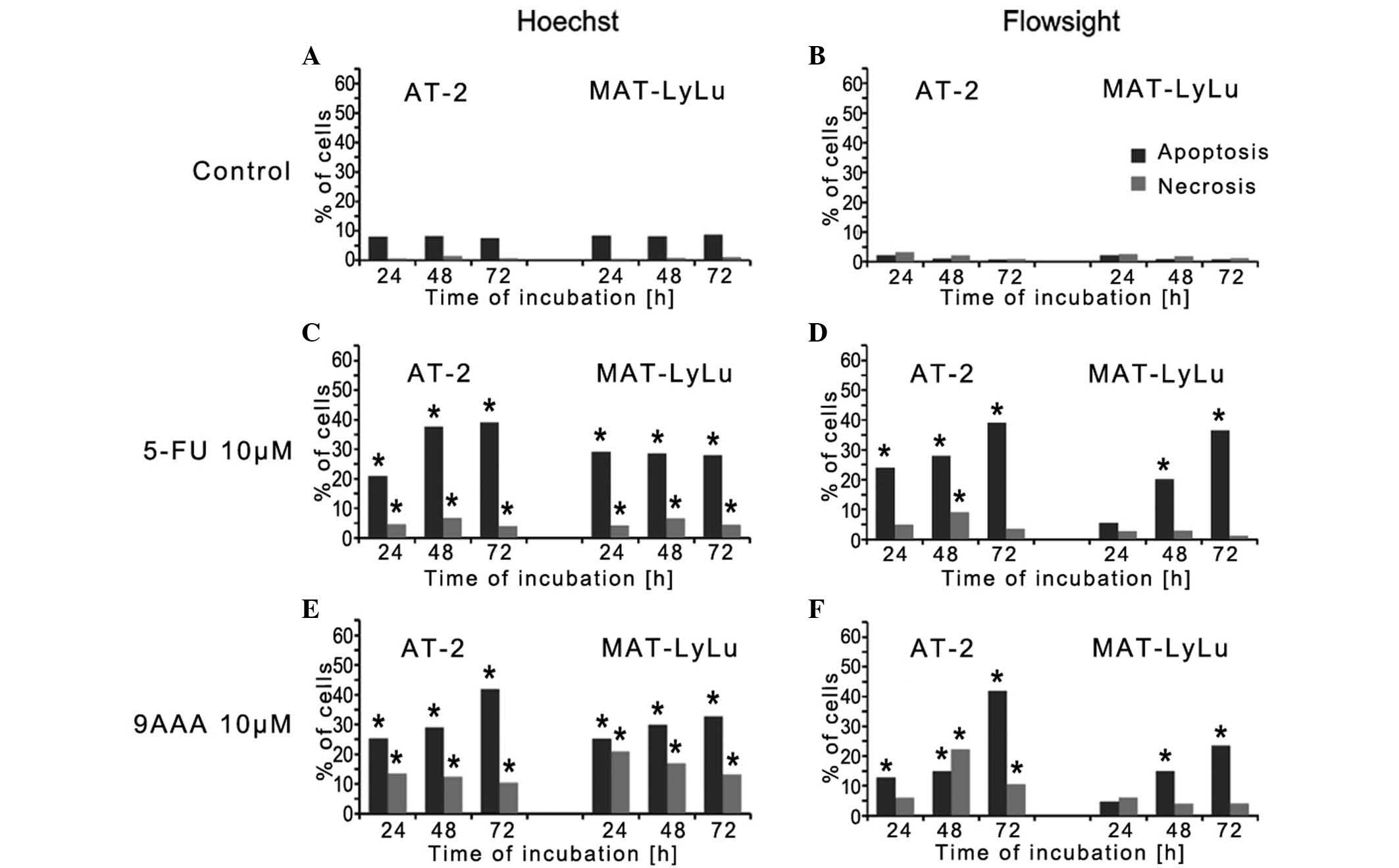
The results obtained using the two methods combined yielded
well-corresponding results (Fig.
2).
FlowSight analysis was used to examine the number of
cells dying by apoptosis and necrosis in the prescence or absence
of 5-FU or 9-AAA (Fig. 3). In HSFs,
in the absence of the tested substances, the number of dying cells
did not exceed 0.5% (Fig. 3A). In the
presence of 10 µM 9-AAA and after 3 days of culture, the number of
dying cells increased, but remained <3%, and more cells died by
apoptosis compared with necrosis (Fig. 3D
and G). By contrast, the cells of the 2 rat prostate cancer
AT-2 and MAT-LyLu cells began to die after growing for 24 h in the
presence of the tested inhibitors (Fig.
3B, C, E, F, H and I; P=0.01). In the rat prostate cancer AT-2
and Mat-LyLu cell lines, which differ in malignancy, the observed
effects of each inhibitor were almost the same. The numbers of
dying cells increased with time and after 3 days of cell culture
reached >40%. The proportion of cells dying by apoptosis was
much greater compared with the cells dying by necrosis. AT-2 cells
were found to be slightly more sensitive to the 9-AAA and 5-FU
compared with Mat-LyLu. In the presence of inhibitors, despite the
great proportion of dying cells, dividing cells were observed via
direct observation after staining with Hoechst, in particular, when
the inhibitors were applied at the 5 µM concentration.
Lack of differential effects of 5-FU
and 9-AAA on cancer and normal cell movement
Since cancer cell malignancy and the capacity of
cells to metastasize is usually correlated with cell motile
activity, the present study examined the effect of 9-AAA on the
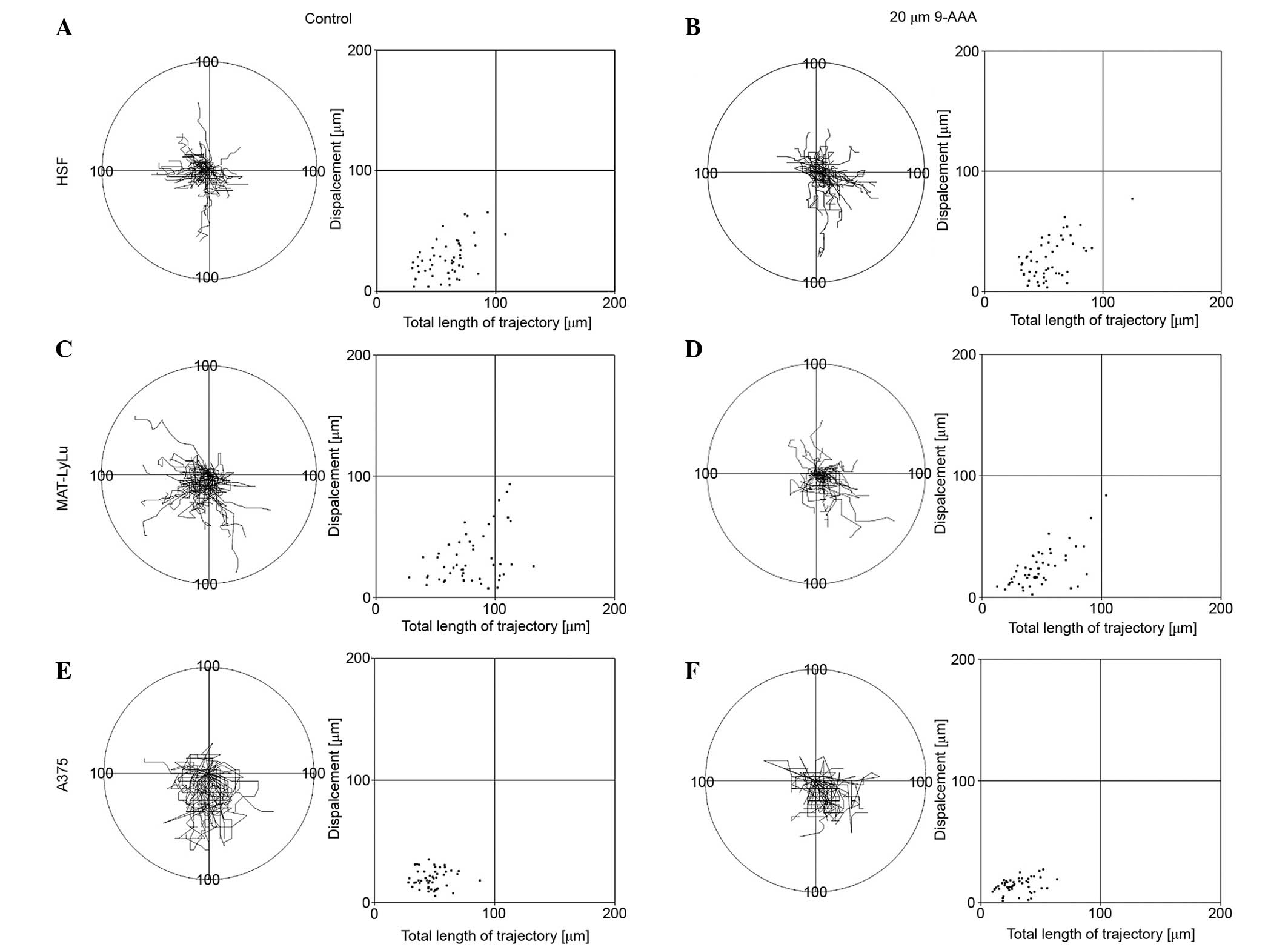
migration of 3 cell lines, including normal HSFs, rat prostate
malignant cancer Mat-LyLu and human melanoma A375 cells. In
previous experiments, a single-cell approach and computer-aided
methods were applied for recording and analyzing cell movement
trajectories in the absence and in the presence of 9-AAA (30,33). Cell
movement was recorded 24 h after cells were seeded. The control
experiments were carried out in cell culture medium. The effects of
9-AAA on cell movement were examined at a concentration of 20 µM,
the highest examined in experiments concerning cell growth. The
results of the cell movement records in the absence or in the
presence of the highest concentration tested are shown in Fig. 4. Even at the highest concentration of
9-AAA, which inhibited the growth of human melanoma cells and rat
prostate malignant cancer Mat-LyLu cell line (but not the growth of
HSFs), the 9-AAA had no statistically significant different effect
on the speed of cell movement/length of cell trajectories, the
shape of the cell trajectories, or the cell turning frequency of
the normal HSF or 2 cancer cell lines (Fig. 4). In all 3 cell lines the presence of
9-AAA in cell medium similarly affected the speed of cell movement
and the length of cell trajectories.
Discussion
The results presented in the current study show that
9-AAA and 5-FU, when present at low concentrations (1 or 5 µM,
respectively) in cell culture medium, efficiently inhibited the
growth of 2 rat prostate cancer cell lines, AT-2 and Mat-LyLu, but
had no influence on the viability and growth of HSFs. Human
melanoma A375 cells were inhibited at a concentration of 5, 10 and
15 µM 9-AAA. Even greater concentrations of these substances (20
and 30 µM) did not inhibit growth of the normal HSFs.
The growth inhibition in the examined cancer cell
lines was caused by 9-AAA and 5-FU at concentrations that did not
affect the cell viability if applied for 8 h. The microscopic
observation of cancer cells, for which proliferation was retarded
or inhibited after 3 days of cell culture in the presence of 9-AAA
or 5-FU, showed dividing cells among cells undergoing death by
necrosis or by apoptosis (formation of apoptotic bodies). One can
expect that, in heterogeneous cell populations such as populations
of cancer cell lines (32,33), the cells that proliferate are present
alongside cells that die by apoptosis or necrosis. The present
study demonstrated that the inhibition of increased cell numbers in
cancer cell cultures in the presence of 5-FU and 9-AAA resulted
from an increased proportion of dying to proliferating cells over
time.
Therefore, the capacity of 9-AAA and 5-FU to induce
apoptosis was compared in normal HSFs and in rat prostate cell
lines. The application of two methods that permitted the
discrimination of cells dying by apoptosis and necrosis yielded the
same results. Both 9-AAA and 5-FU induced an increasing number of
cells dying by apoptosis in cancer cell populations, but did not
markedly change the proportion of apoptotic cells in normal HSF
cultures.
Numerous research concerning the effects of 9-AAA on
cancer cells in vitro have been reported, however, effects
were observed within 3–8 h of 9-AAA administration. The tested
substances were applied for a short time and results were followed
for a few hours, usually in one type of cancer cell. Nevertheless,
among the numerous reports concerning the impact of 9-AAA and its
derivatives (for example quinacrine) on cancer cells, certain
studies appear to have demonstrated similar results compared with
the findings indicated in the present study.
Gurova et al (34) reported that 9-AAA pro-apoptotic
activity is associated with inhibition of the transition of cells
from G1 phase to the S phase of the cell cycle. This may partly
explain why the inhibition of cancer cell proliferation in the
presence of low concentrations of 9-AAA is observed with delay,
only after 24–48 h. In asynchronous cell culture, only a small
fraction of cells may be susceptible to the inhibitory activity of
the inhibitor in a short window of time. However, there is a lack
of studies on this topic of research, which may explain why there
are such large differences in the cellular response to low
concentrations of 9-AAA or 5-FU between the tested cancer cell
lines and normal HSFs. These differences may be associated with the
observations made by Wang et al (22), Gurova et al (34) and Guo et al (35), regarding the effects of 9-AAA on
proteins, including tumor protein 53, Bcl2 associated X protein and
transcription factor nuclear factor-κ B, and/or by Teitelbaum et
al (36) on topoisomerase II and
topoisomerase I (37). Expression of
these proteins usually differs in normal and cancer cells (34). However, the explanation of the results
observed for cancer cells in vitro and in vivo,
reported by Etchison et al (25) and Guo et al (35), requires supplementation by results
from parallel, simultaneous experiments involving cancer and normal
cells, differing in their responses to the tested drugs.
The difference in reactions among various cells
could also be partly due to the differences in electrochemical
properties of the surface of cancer and normal cells (38–42). As a
cationic substance, 9-AAA may more strongly affect the cancer
cells, whose surface is more negatively electrically charged
compared with the surface of normal cells, as the effect of 9-AAA
on cell membranes was previously found to depend upon the negative
electric charging of cell surfaces and liposomes (14,16,17,43).
This phenomenon may result in varying responses of cells, differing
in membrane electric properties. In addition, 9-AAA was reported to
affect electric charges on cell surface and ion fluxes across
membrane channels (8–12,14–17). This
may modify the penetration of 9-AAA into cells or an operation of
signaling pathways and interactions among cells. Therefore, the
differences between the responses of cancer and normal cells do not
appear to result solely and primarily from the often investigated
intercalation of 9-AAA to DNA and its mutagenic activity.
It is well documented that the capacity of cancer
cells to metastasize correlates with their motile activity
(30,44,45). The
results of the current demonstrated that 9-AAA and 5-FU similarly
affected the movement of cancer and normal cells, at least within
the first few hours of its application to the cells. This shows
that the agents that can affect cancer cell malignancy and the
capacity of cells to metastasize, i.e. influence cell motile
behavior, are not necessarily the same agents that can result in
the inhibition of cell growth, in particular if they induce cell
death by apoptosis.
Teitelbaum et al (36) reported that 9-AAA derivatives in
micromolar concentration can be efficient against mouse
glioblastoma, and Etchison et al (25) suggested that the 9-AAA derivatives can
be effective against small cell lung cancer. Overall, these
observations suggest that 9-AAA and its derivatives may be
promising targets for the chemotherapy of types of cancer that are
considered to resistant to the majority of the presently applied
anticancer drugs (25,36,46).
In conclusion, the results of the present study,
which regards the effectiveness of specific cancer cell growth
inhibition by 5-FU and 9-AAA, indicate the requirement for
additional studies on the molecular mechanisms responsible for the
varied responses of normal and cancer cells to 9-AAA and its
derivatives, and suggest that short term cytotoxicity evaluation
can lead to potential anticancer compounds being overlooked. The
results presented show that 9-AAA at low concentrations
specifically inhibits growth and induces apoptosis in human
melanoma A375 cells and 2 rat prostate cancer cells lines, but has
no effect on the survival and growth of HSFs in tissue culture.
Acknowledgements
The present study was financially supported by the
Polish National Science Centre (grant no., 2012/07/B/NZ3/02909).
The Faculty of Biochemistry, Biophysics and Biotechnology of
Jagiellonian University is also a partner of the Leading National
Research Center, supported by the Ministry of Science and Higher
Education.
References
|
1
|
Grys M, Madeja Z and Korohoda W:
Decreasing the thresholds for electroporation by sensitizing cells
with local cationic anesthetics and substances that decrease the
surface negative electric charge. Cell Mol Biol Lett. 19:65–76.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wainwright M: Acridine-a neglected
antibacterial chromophore. J Antimicrob Chemother. 47:1–13. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sebestic J, Hlavácek J and Stibor I: A
role of the 9-aminoacridines and their conjugates in a life
science. Curr Protein Pept Sci. 8:471–483. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Valdés A: Acridine and acridinones: Old
and new structures with antimalarial activity. Open Med Chem J.
5:11–20. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kopsidas G and MacPhee D: Glucose
inhibition of mutagenesis by 9-aminoacridine in Salmonella
typhimurium. Mutat Res. 285:101–108. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kopsidas G and MacPhee D: Frameshift
mutagenesis by 9-aminoacridine: Antimutagenic effects of adenosine
compounds. Mutat Res. 352:135–142. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Acharya N, Abu-Nasr N, Kawaguchi G, Imai M
and Yamamoto K: Frameshift mutations produced by 9-aminoacridine in
wild-type, uvrA and recA strains of Escherichia coli; specificity
within a hotspot. J Radiat Res. 48:361–368. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Galluce M, Agar G, Aslan A, Karadayi M,
Bozari S and Orhan F: Protective effects of methanol extracts from
Cladonia rangiformis and Umbilicaria vellea against known mutagens
sodium azide and 9-aminoacridine. Toxicol Ind Health. 27:675–682.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hoffmann GR, Laterza AM, Sylvia KE and
Tartaglione JP: Potentation of the mutagenicity and
recombinagenicity of bleomycin in yeast by unconventional
intercalating agents. Environ Mol Mutagen. 52:130–144. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Koschelev SG and Khodorov BI: Blockade of
open NMDA channel by tetrabutylammonium, 9-aminoacridine and
tacrine prevents channels closing and desensitization.
Biologicheskie Membrany. 9:93–110. 1995.
|
|
11
|
Kim KH, Gmiro VE, Tikhonov DB and
Magazanik LG: Mechanism of blockade of glutamate receptor ionic
channels: Paradox of 9-aminoacridine. Biochemistry (Moscow)
Supplement Series A: Membrane and Cell Biology. 1:88–95. 2007.
View Article : Google Scholar
|
|
12
|
Barygin OI, Luchkina NV, Gmiro VE and
Tikhonov DB: Different mechanisms of the 9-aminoacridine block of
NMDA-and AMPA-receptor ion channels. Biologicheskie Membrany.
26:280–286. 2009.
|
|
13
|
Muravenko OV, Amosova AV, Samatadze TE,
Popov KV, Poletaev AI and Zelenin AV: 9-aminoacridine: An efficient
reagent to improve human and plant chromosome banding patterns and
to standardize chromosome image analysis. Cytometry A. 51:52–57.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Theuvenet AP, Van De Wijngaard WM, Van De
Rijke JW and Borst-Pauwels GW: Application of 9-aminoacridine as a
probe of the surface potential experienced by cation transporters
in the plasma membrane of yeast cells. Biochim Biophys Acta.
775:161–168. 1984. View Article : Google Scholar
|
|
15
|
Ivanov AG and DiCosmo F:
Microelectrophoretic and 9-aminoacridine fluorescence study of the
surface electrical properties of suspension cultured Catharanthus
roseus cells and isolated protoplasts: Effects of abscinic acid
treatment. Plant Cell Physiol. 36:709–715. 1995.
|
|
16
|
Gage RA, Theuvenet AP and Borst-Pauwels
GW: Effect of plasmolysis upon monovalent cation uptake,
9-aminoacridine binding and the zeta potential of yeast cells.
Biochim Biophys Acta. 854:77–83. 1986. View Article : Google Scholar
|
|
17
|
Aharon D, Weitman H and Ehrenberg B: The
effect of liposomes' surface electric potential on the uptake of
hematoporphyrin. Biochim Biophys Acta. 1808:2031–2035. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Radzikowski C, Ledóchowski Z, Ledóchowski
A, Wrzołek S, Hrabowska M and Konopa J: The evaluation of antitumor
properties of acridine derivatives on the basis of the results from
some in vivo and in vitro tests. Arch Immunol Ther Exp (Warsz).
15:126–128. 1967.PubMed/NCBI
|
|
19
|
Mendecki J, Więckowska Z and Chorąży M:
Inhibition of RNA synthesis by 9-aminoacridine in regenerating rat
liver and cell culture. Acta Biochim Pol. 16:253–262.
1969.PubMed/NCBI
|
|
20
|
Rehn C and Pindur U: Molecular modeling of
intercalation complexes of antitumor active 9-aminoacridine and a
[d, e]-anellated isoquinoline derivative with base paired
deoxytetranucleotides. Monatshefte für Chemie. 127:645–658. 1996.
View Article : Google Scholar
|
|
21
|
Murza A, Sánchez-Cortéz S, Garcia-Ramos
JV, Guisan JM, Alfonso C and Rivas G: Interaction of the antitumor
drug 9-aminoacridine with guanidinobenzoatase studied by
spectroscopic methods: A possible tumor marker probe based on the
fluorescence exciplex emission. Biochemistry. 39:10557–10565. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Temple MD, Recabarren P, McFadyen WD,
Holmes RJ, Denny WA and Murray V: The interaction of DNA-targeted
9-aminoacridine-4-carboxamide platinum complexes with DNA in intact
human cells. Biochim Biophys Acta. 1574:223–230. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kumar P, Kumar R and Prasad DN: Synthesis
and biological evaluation of new 9-aminoacridine-4-carboxamide
derivatives as anticancer agents: 1st Cancer Update. Arab J Chem.
6:59–65. 2013. View Article : Google Scholar
|
|
24
|
Wang WG, Ho WC, Dicker DT, MacKinnon C,
Winkler JD, Marmorstein R and El-Deiry WS: Acridine derivatives
activate p53 and induce tumor cell death through Bax. Cancer Biol
Ther. 4:893–898. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ryan E, Blake AJ, Benoit A, David MF and
Robert AK: Efficacy of substituted 9-aminoacridine derivatives in
small cell lung cancer. Invest New Drugs. 31:285–292. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Reiss K and Korohoda W: The formation of
myotubes in cultures of chick embryo myogenic cells in serum-free
medium is induced by the insulin pulse treatment. Folia Histochem
Cytobiol. 26:133–141. 1988.PubMed/NCBI
|
|
27
|
Reiss K, Kajstura J and Korohoda W: The
insulin signal initiating cellular differentiation is preserved by
chick embryo myoblasts incubated at 2°C. Europ J Cell Biol.
53:42–47. 1990.PubMed/NCBI
|
|
28
|
Longley DB, Harkin DP and Johnston PG:
5-fluorouracil: Mechanisms of action and clinical strategies. Nat
Rev Cancer. 3:330–338. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Raghunathan K and Priest DG: Modulation of
fluorouracil antitumor activity by folic acid in a murine model
system. Biochem Pharmacol. 58:835–839. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Djamgoz MBA, Mycielska M, Madeja Z, Fraser
SP and Korohoda W: Directional movement of rat prostate cancer
cells in direct-current electric field. Involvement of voltagegated
Na+ channel activity. J Cell Sci. 114:2697–2705.
2001.PubMed/NCBI
|
|
31
|
Waligórska A, Wianecka-Skoczeń M, Nowak P
and Korohoda W: Some difficulties in research into cell motile
activity under isotropic conditions. Folia Biol (Kraków). 55:9–16.
2007. View Article : Google Scholar
|
|
32
|
Musialik E, Ryszawy D, Madeja Z and
Korohoda W: Morpho-physiological heterogeneity of cells within two
rat prostate carcinoma cell lines AT-2 and MAT-LyLu differing in
the degree of malignancy observed by cell cloning and the effects
of caffeine, theophylline and papaverine upon a proportion of the
clones. Oncol Rep. 29:1789–1796. 2013.PubMed/NCBI
|
|
33
|
Ryszawy D, Sarna M, Rak M, Szpak K,
Kędracka-Krok S, Michalik M, Siedlar M, Zuba-Surma E, Burda K,
Korohoda W, et al: Functional links between Snail-1 and Cx43
account for the recruitment of Cx43-positive cells into the
invasive front of prostate cancer. Carcinogenesis. 35:1920–1930.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gurova KV, Hill JE, Guo C, Prokvolit A,
Burdelya LG, Samoylova E, Khodyakova AV, Ganapathi R, Ganapathi M,
Tararova ND, et al: Small molecules that reactivate p53 in renal
cell carcinoma reveal a NF-kappaB-dependent mechanism of p53
suppression in tumors. Proc Natl Acad Sci USA. 102:17448–17453.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Guo C, Gasparian AV, Zhuang Z, Bosykh DA,
Komar AA, Gudkov AV and Gurova KV: 9-aminoacridine-based anticancer
drugs target the PI3K/AKT/mTOR, NF-kappaB and p53 pathways.
Oncogene. 28:1151–1161. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Teitelbaum AM, Gallardo JI, Bedi J, Giri
R, Renoit AR, Olin MR, Morizio KM, Ohlfest JR, Remmel RP and
Ferguson DM: 9-Amino acridine pharmacokinetics, brain distribution
and in vitro/in vivo efficacy against malignant glioma. Cancer
Chemother Pharmacol. 69:1519–1527. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Galvez-Peralta M, Hackbarth JS, Flatten
KS, Kaufmann SH, Hiasa H, Xing C and Ferguson DM: On the role of
topoisomerase I in mediating the cytotoxicity of
9-aminoacridine-based anticancer agents. Bioorg Med Chem Lett.
19:4459–4462. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Abercrombie M and Ambrose EJ: The surface
properties of cancer cells: A review. Cancer Res. 22:525–548.
1962.PubMed/NCBI
|
|
39
|
Carter HB and Coffey DS: Cell surface
charge in predicting metastatic potential of aspirated cells from
the Dunning rat prostatic adenocarcinoma model. J Urol.
140:173–175. 1988.PubMed/NCBI
|
|
40
|
Mehrishi JN: Molecular aspects of the
mammalian cell surfaceProgress in Biophysics and Molecular Biology.
Butler JAV and Noble D: Pergamon Press; Oxford: pp. 3–70. 1972
|
|
41
|
Mehrishi JN and Bauer J: Electrophoresis
of cells and the biological relevance of surface charge.
Electrophoresis. 23:1984–1994. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Korohoda W and Wilk A: Cell
electrophoresis-a method for cell separation and research into cell
surface properties. Cell Mol Biol Lett. 13:312–326. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Searle GF and Barber J: The involvement of
the electrical double layer in the quenching of 9-aminoacridine
fluorescence by negatively charged surfaces. Biochim Biophys Acta.
502:309–320. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Doyle GM and Mohler JL: Prediction of
metastatic potential of aspirated cells from the Dunning R-3327
prostatic carcinoma model. J Urol. 147:756–759. 1992.PubMed/NCBI
|
|
45
|
Wyckoff JB, Segall JE and Condeelis JS:
The collection of the motile population of cells from a living
tumor. Cancer Res. 60:5401–5404. 2000.PubMed/NCBI
|
|
46
|
Preet R, Mohapatra P, Mohanty S, Sahu SK,
Choudhuri T, Wyatt MD and Kundu CN: Quinacrine has anticancer
activity in breast cancer cells through inhibition of topoisomerase
activity. Int J Cancer. 130:1660–1670. 2012. View Article : Google Scholar : PubMed/NCBI
|