Introduction
The use of natural compounds for treatment is one of
the strategies used for cancer therapy and prevention.
Styryl-lactone compounds are one type of bioactive compound showing
cytotoxic activity towards several cancer cell lines (1,2). These
secondary metabolites are found ubiquitously in the
Goniothalamus plant genus, indigenous to South East Asia.
Goniothalamin is a major styryl-lactone compound extracted from
Goniothalamus macrophyllus (Blume) Hook.f. & Thomson
(3). The cytotoxicity of
styryl-lactones towards cancer cells is specific, as these
compounds have been reported to have no significant effects on
normal cell lines, including liver, kidney and fibroblast cell
lines (4). Previous studies have
shown that goniothalamin induces apoptosis predominantly through
the intrinsic pathway. However, the detailed mechanism remains to
be fully elucidated (5–11).
Endoplasmic reticulum (ER) stress is stress in the
ER caused by the accumulation of unfolded/misfolded proteins. It
triggers a response to restore homeostasis in the ER, termed the
unfolded protein response (UPR). In mild ER stress, the UPR
triggers and promotes ER-associated protein degradation to remove
misfolded proteins and then restores normal ER function. In
prolonged or severe ER stress, the UPR triggers the cell to commit
suicide, usually in the form of apoptosis, also termed ER
stress-induced apoptosis. It stimulates the apoptotic-associated
signaling of ER stress sensor transmembrane proteins, including
protein kinase RNA-like ER kinase (PERK), activating transcription
factor 6 (ATF6) and inositol-requiring enzyme 1 α (IRE1α), and
increases the expression of C/EBP homologous protein (CHOP), a
critical molecule of ER stress-induced apoptosis (12,13).
Apoptosis is a crucial mechanism of anticancer
drug-induced cell death. The majority of chemotherapeutic agents
inhibit tumors by triggering cancer cell apoptosis. Apoptosis can
be activated either by cell surface death receptor- or
mitochondria-mediated apoptosis signaling pathways (14,15).
However, ER stress is primarily associated with
mitochondria-mediated apoptosis. In a number of studies on ER
stress, several signaling mechanisms of crosstalk between ER stress
and mitochondria-mediated apoptosis have been suggested. These
include IRE1α → tumor necrosis factor receptor-associated factor 2
→ apoptosis signal-regulating kinase 1 → c-Jun
NH2-terminal kinase (JNK) and PERK → eukaryotic
initiation factor 2 α → ATF4 → CHOP, and result in the induction of
mitochondria-mediated apoptosis (12,13,16). The
activation of IRE1α also activates the endonuclease domain, which
splices mRNA of X-box binding protein 1 (XBP1) and results in
expression of the spliced form of the UPR gene (12,13). In
addition, the activation of IRE1α leads to the phosphorylation of
JNK, which is one of three major mitogen-activated protein kinase
(MAPK) pathways that have generally been associated with
pro-apoptotic action in several cell types (16–18). Thus,
this information indicates that JNK activation is sustained in
severe ER stress-induced apoptotic cell death.
Cervical cancer is the fourth most common type of
cancer among women and the seventh most common worldwide (19,20).
Annual incidence rates of >450,000 and >240,000 cases have
been estimated in low- and middle-income countries, respectively,
with the mortality rates in these countries estimated to reach
>88% and predicted to increase to at least 91.5% by 2030
(21). This emphasizes the
requirement for identifying effective and non-cytotoxic chemical
agents for chemoprevention and treatment. The HeLa cell line is
most widely used as a cervical cancer model for investigating human
cellular and molecular biology (22).
The present study investigated the induction of
mitochondria-mediated apoptosis associated with the ER
stress-induced activation of JNK caused by goniothalamin treatment
in HeLa cells. As the first investigation of the effects of
goniothalamin on ER stress-induced activation of JNK-associated
apoptosis on cancer cells, the results may be useful for enabling
further investigations of the drug action of styryl-lactone
compounds and indicate the potential application of goniothalamin
as an anticancer agent for the treatment of cervical cancer.
Materials and methods
Chemicals and antibodies
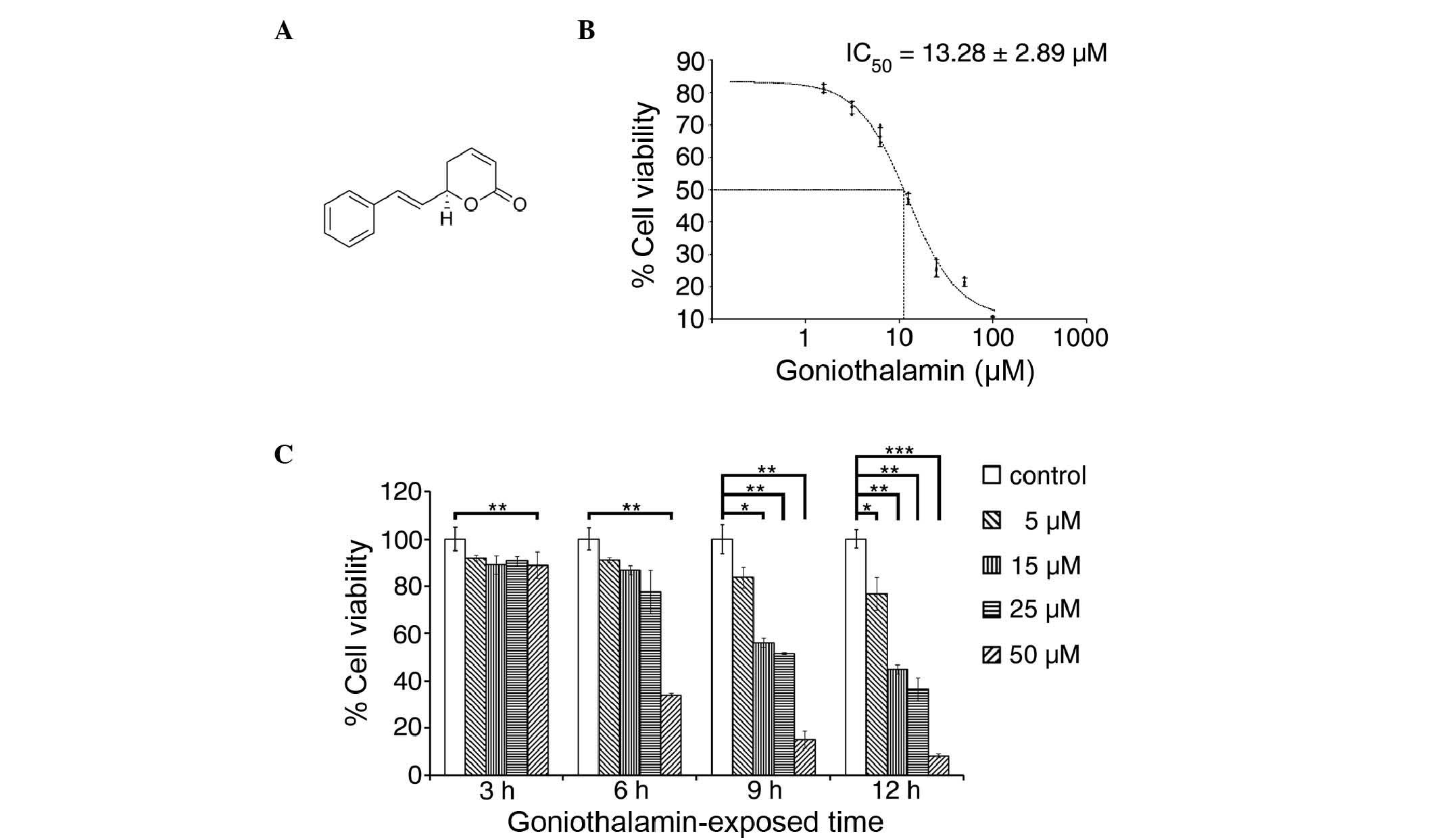
Goniothalamin was obtained from Professor Wilawan
Mahabusarakam of the Faculty of Science, Prince of Songkla
University (Songkhla, Thailand) in purified powder form, the
structure of which is shown in Fig.
1A. The stems of Goniothalamus macrophyllus were
collected from Songkhla province in the southern region of Thailand
in September 2007. Identification was performed by Mr. Ponlawat
Pattarakulpisutti of the Department of Biology, Faculty of Science,
Prince of Songkla University. The specimen (Uraiwan 01) was
deposited in the Herbarium of the Department of Biology, Faculty of
Science, Prince of Songkla University. Antibodies (Abs) for
immunoblotting analysis, including mouse monoclonal Abs against
CHOP, and rabbit monoclonal Abs against glucose-regulated protein
78 (GRP78), poly ADP ribose polymerase (PARP), caspase-3,
caspase-9, p38, phosphorylated (phospho)-p38 at Thr180/Tyr182,
stress-activated protein kinase (SAPK)/JNK, phospho-SAPK/JNK at
Thr183/Tyr185, p44/42 MAPK [extracellular signal-regulated kinase
(Erk1/2)], phospho-p44/42 MAPK (Erk1/2) at Thr202/Tyr204, p53, B
cell lymphoma 2 (Bcl2), phospho-Bcl2 at Ser70, Bcl2-associated X
protein (Bax), Bcl2-associated death promoter (Bad) and β-actin,
and anti-mouse immunoglobulin G and anti-rabbit immunoglobulin G
horseradish peroxidase-conjugated secondary antibodies were
obtained from Cell Signaling Technology, Inc. (Danvers, MA,
USA).
Cell culture
The HeLa human cervical cancer cell line was
obtained from the American Type Culture Collection (Manassas, VA).
The cells were maintained in Dulbecco's modified Eagle's medium
(DMEM; Gibco Life Technologies; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) supplemented with 10% fetal bovine serum (GE
Healthcare Life Sciences, Little Chalfont, UK), 100 U/ml penicillin
and 100 µg/ml streptomycin (GE Healthcare Life Sciences) at 37°C in
a humidified 5% CO2 atmosphere, and were used for assays
during the exponential phase of growth.
Cell viability assessment using an MTT
assay
The cells were plated at a density of
5×103 cells/well in 96-well plates and allowed to grow
for 24 h. The cells were then treated with goniothalamin at serial
concentrations of 100, 50, 25, 12.5, 6.25, 3.125 and 1.562 µM, and
the control group was treated with 0.5% DMSO. The cytotoxicity of
goniothalamin was determined by cell proliferation analysis using
an MTT assay, as described by Denizot and Lang (23). Briefly, the cells were incubated at
37°C with the indicated concentration of goniothalamin for 24 h to
determine the half maximal inhibitory concentration
(IC50) value, or at different time points (3, 6, 9 and
12 h), to investigate the effect of time and dose on cell
viability. Following the indicated treatment, 0.5 mg/ml of MTT
solution (Sigma-Aldrich; Merck Millipore, Darmstadt, Germany)
dissolved in culture medium was added and the cells were incubated
for 2 h at 37°C with 5% CO2. The MTT solution was then
aspirated and 100 µl of DMSO was added to each well to dissolve the
formazan crystals, a product of cell respiration reacting with MTT
tetrazolium compound to indicate viable cells. The absorbance at
540 nm was quantified using an Epoch™ microplate spectrophotometer
and analyzed using Gen5™ data analysis software (BioTek
Instruments, Inc., Winooski, VT, USA).
Hoechst 33342 staining analysis for
chromatin condensation
The fluorescent dye, Hoechst 33342, was used to
detect chromatin condensation, which is a characteristic of
apoptotic cells. The protocol was modified from Oberhammer et
al (24). The HeLa cells were
plated at a density of 2×105 cells/well in 6-well plates
and treated with 15 µM of goniothalamin for 0, 3, 6, 9 and 12 h at
37°C with 5% CO2. The treated cells were then washed
with PBS and fixed with 4% paraformaldehyde for 15 min at room
temperature. The fixed cells were washed with PBS and stained with
5 µg/ml of Hoechst 33342 solution (Invitrogen™; Thermo Fisher
Scientific, Inc.) for 15 min. The cells were washed with PBS and
the plates were observed using a fluorescence microscope (IX73;
Olympus Corporation, Tokyo, Japan) using U-MWU2 mirror units for
ultraviolet excitation.
Cell cycle determination
The HeLa cells were plated at a density of
2×105 cells/well in 6-well plates. The cells were
incubated with 15 µM of goniothalamin for various durations of 0,
3, 6, 9 and 12 h at 37°C with 5% CO2. The protocol used
for the flow cytometric analysis of cell cycle distributions using
propidium iodide was modified from Krishan (25). Following treatment, the whole cells
were collected and fixed with ethanol (70% final concentration).
The fixed cells were washed with PBS and stained with 50 µg/ml
propidium iodide solution (Invitrogen™; Thermo Fisher Scientific,
Inc.). The stained cells were incubated at 4°C in the dark, and
were then sorted and analyzed for DNA content using the CyAn™ ADP
Beckman Coulter International S.A. Flow cytometer and
Kaluza® flow analysis software (Beckman Coulter, Inc.,
Brea, CA, USA), respectively.
Cell surface phosphatidyl-serine
determination
The HeLa cells were plated at a density of
2×105 cells/well in 6-well plates. The cells were
incubated with 15 µM goniothalamin for 0, 3, 6, 9 and 12 h at 37°C,
5% CO2. DMSO-treated HeLa cells at a 0.5% final
concentration were used as a control. The whole cells were
collected and stained according to the manufacturer's protocol of
the fluorescein isothiocyanate (FITC) Annexin V/Dead Cell apoptosis
kit for flow cytometry (Invitrogen™; Thermo Fisher Scientific,
Inc.). Following staining, the stained cells were sorted and
analyzed for outer membrane phosphatidylserine content using the
CyAn™ ADP Beckman Coulter International S.A. flow cytometer and
Kaluza® flow analysis software (Beckman Coulter, Inc.),
respectively.
Analysis of the loss of mitochondrial
membrane potential
The loss of mitochondrial membrane potential was
detected using JC-1 dye. The protocol for the observation of
mitochondrial membrane potential under a fluorescence microscope
using JC-1 dye was modified from Perelman et al (26). The HeLa cells were treated with 15 µM
goniothalamin for 3 and 6 h, and DMSO-treated HeLa cells at a final
concentration of 0.5% were used as a control. The treated cells
were stained with 10 µg/ml JC-1 (Invitrogen™; Thermo Fisher
Scientific, Inc.) and washed with PBS to remove excess dye.
Subsequently, the stained cells were qualitatively analyzed by
observation under a fluorescence microscope (IX73; Olympus
Corporation) with U-MWB2 mirror units for excitation at 480 nm. The
aggregated form is emitted at red light (590 nm), indicating
healthy mitochondria, whereas monomer formation is emitted at green
light (525 nm), indicating damaged mitochondria or loss of
mitochondrial membrane potential.
Analysis of mRNA levels of spliced
XBP1 using reverse transcription-polymerase chain reaction (RT-PCR)
analysis
The HeLa cells were plated at a density of
5×104 cells/well in 12-well plates. The cells were
incubated with 15 µM goniothalamin for 0, 3, 6, 9 and 12 h at 37°C,
5% CO2. DMSO-treated HeLa cells at a final concentration
of 0.5% were used as a control. The whole cells were collected and
their RNA was extracted using QIAzol™ lysis reagent (Qiagen N.V.,
Venlo, The Netherlands) and cDNA was synthesized by RT using a
RevertAid™ First Strand cDNA Synthesis kit (Fermentas™; Thermo
Fisher Scientific, Inc.) with 2 µg of total RNA from each sample.
These steps were performed according to the manufacturer's
protocol. The PCR step was performed using Taq polymerase
(Vivantis Technologies Sdn. Bhd., Selangor Darul Ehsan, Malaysia)
using a pair of primers corresponding to the spliced site of the
XBP1 gene; forward 5′-AATGAAGTGAGGCCAGTGGCC-3′ and reverse
5′-AATACCGCCAGAATCCATGGG −3′. In detail, the PCR step was performed
in a 20 µl reaction volume using 1 µl of cDNA with a PCR mixture
containing 0.3 µM forward primer, 0.3 µM reverse primer, 0.25 mM
deoxynucleotide triphosphate, 10 mM Tris-HCl, 50 mM KCl, 0.01%
Triton X-100, 1.5 mM MgCl2 and 2 units of Taq
polymerase. The PCR thermal cycler conditions used were as follows:
94°C for 2 min, followed by 35 cycles at 94°C for 15 sec, 55°C for
30 sec and 72°C for 30 sec; final extension was not included. The
PCR products were analyzed using 8% polyacrylamide gel
electrophoresis with ethidium bromide staining. The unspliced- and
spliced- forms of the XBP1 gene were indicated at 125 and 99 base
pairs, respectively.
Analysis of ER stress-mediated mRNA
expression using RT-quantitative PCR (RT-qPCR) analysis
The HeLa cells were plated at a density of
5×104 cells/well in 12-well plates. The cells were
incubated with 15 µM goniothalamin for 0, 3, 6, 9 and 12 h at 37°C,
5% CO2. DMSO-treated HeLa cells at a final concentration
of 0.5% were used as a control. The whole cells were collected and
the RNA was extracted using QIAzol™ lysis reagent (Qiagen N.V.).
The cDNA was synthesized by RT using the RevertAid™ First Strand
cDNA Synthesis kit (Fermentas™; Thermo Fisher Scientific, Inc.)
with 2 µg of total RNA from each sample, and the synthesized cDNA
was diluted 20X prior to use in the qPCR step. The subsequent qPCR
step was performed in a 10-µl reaction volume containing 1 µl
diluted cDNA, SYBR® Select Master Mix (Applied
Biosystems; Thermo Fisher Scientific, Inc.), 0.2 µM forward primer
and 0.2 µM reverse primer. The following primers were used for
amplification: CHOP, forward 5′-GCGCATGAAGGAGAAAGAAC-3′ and reverse
5′-TCACCATTCGGTCAATCAGA-3′; ER-localized Dna J homologue 4 (ERdj4),
forward 5′-AAAATAAGAGCCCGGATGCT-3′ and reverse
5′-CGCTTCTTGGATCCAGTGTT-3′; growth arrest and DNA damage protein 34
(GADD34), forward 5′-AAACCAGCAGTTCCCTTCCT-3′ and reverse
5′-CTCTTCCTCGGCTTTCTCCT-3′; GRP78, forward
5′-GCTCGACTCGAATTCCAAAG-3′ and reverse 5′-GATCACCAGAGAGCACACCA-3′;
and GAPDH, forward 5′-AGGTCGGAGTCAACGGATTT-3′ and reverse
5′-TAGTTGAGGTCAATGAAGGG-3′. The qPCR cycling conditions were
optimized for all the primers and performed according to the
SYBR® Select Master Mix user guide's protocol as
follows: 50°C for 2 min, 95°C for 2 min, and 40 cycles at 95°C for
15 sec and 60°C for 60 sec. The qPCR amplification was analyzed
using the CFX96 Touch™ Real-Time PCR detection system with CFX
Manager™ software (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
The relative quantification (ΔΔCq) method described by Livak and
Schmittgen (27) was used to analyze
the results of gene expression using GAPDH as a reference gene. The
gene expression calculation was performed according to the CFX
Manager™ software manufacturer's protocol.
Analysis of protein expression via
immunoblotting
SDS-PAGE and immunoblotting were used to detect the
expression levels of apoptotic intermediate proteins. The
procedures for SDS-PAGE and immunoblotting were modified from
Taylor et al (28). The HeLa
cells were plated at a density of 2×105 cells/well in
6-well plates and incubated with 15 µM goniothalamin for 0, 3, 6, 9
and 12 h at 37°C with 5% CO2 DMSO-treated HeLa cells at
a final concentration of 0.5% were used as a control. The whole
cells were then collected for protein extraction in RIPA lysis
buffer, containing 50 mM Tris-HCl (pH 7.4), 1% NP-40, 0.5%
C24H39NaO4, 0.1% SDS, 150 mM NaCl,
2 mM EDTA and 50 mM NaF. Protein concentration was determined using
Bio-Rad® Protein Assay kit (Bio-Rad Laboratories, Inc.),
which is based on the Bradford method (29) using bovine serum albumin as the
standard protein. The cell lysates (containing 10 µg of protein)
were separated on 8–15% acrylamide gels by SDS-PAGE and then
transferred onto polyvinylidene difluoride membranes (Merck
Millipore), following which, they were blocked with 5% skimmed-milk
in TBS-Tween buffer for 1 h at room temperature. The membranes were
then incubated with mouse monoclonal Abs against CHOP (1:1,000),
and rabbit monoclonal Abs against GRP78 (1:1,000), PARP (1:1,000),
caspase-3 (1:1,000), caspase-9 (1:1,000), p38 (1:1,000),
phospho-p38 at Thr180/Tyr182 (1:1,000), SAPK/JNK (1:1,000),
phospho-SAPK/JNK at Thr183/Tyr185 (1:1,000), p44/42 MAPK (Erk1/2;
1:1,000), phospho-p44/42 MAPK (Erk1/2) at Thr202/Tyr204 (1:1,000),
p53 (1:1,000), Bcl2 (1:1,000), phospho-Bcl2 at Ser70 (1:1,000), Bax
(1:1,000), Bad (1:1,000) and β-actin (1:5,000) overnight at 4°C.
Following incubation with anti-mouse immunoglobulin G or
anti-rabbit immunoglobulin G horseradish peroxidase-conjugated
secondary antibodies (1:10,000) for 1 h at room temperature, the
signals were developed using Immobilon™ Western Chemiluminescent
HRP substrate (Merck Millipore) and detected using a
chemiluminescent imaging system (GeneGnome gel documentation;
Synoptics Ltd., Cambridge, UK).
Statistical analysis
To compare the data from different treatment groups,
student's t-test was used. Microsoft Excel version 2010
software (Microsoft Corporation, Redmond, WA, USA) was used to
analyze the data. All data presented were obtained from at least
three independent experiments and are presented as the mean ±
standard deviation. P≤0.05 was considered to indicate a
statistically significant difference.
Results
Effect of goniothalamin on HeLa cell
viability and toxicity
The cytotoxicity of goniothalamin towards HeLa cells
was analyzed and the results are shown in Fig. 1B and C. Goniothalamin induced a
cytotoxic effect with an IC50 value of 13.28±2.89 µM at
24 h. Goniothalamin induced these cytotoxic effects in a time- and
dose-dependent manner.
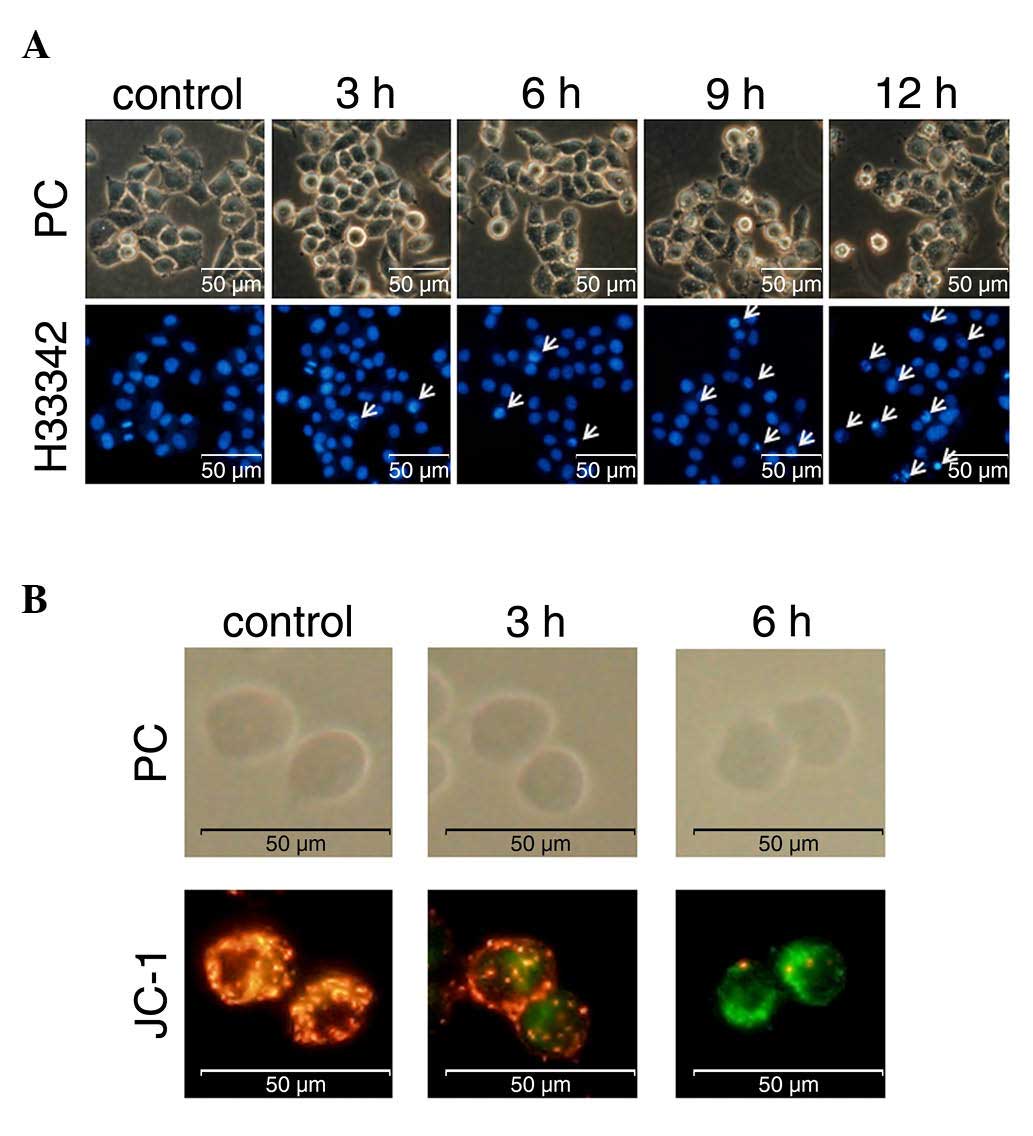
Effect of goniothalamin on chromatin
condensation
An important characteristic of apoptotic cells is
chromatin condensation. Goniothalamin was shown to induce chromatin
condensation and apoptotic body-like formation in the treated HeLa
cells (Fig. 2A). The number of cells
with chromatin condensation following treatment with 15 µM
goniothalamin increased in a time-dependent manner.
Loss of mitochondrial membrane
potential
The loss of mitochondrial membrane potential is one
of the apoptotic characteristics of the intrinsic pathway. The
present study investigated this event using the fluorescent dye,
JC-1, to stain the treated HeLa cells. The results showed that
goniothalamin increased the presence of green puncta, indicating
the monomer form of JC-1 in cells with loss of mitochondrial
membrane potential. Red puncta were observed in the control sample,
indicating the aggregated form of JC-1 in cells with a normal
mitochondrial membrane potential or healthy cells (Fig. 2B). These results indicated that
goniothalamin induced the loss of mitochondrial membrane potential
in the HeLa cells and this was likely due to the intrinsic
apoptotic pathway.
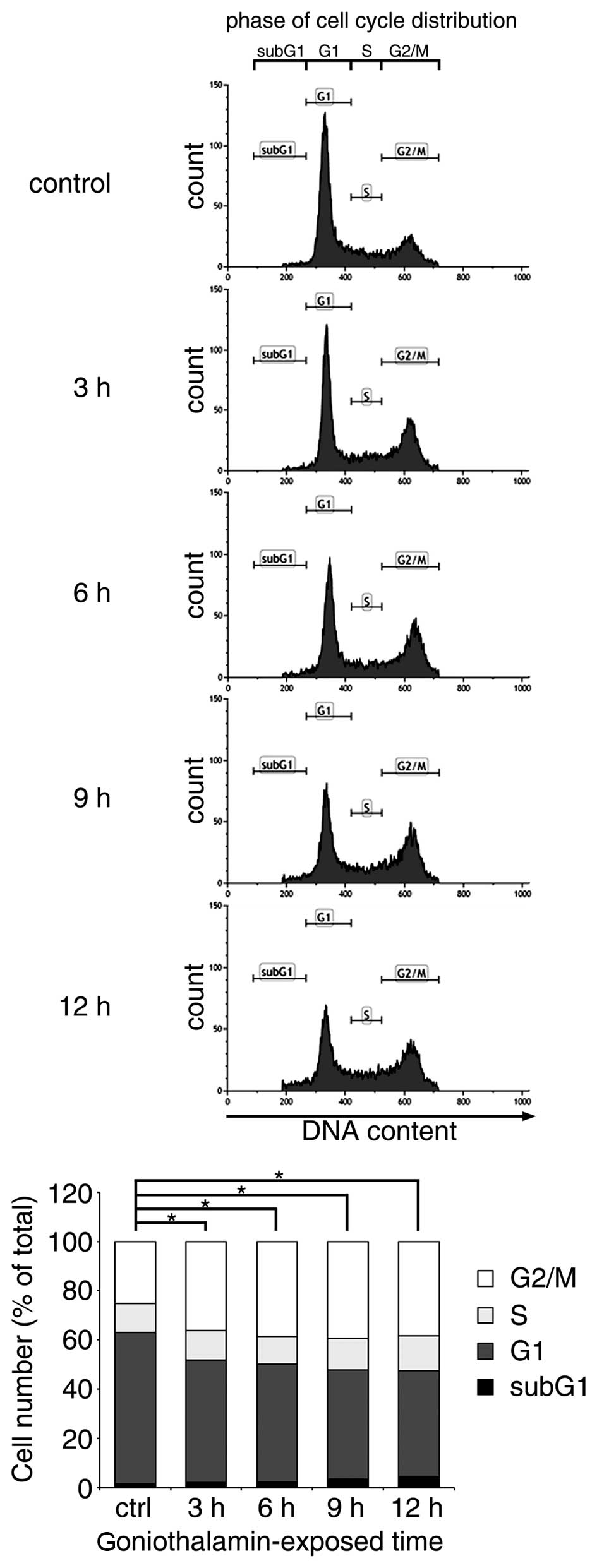
Effect of goniothalamin on the
regulation of cell cycle arrest
The majority of apoptosis-inducing compounds
interrupt cell cycle regulation, which may lead to cell cycle
arrest and subsequent apoptosis. Therefore, the present study
investigated the effect of goniothalamin on the cell cycle of HeLa
cells treated with 15 µM goniothalamin. The results indicated that
goniothalamin predominantly affected cell cycle arrest at the G2/M
phase in a time-dependent manner (Fig.
3). The increase in G2/M arrest was ~15% following treatment
with 15 µM goniothalamin for 12 h.
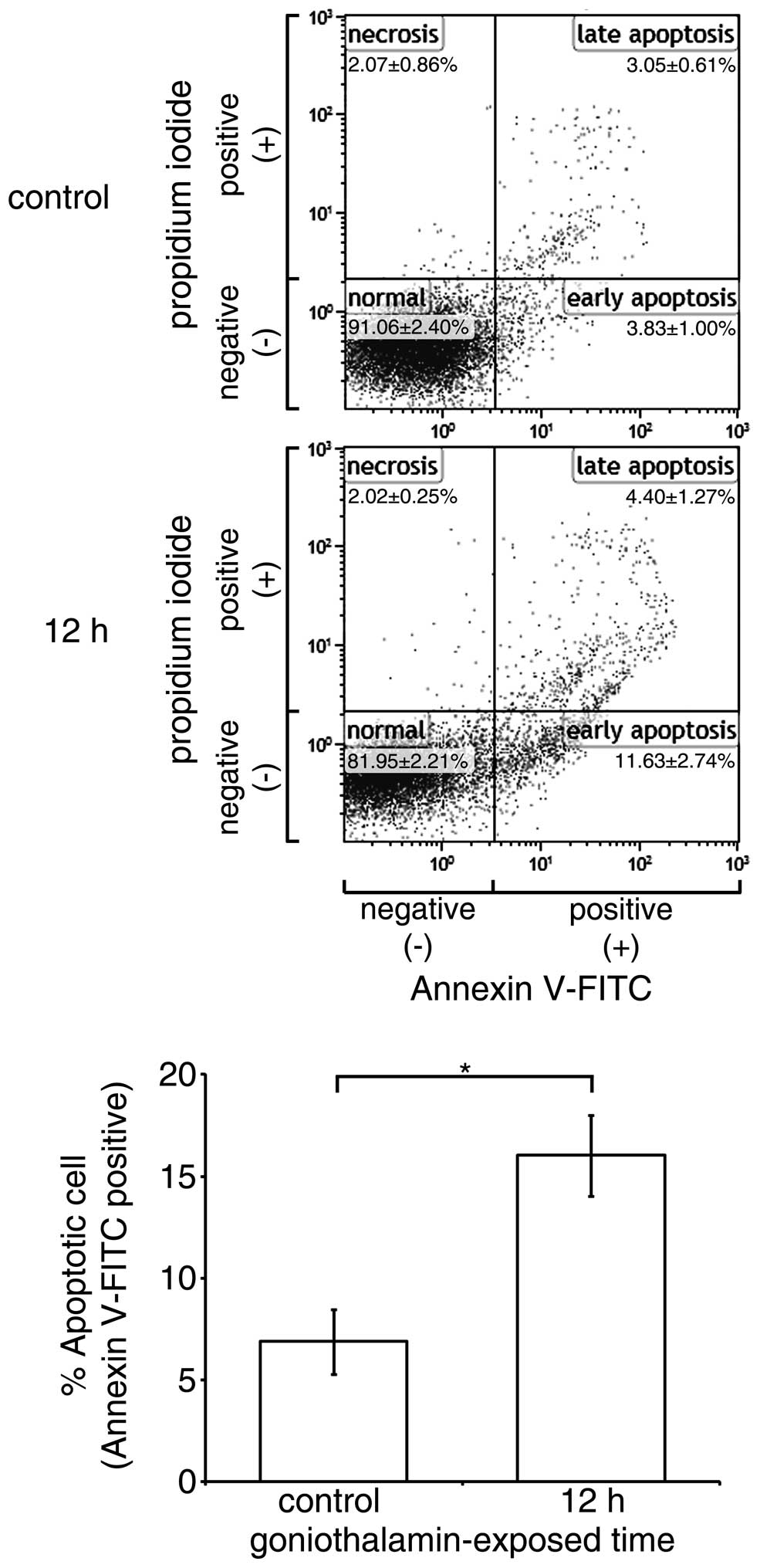
Effect of goniothalamin on cell
surface phosphatidyl-serine presentation
The translocation of phosphatidyl-serine out of the
cell membrane and exposed to annexin V is an important
characteristic in differentiating cell apoptosis from necrosis. In
the present study, goniothalamin predominantly induced HeLa cell
accumulation at the early apoptotic stage (Fig. 4). The results showed that
goniothalamin increased the percentage of total apoptotic HeLa
cells to 16.59±2.4% within 12 h, indicating the induction of
apoptosis.
Effect of goniothalamin on ER
stress
The mRNA and protein expression levels of CHOP
following treatment with goniothalamin are shown in Fig. 5A and B, respectively, which showed
increased mRNA and protein levels of CHOP, the key mediator of ER
stress-induced apoptosis. The increased expression of other ER
stress-associated genes, including ERdj4 and GADD34, were also
detected, with the exception of GRP78. In addition, the results of
the XBP1 splicing analysis (Fig. 5C)
showed an increase in the level of spliced XBP1 following
goniothalamin treatment. These results indicated that goniothalamin
triggered ER stress in the HeLa cells.
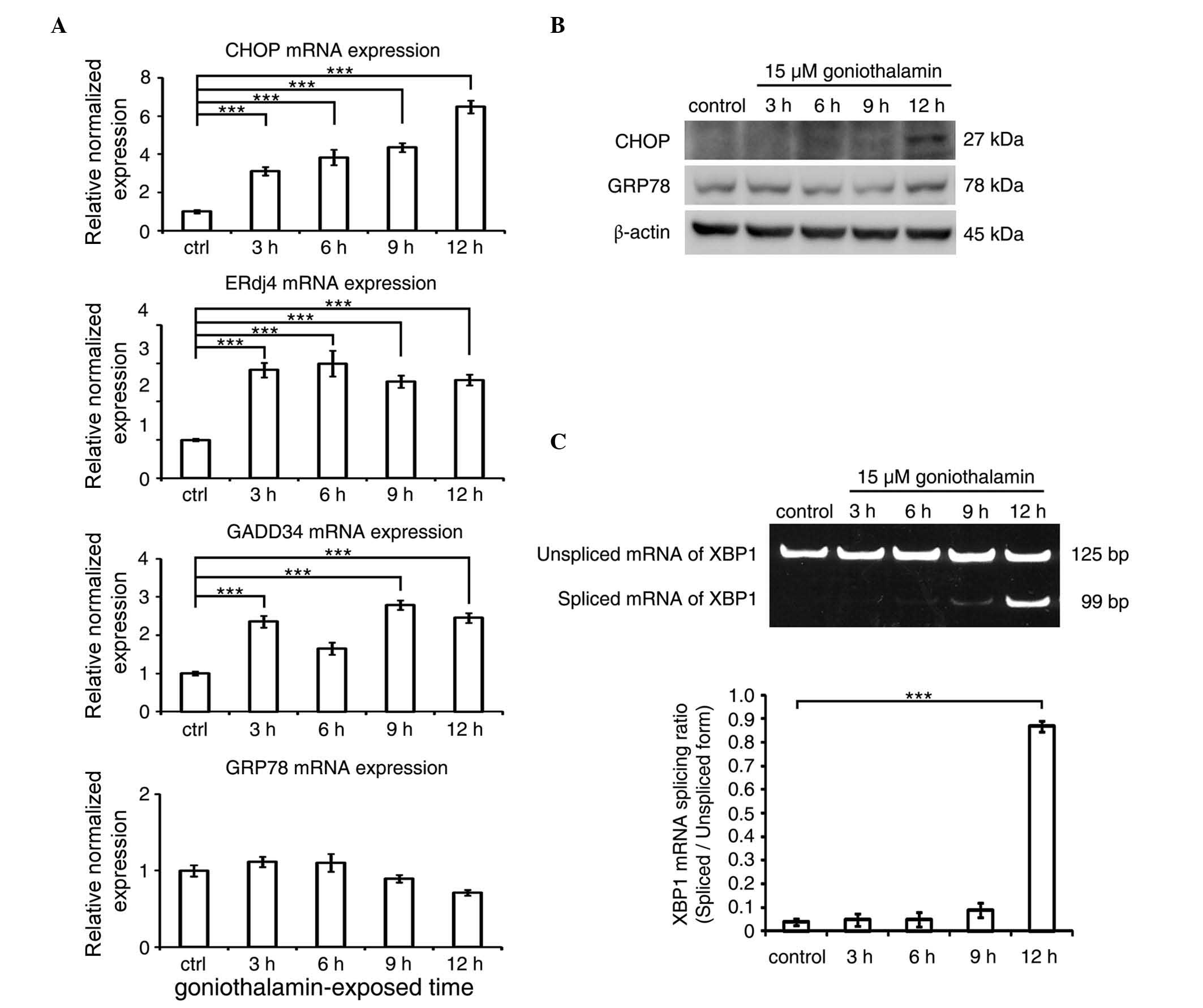 | Figure 5.ER stress is triggered in
goniothalamin-treated HeLa cells. (A) mRNA expression levels of ER
stress-associatead genes, including CHOP, ERdj4, GADD34 and GRP78
in HeLa cells treated with 15 µM of goniothalamin for 3, 6, 9 and
12 h using RT-qPCR analysis. (B) Immunoblotting analysis of ER
stress mediators, CHOP and GRP78. Images shown are representative
from three independent experiments. (C) Analysis of the splicing of
XBP1 mRNA using RT-PCR analysis. Values of fluorescence intensity
are presented as the mean ± standard deviation from at least three
independent experiments. ***P<0.001, vs. ctrl (control). ER,
endoplasmic reticulum; CHOP, C/EBP homologous protein; ERdj4,
ER-localized Dna J homologue 4; GADD34, growth arrest and DNA
damage protein 34; GRP78; glucose-regulated protein 78; RT-qPCR,
reverse transcription-quantitative polymerase chain reaction; ctrl,
control. |
Induction of apoptosis is associated
with the ER stress-induced activation of JNK, which is triggered by
goniothalamin
The protein expression levels of
apoptotic-associated mediators are shown in Fig. 6, which corresponded with induction of
ER stress. The results, as shown in Fig.
6A, revealed that phosphorylation of SAPK/JNK and p38 increased
in a time-dependent manner, which was not observed for ERK. The
inhibition of Bcl2 through phosphorylation was also observed. The
level of phospho-Bcl2 at Ser70, one of the phosphorylated residues
activated by phospho-SAPK/JNK, increased in a time-dependent
manner. The activation of p38 and SAPK/JNK was closely associated
with the inhibition of Bcl2 via phosphorylation, leading to
apoptosis (Fig. 6C). This event led
to mitochondrial dysfunction and the induction of apoptosis via the
intrinsic pathway. Activation of the caspase cascade components
(Fig. 6B), including the initiator
caspase-9, executioner caspase-3 and PARP, were detected upon
treatment with 15 µM goniothalamin, indicating the induction of
apoptosis. These results suggested that goniothalamin induced
mitochondria-mediated apoptosis, which was associated with ER
stress-induced activation of the JNK pathway.
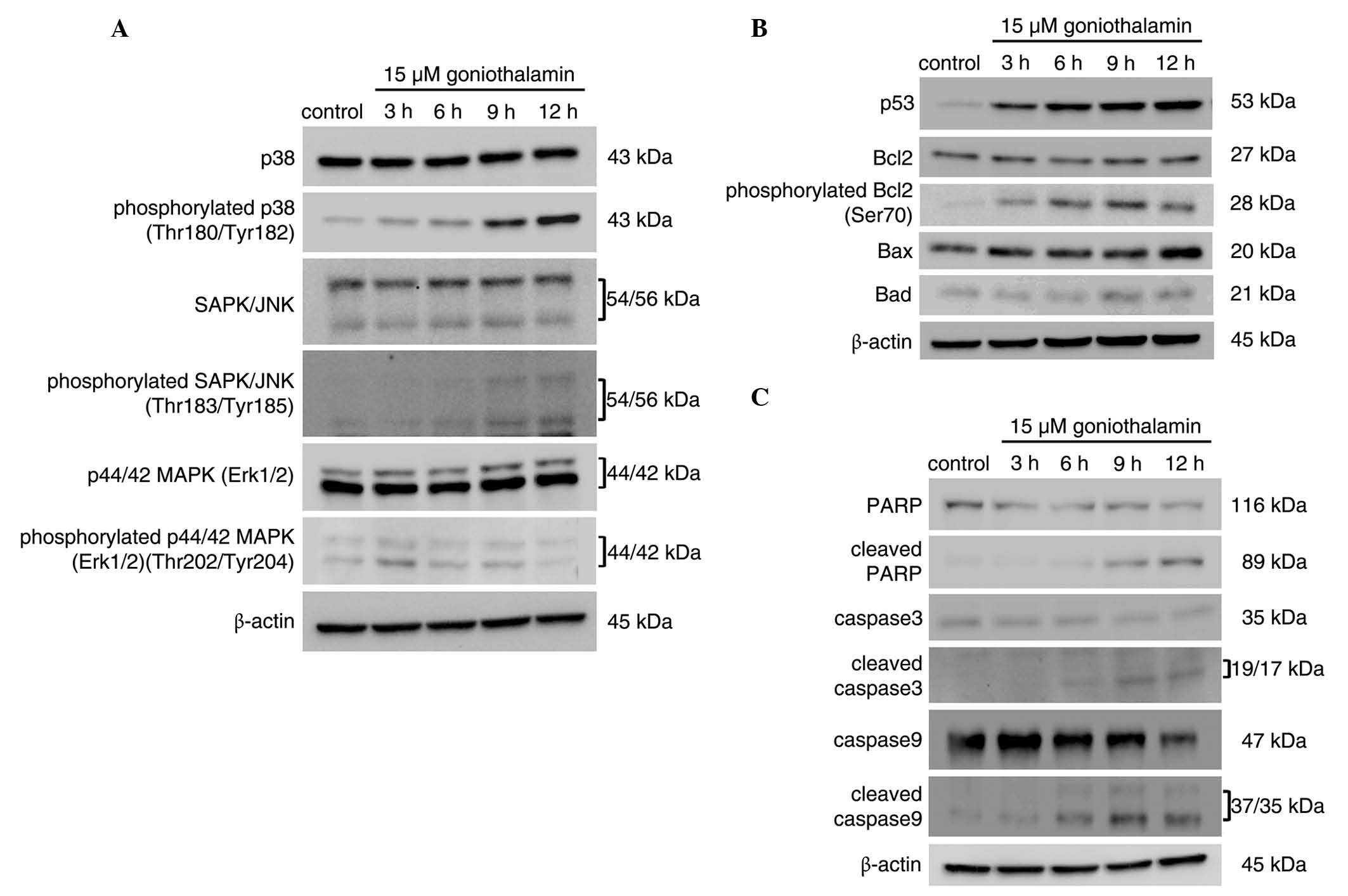 | Figure 6.MAPK activation is associated with
the induction of apoptosis. HeLa cells were treated with 15 µM of
goniothalamin for 3, 6, 9 and 12 h. Immunoblotting assays of (A)
MAPK pathway mediators, (B) apoptosis mediators, and (C) p53,
antiapoptotic Bcl2, proapoptotic Bax and Bad molecules and
phosphorylated Bcl2 (Ser70). The results indicated that
goniothalamin induced apoptosis in HeLa cells, associated with
activation of the MAPK pathway. Images shown are representative
from three independent experiments. MAPK, mitogen-activated protein
kinase; SAPK, stress-activated protein kinase; JNK, c-Jun
NH2-terminal kinase; Erk1/2. extracellular
signal-regulated kinase 1/2; PARP, poly ADP ribose polymerase;
Bcl2, B cell lymphoma 2; Bax, Bcl2-associated X protein; Bad,
Bcl2-associated death promoter. |
Discussion
At present, the use of traditional medicine or
natural compounds extracted from plants is of interest as it may
reduce the adverse effects of conventional therapies (30). Previous studies have reported that
goniothalamin inhibits proliferation and induces apoptosis in
various cancer cell lines, including human cervical cancer
(7–11). Although the effect of goniothalamin on
the induction of apoptosis in the HeLa cell line was reported
previously by Alabsi et al (9,10), the
molecular signaling pathway of goniothalamin-induced apoptosis in
HeLa cells remains to be elucidated. The present study is the
first, to the best of our knowledge, to indicate that ER
stress-induced activation of JNK was associated with
goniothalamin-induced HeLa cervical cancer cell apoptosis. The
results showed that goniothalamin reduced HeLa cell viability with
an IC50 value of 13.28±2.89 µM, and this reduced
viability occurred in a dose- and time-dependent manner. In
addition, the induction of apoptosis by goniothalamin was assessed
by examining chromatin condensation, cell cycle arrest, cell
surface phosphatidyl-serine presentation and caspase cascade
activation in goniothalamin-treated HeLa cells. The effect of
goniothalamin on cell cycle distribution was investigated by
observing DNA content in the treated cells using propidium iodide
staining. The results showed that goniothalamin caused accumulation
at the G2/M phase of arrest in apoptosis (Fig. 3). This response differed from the cell
cycle arrest induced by goniothalamin in Ca9-22 oral cancer cells
and HL-60 promyelocytic leukemia cells, which showed subG1 arrest
(11,31). However, cell cycle arrest in
MDA-MB-231 breast cancer cells showed accumulation at the G2/M
phase of arrest (32). This suggested
that differences in cell cycle arrest depend on the type of cancer
cell. In addition, the accumulation of cells at the G2/M phase
arrest can trigger p53-dependent p38 MAPK activation (33), and the goniothalamin-induced G2/M
phase arrest was associated with p38 MAPK phosphorylation and
increased expression of p53 (Fig. 6A and
C). In early apoptosis, phosphatidyl-serine is translocated
from the inner surface to the outer surface of the cellular
membrane due to the loss of membrane asymmetry, which can be
detected by Annexin V (34–37). In the present study, it was observed
that the numbers of positively-stained Annexin V cells were
increased in the goniothalamin-treated HeLa cells, compared with
the control (Fig. 4). This effect of
goniothalamin on the HeLa cells correlated with previous reports,
which showed goniothalamin-induced increases in positively-stained
Annexin V cells in cell lines, including human leukemia cells,
hepatoblastoma and urinary bladder cancer cells (5,11,38,39). These
results also confirmed the ability of goniothalamin to induce
apoptosis.
During apoptosis, the loss of mitochondrial membrane
potential is a characteristic of early apoptosis, which triggers
the mitochondria-mediated pathway. In this process, following
mitochondrial membrane collapse, the mitochondrial permeability
transition pore (MPTP) is opened by pro-apoptotic signaling
proteins, including Bax and Bid, resulting in decreased
mitochondrial membrane potential, following which cytochrome
c is released into the cytoplasm and induces activation of
the apoptosome-dependent apoptosis cascade (40). In the present study, the loss of
mitochondrial membrane potential was detected by staining cells
with specific fluorescent dye, JC-1, which can selectively enter
mitochondria through the MPTP and alters in color from red to green
as the membrane potential decreases. The results of the present
study indicated that goniothalamin induced the loss of
mitochondrial membrane potential in HeLa cells (Fig. 2B). In addition, activation of the
caspase cascade was triggered and resulted in apoptotic cell death.
Following the loss of mitochondrial membrane potential in the
apoptotic cells, the released cytochrome c interacts with
Apaf-1 and forms the apoptosome, which activates caspase-9 and then
caspase-3, and destroys PARP (41–43). This
results in cells undergoing apoptotic cell death. In the present
study, the activation of initiator caspase-9 and executioner
caspase-3, and the inactivation of PARP were investigated The
results (Fig. 6B) showed increases in
the cleaved form of caspase-9, caspase-3 and PARP in the
goniothalamin-treated HeLa cells in a time-dependent manner. Thus,
goniothalamin may have induced apoptosis through the
mitochondria-mediated pathway or intrinsic pathway in the HeLa
cells. The activation of initiator caspase-8, which is a mediator
of the death receptor-mediated pathway or extrinsic pathway, was
observed in the present study; however, the active form of
caspase-8 was not detected (data not shown). In a previous report
by Petsoponsakul et al (11),
it was shown that goniothalamin increased the activation of
caspase-8 in human leukemic HL-60 cells, but not in human leukemic
U937 cells. Thus, whether goniothalamin induced apoptosis through
the intrinsic or extrinsic pathway was dependent on the specific
cell type.
The present study also investigated the ER
stress-associated mitochondrial-mediated apoptosis signaling
pathway. Generally, the ER stress response of cells depends on its
severity. The ER chaperone, GRP78, usually binds to the
transmembrane ER proteins, including PERK, ATF6 and IRE1α,
preventing their activation by dimerization or polymerization.
However, when unfolded proteins accumulate in the ER, GRP78 is
released from transmembrane ER proteins to target the accumulated
unfolded protein, and these transmembrane ER proteins are then
activated by dimerization or polymerization to initiate the UPR. In
initial or mild ER stress, the UPR is triggered to recover
homeostasis in ER and reestablish ER function. Prolonged or severe
ER stress triggers ER stress-induced apoptosis (12,13). One
ER stress-associated mitochondria-mediated apoptosis signaling
pathway is the cascade of IRE1α → spliced XBP1 → JNK → loss of
mitochondrial membrane activation (16,44). The
activated IRE1α has ribonuclease and kinase activities, and one
signaling pathway of IRE1α activation is to promote the mRNA
splicing of XBP1 via its ribonuclease activity. The spliced XBP1
mRNA is translated to spliced XBP1 protein, an active transcription
factor, which regulates the transcription of several UPR genes,
including ER chaperones and genes encoding the components of
ER-associated degradation, including ERdj4 (45). Another signaling pathway of IRE1α
activation is the activation of JNK via phosphorylation, resulting
in the JNK-mediated apoptotic pathway (46,47). The
JNK mediator is one of the MAPK signaling molecules, which is
critical in the determination of cell fate between proliferation
and death. Several studies have reported that the mechanisms
underlying the induction of apoptosis in cancer cells by anticancer
agents are regulated through the MAPK signaling pathway (17,18,48).
Another ER stress-mediated apoptosis signaling pathway is the
activation of PERK. Activated PERK signaling leads to the increase
translation of specific mRNAs, including ATF4, which is a
transcription factor for pro-apoptotic CHOP and GADD34 proteins,
resulting in the initiation of apoptosis signaling (49).
To the best of our knowledge, the present study was
the first to demonstrate that ER stress- and MAPK
signaling-associated apoptosis were activated in
goniothalamin-treated HeLa cells. The results showed that
goniothalamin induced the mRNA splicing of XBP1 (Fig. 5B). The ratio of spliced:unspliced XBP1
increased following goniothalamin treatment for 12 h. In addition,
goniothalamin treatment upregulated the mRNA expression levels of
ER stress-associated genes, including CHOP, ERdj4 and GADD34.
However, GRP78 did not respond to goniothalamin treatment at either
the mRNA or protein levels, although other evidence supported that
goniothalamin treatment induced ER stress in the HeLa cells
(Fig. 5A and C). These finding
suggested that goniothalamin may induce ER stress in HeLa cells by
a different mechanism to that observed in several other ER stress
inducers, including tunicamycin, thapsigargin and brefeldin A
(50). Furthermore, the present study
found the MAPK pathway, was activated, particularly through JNK
phosphorylation, as were associated apoptosis-associated events
downstream of this activation, including the phosphorylation of
Bcl2, triggering the loss of mitochondrial membrane potential.
These results corresponded with the IRE1α activation pathway
(51). The results of the present
study are the first, to the best of our knowledge, to show that
goniothalamin triggered the ER stress-associated activation of
IRE1α, and activated JNK through phosphorylation associated with
the mitochondria-mediated induction of apoptosis.
In conclusion, although goniothalamin-induced
apoptosis in the HeLa cell line has been previously reported, as
mentioned above, the present study is the first, to the best of our
knowledge, to show that the induction of apoptosis in the HeLa cell
line by goniothalamin was associated with the ER stress-induced
activation of JNK. The effect of goniothalamin on the ER
stress-induced activation of JNK may be useful for further
investigations of the drug action of styryl-lactone compounds, and
suggests a potential candidate for preventive and therapeutic
applications in the treatment of cervical cancer.
Acknowledgements
The present study was supported by The Royal Golden
Jubilee Ph.D. Program (grant no. PHD/0214/2551), Thailand Research
Fund, Thailand and Center of Excellence in Biological Activities of
Bioactive Compounds, the Strategic Wisdom and Research Institute
(grant no. 127/2558) and Srinakharinwirot University (Bangkok,
Thailand).
References
|
1
|
Mereyala HB and Joe M: Cytotoxic activity
of styryl lactones and their derivatives. Curr Med Chem Anticancer
Agents. 1:293–300. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
de Fátima Â, Modolo LV, Conegero LS, Pilli
RA, Ferreira CV, Kohn LK and de Carvalho JE: Styryl lactones and
their derivatives: Biological activities, mechanisms of action and
potential leads for drug design. Curr Med Chem. 13:3371–3384. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jewers K, Davis JR, Dougan J, Machanda AH,
Blunden G, Kyi A and Wetchapinan S: Goniothalamin and its
distribution in four goniothalamus species. Phytochemistry.
11:2025–2030. 1972. View Article : Google Scholar
|
|
4
|
Seyed MA, Jantan I and Bukhari SN:
Emerging anticancer potentials of goniothalamin and its molecular
mechanisms. Biomed Res Int. 2014:5365082014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Inayat-Hussain SH, Annuar BO, Din LB, Ali
AM and Ross D: Loss of mitochondrial transmembrane potential and
caspase-9 activation during apoptosis induced by the novel
styryl-lactone goniothalamin in HL-60 leukemia cells. Toxicol In
Vitro. 17:433–439. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rajab NF, Hamid ZA, Hassan H, Ali MA, Din
LB and Inayat-Hussain SH: Evaluation of the cytotoxic and genotoxic
effects of goniothalamin in leukemic cell lines. Environ Mutagen
Res. 27:161–164. 2005. View Article : Google Scholar
|
|
7
|
Wattanapiromsakul C, Wangsintaweekul B,
Sangprapan P, Itharat A and Keawpradub N: Goniothalamin, a
cytotoxic compound, isolated from Goniothalamus macrophyllus
(Blume) Hook. f. & Thomson var. macrophyllus. Songklanakarin
Journal of Science and Technology. 27:480–487. 2005.
|
|
8
|
de Fátima A, Kohn LK, Antônio MA, de
Carvalho E and Pilli RA: (R)-Goniothalamin: Total syntheses and
cytotoxic activity against cancer cell lines. Bioorg Med Chem.
13:2927–2933. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Alabsi AM, Ali R, Ali AM, Al-Dubai SAR,
Harun H, Abu Kasim NH and Alsalahi A: Apoptosis induction, cell
cycle arrest and in vitro anticancer activity of goniothalamin in a
cancer cell lines. Asian Pac J Cancer Prev. 13:5131–5136. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Alabsi AM, Ali R, Ali AM, Harun H,
Al-Dubai SA, Ganasegeran K, Alshagga MA, Salem SD and Abu Kasim NH:
Induction of caspase-9, biochemical assessment and morphological
changes caused by apoptosis in cancer cells treated with
goniothalamin extracted from Goniothalamus macrophyllus. Asian Pac
J Cancer Prev. 14:6273–6280. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Petsophonsakul P, Pompimon W and
Banjerdpongchai R: Apoptosis induction in Human leukemic
promyelocytic HL-60 and monocytic U937 cell lines by goniothalamin.
Asian Pac J Cancer Prev. 14:2885–2889. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xu C, Bailly-Maitre B and Reed JC:
Endoplasmic reticulum stress: Cell life and death decisions. J Clin
Invest. 115:2656–2664. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Szegezdi E, Logue SE, Gorman AM and Samali
A: Mediators of endoplasmic reticulum stress-induced apoptosis.
EMBO Rep. 7:880–885. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Elmore S: Apoptosis: A review of
programmed cell death. Toxicol Pathol. 35:495–516. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nikoletopoulou V, Markaki M, Palikaras K
and Tavernarakis N: Crosstalk between apoptosis, necrosis and
autophagy. Biochim Biophys Acta. 1833:3448–3459. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Win S, Than TA, Fernandez-Checa JC and
Kaplowitz N: JNK interaction with Sab mediates ER stress induced
inhibition of mitochondrial respiration and cell death. Cell Death
Dis. 5:e9892014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yuan L, Wang J, Xiao H, Wu W, Wang Y and
Liu X: MAPK signaling pathways regulate mitochondrial-mediated
apoptosis induced by isoorientin in human hepatoblastoma cancer
cells. Food Chem Toxicol. 53:62–68. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sui X, Kong N, Ye L, Han W, Zhou J, Zhang
Q, He C and Pan H: p38 and JNK MAPK pathways control the balance of
apoptosis and autophagy in response to chemotherapeutic agents.
Cancer Lett. 344:174–179. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Saxena U, Sauvaget C and Sankaranarayanan
R: Evidence-based screening, early diagnosis and treatment strategy
of cervical cancer for national policy in low- resource countries:
Example of India. Asian Pac J Cancer Prev. 13:1699–1703. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Landry JJ, Pyl PT, Rausch T, Zichner T,
Tekkedil MM, Stütz AM, Jauch A, Aiyar RS, Pau G, Delhomme N, et al:
The genomic and transcriptomic landscape of a HeLa cell line. G3
(Bethesda). 3:1213–1224. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Denizot F and Lang R: Rapid colorimetric
assay for cell growth and survival: Modifications to the
tetrazolium dye procedure giving improved sensitivity and
reliability. J Immunol Methods. 89:271–277. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Oberhammer FA, Hochegger K, Fröschl G,
Tiefenbacher R and Pavelka M: Chromatin condensation during
apoptosis is accompanied by degradation of Lamin A+B, without
enhanced activation of cdc2 kinase. J Cell Biol. 126:827–837. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Krishan A: Rapid flow cytofluorometric
analysis of mammalian cell cycle by propidium iodide staining. J
Cell Biol. 66:188–193. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Perelman A, Wachtel C, Cohen M, Haupt S,
Shapiro H and Tzur A: JC-1: Alternative excitation wavelengths
facilitate mitochondrial membrane potential cytometry. Cell Death
Dis. 22:e4302012. View Article : Google Scholar
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2-ΔΔCT method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Taylor SC and Posch A: The design of a
quantitative western blot experiment. Biomed Res Int.
2014:3615902014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bradford MM: A rapid and sensitive method
for the quantitation of microgram quantities of protein utilizing
the principle of protein-dye binding. Anal Biochem. 72:248–254.
1976. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shankar S, Kumar D and Srivastava RK:
Epigenetic modifications by dietary phytochemicals: Implications
for personalized nutrition. Pharmacol Ther. 138:1–17. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yen CY, Chiu CC, Haung RW, Yeh CC, Huang
KJ, Chang KF, Hseu YC, Chang FR, Chang HW and Wu YC:
Antiproliferative effects of goniothalamin on Ca9-22 oral cancer
cells through apoptosis, DNA damage and ROS induction. Mutat Res.
747:253–258. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen WY, Wu CC, Lan YH, Chang FR, Teng CM
and Wu YC: Goniothalamin induces cell cycle-specific apoptosis by
modulating the redox status in MDA-MB-231 cells. Eur J Pharmacol.
522:20–29. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Thornton TM and Rincon M: Non-classical
p38 map kinase functions: Cell cycle checkpoints and survival. Int
J Biol Sci. 5:44–51. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Fadok VA, Bratton DL, Frasch SC, Warner ML
and Henson PM: The role of phosphatidylserine in recognition of
apoptotic cells by phagocytes. Cell Death Diff. 5:551–562. 1998.
View Article : Google Scholar
|
|
35
|
Denecker G, Dooms H, Van Loo G, Vercammen
D, Grooten J, Fiers W, Declercq W and Vandenabeele P: Phosphatidyl
serine exposure during apoptosis precedes release of cytochrome c
and decrease in mitochondrial transmembrane potential. FEBS Lett.
465:47–52. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Rello S, Stockert JC, Moreno V, Gámez A,
Pacheco M, Juarranz A, Cañete M and Villanueva A: Morphological
criteria to distinguish cell death induced by apoptotic and
necrotic treatments. Apoptosis. 10:201–208. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hanshaw RG and Smith BD: New reagents for
phosphatidylserine recognition and detection of apoptosis. Bioorg
Med Chem. 13:5035–5042. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Al-Qubaisi M, Rosli R, Subramani T, Omar
AR, Yeap SK, Ali AM and Alitheen NB: Goniothalamin selectively
induces apoptosis on human hepatoblastoma cells through caspase-3
activation. Nat Prod Res. 27:2216–2218. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Luo X, Budihardio I, Zou H, Slaughter C
and Wang X: Bid, a Bcl-2 interacting protein, mediates cytochrome c
release from mitochondria in response to activation of cell surface
death receptors. Cell. 94:481–490. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yen HK, Fauzi AR, Din LB, McKelvey-Martin
VJ, Meng CK, Inayat-Hussain SH and Rajab NF: Involvement of
Seladin-1 in goniothalamin-induced apoptosis in urinary bladder
cancer cells. BMC Complement Altern Med. 14:2952014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Boatright KM and Salvesen GS: Mechanisms
of caspase activation. Curr Opin Cell Biol. 15:725–731. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Riedl SJ and Shi Y: Molecular mechanisms
of caspase regulation during apoptosis. Nat Rev Mol Cell Biol.
5:897–907. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Pop C, Timmer J, Sperandio S and Salvesen
GS: The apoptosome activates caspase-9 by dimerization. Mol Cell.
22:269–275. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lee H, Park M, Choi B, Oh E, Song M, Lee
J, Kim C, Lim BU and Park HJ: Endoplasmic reticulum stress-induced
JNK activation is a critical event leading to mitochondria-mediated
cell death caused by β-lapachone treatment. PLoS One. 6:e215332011.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Chen Y and Brandizzi F: IRE1: ER stress
sensor and cell fate executor. Trends Cell Biol. 23:547–555. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Selimovic D, Ahmad M, El-Khattouti A,
Hannig M, Haïkel Y and Hassan M: Apoptosis-related protein-2
triggers melanoma cell death by a mechanism including both
endoplasmic reticulum stress and mitochondrial dysregulation.
Carcinogenesis. 32:1268–1278. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Urano F, Wang X, Bertolotti A, Zhang Y,
Chung P, Harding HP and Ron D: Coupling of stress in the ER to
activation of JNK protein kinases by transmembrane protein kinase
IRE1. Science. 287:664–666. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Wada T and Penninger JM: Mitogen-activated
protein kinases in apoptosis regulation. Oncogene. 23:2838–2849.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Novoa I, Zeng H, Harding HP and Ron D:
Feedback inhibition of the unfolded protein response by
GADD34-mediated dephosphorylation of eIF2alpha. J Cell Biol.
153:1011–1022. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Shinjo S, Mizotani Y, Tashiro E and Imoto
M: Comparative analysis of the expression patterns of UPR-target
genes caused by UPR-inducing compounds. Biosci Biotechnol Biochem.
77:729–735. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Annis MG, Yethon JA, Leber B and Andrews
DW: There is more to life and death than mitochondria: Bcl-2
proteins at the endoplasmic reticulum. Biochim Biophys Acta.
1644:115–123. 2004. View Article : Google Scholar : PubMed/NCBI
|