Introduction
External radiotherapy for primary solid tumors is
generally effective as it is non-invasive and improves local
control in the target region. However, certain dispersal targets in
radiotherapy induce poor prognosis, including distant metastasis
and radiation resistance, in human fibrosarcoma HT1080 cells
(1). Thus, research has focused on
the metastasis-inducible factor hyaluronan (HA) to resolve these
concerns pertaining to radiotherapy. HA is the primary component of
the extracellular matrix and may promote cellular motility by
activating the epidermal growth factor receptor through Akt
phosphorylation signaling (2). In
addition, HA regulates the expression of matrix metalloproteinases
(MMP)-2 and −9, which belong to the type IV collagenase family
(3). A previous report has clarified
that the mutant overexpression of MMP-2 and −9 promotes distant
metastasis and invasion (4). An
inhibitor of HA synthesis, 4-methylumbelliferone (MU), was
identified as a possible countermeasure in prostate, lung and
breast cancer cell models (5–7). To the best of our knowledge, there have
been no published studies investigating the administration of MU in
conjunction with the exposure of cancer cells to ionizing radiation
(IR); however, the chemotherapy drugs cisplatin and paclitaxel have
been clinically used along with radiotherapy in various solid
tumors (8–10).
It was hypothesized that if the mechanism underlying
the synergistic anti-tumor effect of IR with MU was clarified, a
novel countermeasure to distant metastases may be proposed.
Therefore, in the present study, the effect of the administration
of MU with IR on cell survival fraction, HA synthesis, and MMP
expression was analyzed in HT1080 cells.
Materials and methods
Reagents
MU, an HA inhibitor, was purchased from Nacalai
Tesque, Inc., (Kyoto, Japan) and diluted in dimethyl sulfoxide
(DMSO; Wako Pure Chemical Industries, Ltd., Osaka, Japan). A
Gelatin Zymography kit for detecting MMP was purchased from Cosmo
Bio Co., Ltd., (Tokyo, Japan). The Hyaluronan
Quantikine® ELISA kit for quantifying HA concentration
was purchased from R&D Systems, Inc., (Minneapolis, MN,
USA).
Cell culture
Human fibrosarcoma HT1080 cells were purchased from
the American Type Culture Collection (Manassas, VA, USA) and
cultured in RPMI 1640 medium (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) supplemented with 10% heat-inactivated fetal
bovine serum (Japan Bio Serum, Fukuyama, Japan) and 1%
penicillin/streptomycin (Thermo Fisher Scientific, Inc.) at 37°C in
a humidified atmosphere with 5% CO2. The number of
viable cells was counted using the trypan blue (Sigma-Aldrich, St.
Louis, MO, USA) dye exclusion method. The clonogenic potency of
cells was estimated by colony formation assay and appropriate
numbers of cells were seeded. Following 8–10 days of incubation,
the cell colonies were fixed with methanol, stained with Giemsa
(both Wako Pure Chemical Industries, Ltd.) and counted.
Exposure of cells to IR
IR was performed using an X-ray generator
(MBR-1520R-3; Hitachi Medical Co. Ltd., Tokyo, Japan) with 0.5 mm
aluminum and 0.3 mm copper filters at a distance of 45 cm between
the focus and the target. Radiation was carried out at 150 kV, 20
mA, 0.9 Gy/min. During X-ray exposure, the total dose and dose rate
were monitored with a thimble ionization chamber placed next to the
sample.
Analysis of viable and apoptotic
cells
The viability of each cell culture was assessed
using an annexin V and propidium iodide (PI) staining kit
(BioLegend, Tokyo, Japan). The cell cycle distribution was analyzed
by Hoechst 33342 staining. Fluorescence data were collected using
the Cell Lab Quanta™ SC MPL Flow Cytometer (Beckman Coulter, Inc.,
Brea, CA, USA).
Invasion assay
Invasion potential was evaluated using a BioCoat
Matrigel invasion chamber (BD Biosciences, San Jose, CA, USA). The
HT1080 cell suspension (2.5×104 cells) was added to
24-well chambers and incubated for 22 h at 37°C in a humidified
tissue culture incubator at 37°C with 5% CO2. Invasive
cells were fixed and stained using a Diff-Quik staining kit (Dade
Behring, Inc.; Siemens Healthcare GmbH, Erlangen, Germany). The
number of cells in a predetermined field of view (876×659 µm) were
counted, allowing a calculation of the invasion rate to be
performed using the formula: Invasion rate = (number of invasion
cells) / (number of migrated cells) × 100.
HA density quantitation
The Hyaluronan Quantikine® ELISA kit
(R&D Systems, Inc.) was used to analyze the HA concentration of
the supernatants. At near confluence, 30 µl aliquots of the HT1080
cell culture supernatants were prepared, and 210 µl aliquots of the
Calibrator Diluent RD5-18 buffer was added to the kit. The samples
were subsequently added and buffer from the kit (HA conjugate and
substrate solution) was added to each well. Finally, stop solution
was added, and the absorbance of each well was measured at a
wavelength of 540 nm using a microplate reader (10043; Bio-Rad
Laboratories, Inc., Hercules, CA, USA). HA concentration was
calculated from a standard curve of the measured absorbance.
MMP protein and gene expression
analysis
Reverse transcription-quantitative polymerase chain
reaction (RT-qPCR) was performed to quantify MMP mRNA expression.
Total RNA was extracted from HT1080 cells using an
RNeasy® Micro kit (Qiagen, Inc., Valencia, CA, USA) and
quantified using a NanoDrop Spectrophotometer (NanoDrop
Technologies; Thermo Fisher Scientific, Inc., Wilmington, DE, USA).
First strand cDNAs were synthesized using the iScript™ cDNA
Synthesis kit (Bio-Rad Laboratories, Inc.) according to the
manufacturer's instructions. The expression of MMP-2, −9 and GAPDH
were measured by RT-qPCR using the TaqMan® assay
(Applied Biosystems; Thermo Fisher Scientific., Inc.) with
Hs01548727_m1 for MMP-2, Hs0095755_m1 for MMP-9, and Hs99999905_m1
for GAPDH. RT-qPCR was performed using the StepOnePlus™ Real-Time
PCR system (Applied Biosystems; Thermo Fisher Scientific., Inc.)
under the conditions of 10 min at 95°C, followed by 40 cycles at
95°C for 15 sec and 60°C for 60 sec. Relative differences in
expression were determined by the ΔΔCq method. GAPDH was used as an
internal control for each reaction and the mRNA expression of the
control was defined as the baseline.
The extracellular protein expression of MMP-2 and −9
were measured using the Gelatin Zymography protease assay kit
(Cosmo Bio Co., Ltd.). At near confluence, 70 µl supernatant of
serum-free cell culture was mixed with 30 µl sample buffer [8.7%
sodium dodecyl sulfate (SDS); 0.5 M Tris-HCl (pH 6.8), 70% glycerol
and 0.05% bromophenol blue] and loaded on the gel. Prepared samples
were then subjected to electrophoresis on 10% SDS-polyacrylamide
gels containing 0.417% gelatin. Following electrophoresis, the gels
were washed in 2.5% Triton X-100 for 1 h at room temperature to
remove SDS. The gels were subsequently incubated at 37°C for 18 h
in a substrate buffer containing 50 mM Tris-HCl (pH 7.5) and 10 mM
CaCl2 and stained with 0.25% Coomassie Brilliant Blue
R250 in 45% methanol and 10% acetic acid. The section of the
substrate degraded by proteases was detected as clear bands. The
band sizes were determined by gel densitometry using the program
ImageJ ver. 1.44b (National Institutes of Health, Bethesda, MD,
USA).
Statistical analysis
The significance of the differences between the
control and experimental cultures was determined using the
Tukey-Kramer test. Statistical analysis was performed using
Microsoft Excel 2010 (Microsoft Corporation, Redmond, WA, USA) with
the add-on software Statcel (version 3; OMS Publishing, Inc.,
Saitama, Japan). P<0.05 was considered to indicate a
statistically significant difference. Dose survival curves were
fitted using the Boltzmann function.
Results
Cell viability following MU and
X-irradiation
MU was dissolved in DMSO prior to being added to the
cell culture medium. Cells cultured with 0.1% DMSO were considered
controls as the viability of HT1080 and WI38 cells (non-cancer
fibroblast cells) with 0.1% DMSO added to the cell culture medium
was similar to that of untreated cells. To clarify the toxicity of
the MU concentration, HT1080 and WI38 cells were cultured with DMSO
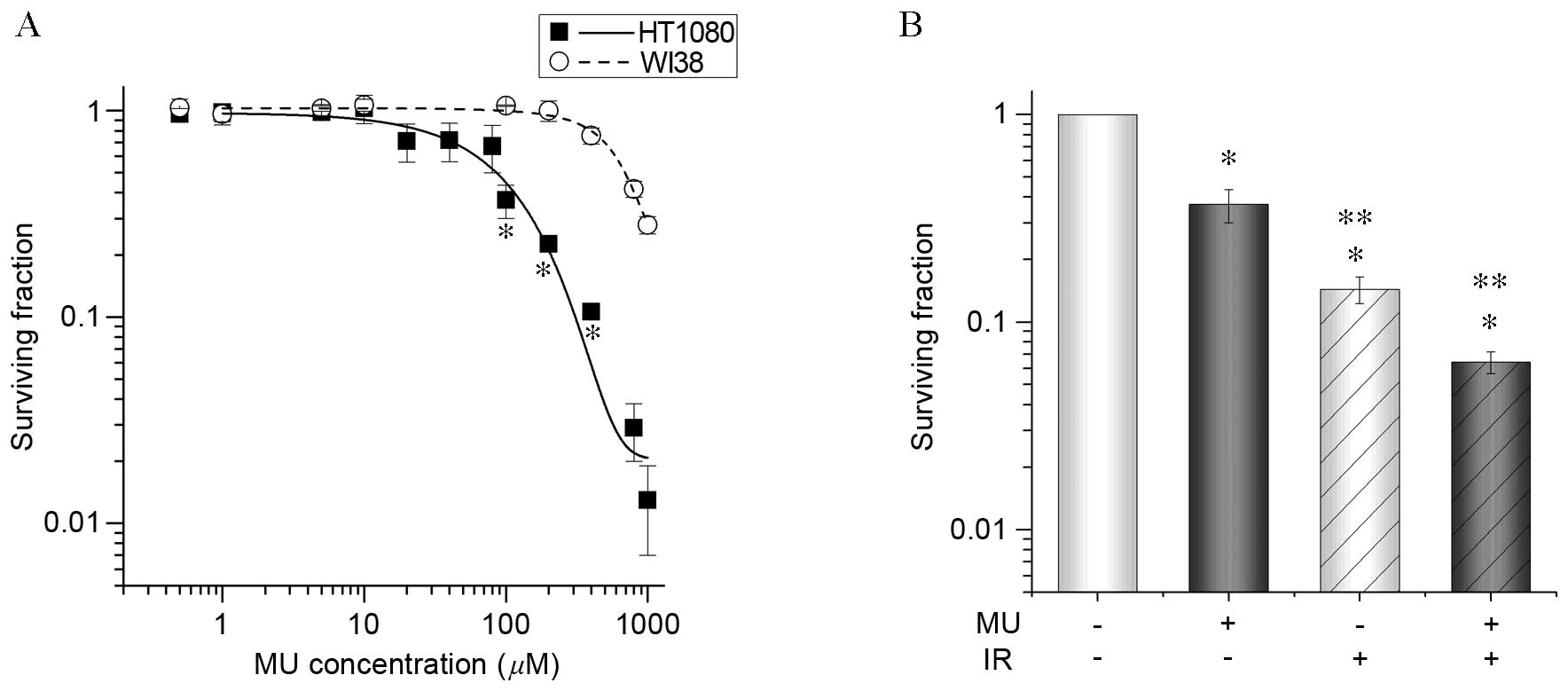
containing MU. The survival fraction of HT1080 cells in >100 µM
MU decreased significantly (100 µM, 0.3±0.1; 200 µM,
8.0±0.4×10−2) compared to WI38 cells (100 µM, 1.1±0.2;
200 µM, 1.0±0.2; Fig. 1A), indicating
that they were more sensitive to MU. The lowest effective
concentration, 100 µM MU, was chosen to evaluate the effect of MU
with 2 Gy IR due to its specificity for tumor cells.
A clonogenic potency assay was conducted to examine
the effect of 100 µM MU with 2 Gy IR on HT1080 cells (Fig. 1B). There was a significantly greater
decrease (3.6±0.7×10−1) in the survival of cells treated
with 100 µM MU decreased compared with controls (no decrease;
Fig. 2B). Furthermore, in cells
treated with 100 µM MU and 2 Gy IR, there was a significantly
greater decrease (6.4±0.8×10−2) in cell survival
compared with cells treated with 2 Gy IR or MU alone. These results
indicate that MU with 2 Gy IR is a more effective anti-tumor
treatment than 2 Gy IR alone.
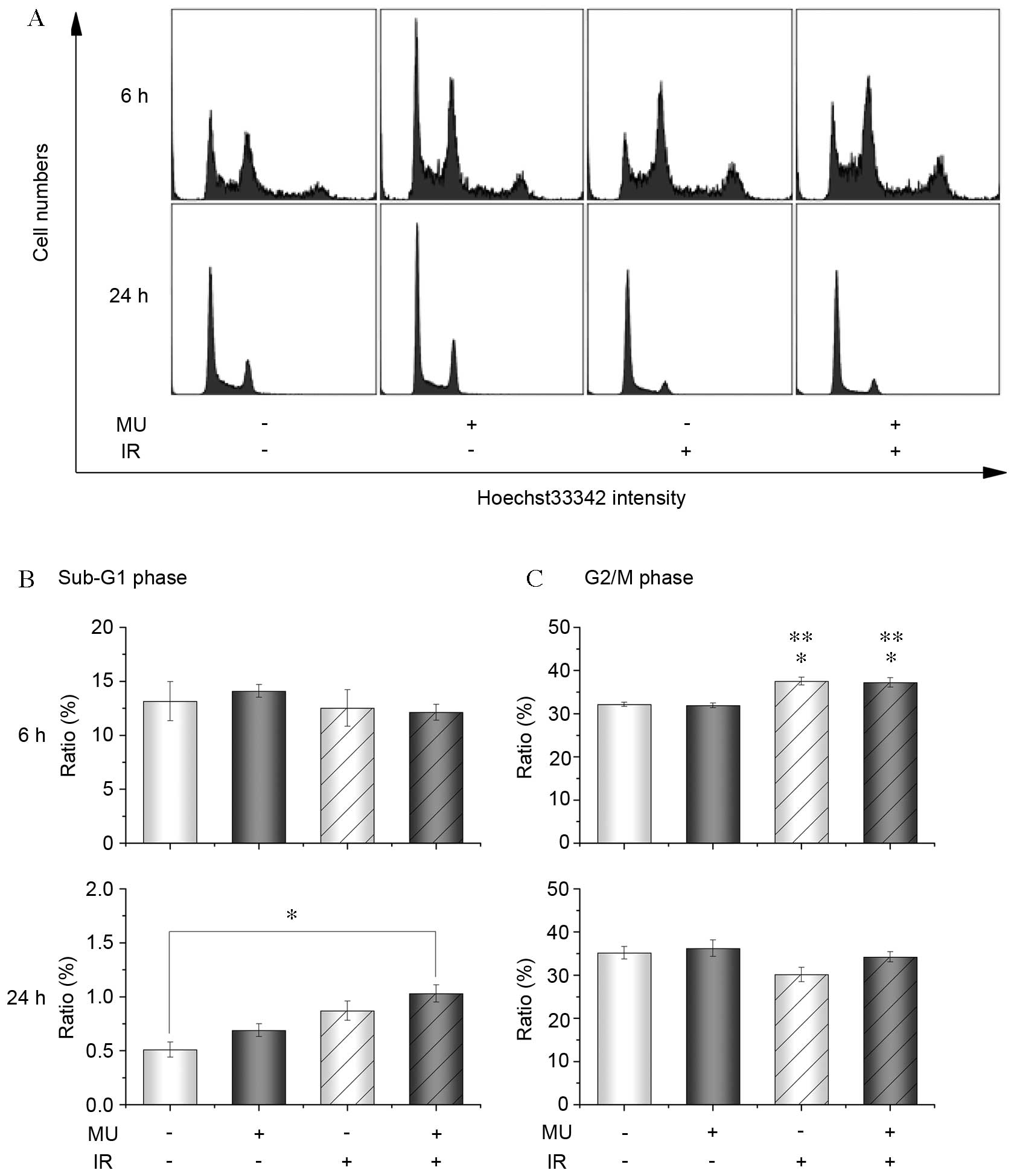
To clarify whether the decrease in cell survival
occurred by cell death and/or quiescence, cell cycle distribution
and apoptosis markers were assessed. Following 2 Gy IR and MU
treatment, there was a significant increase of the number of cells
in the G2/M phase of the cell cycle after 6 h (control, 32.2±0.5%;
2 Gy IR, 37.6±0.9%; 2 Gy IR and MU, 37.3±1.1%) and in the sub-G1
phase after 24 h (control, 0.5±0.1%; 2 Gy IR and MU, 1.0±0.1%;
Fig. 2). Furthermore, there was a
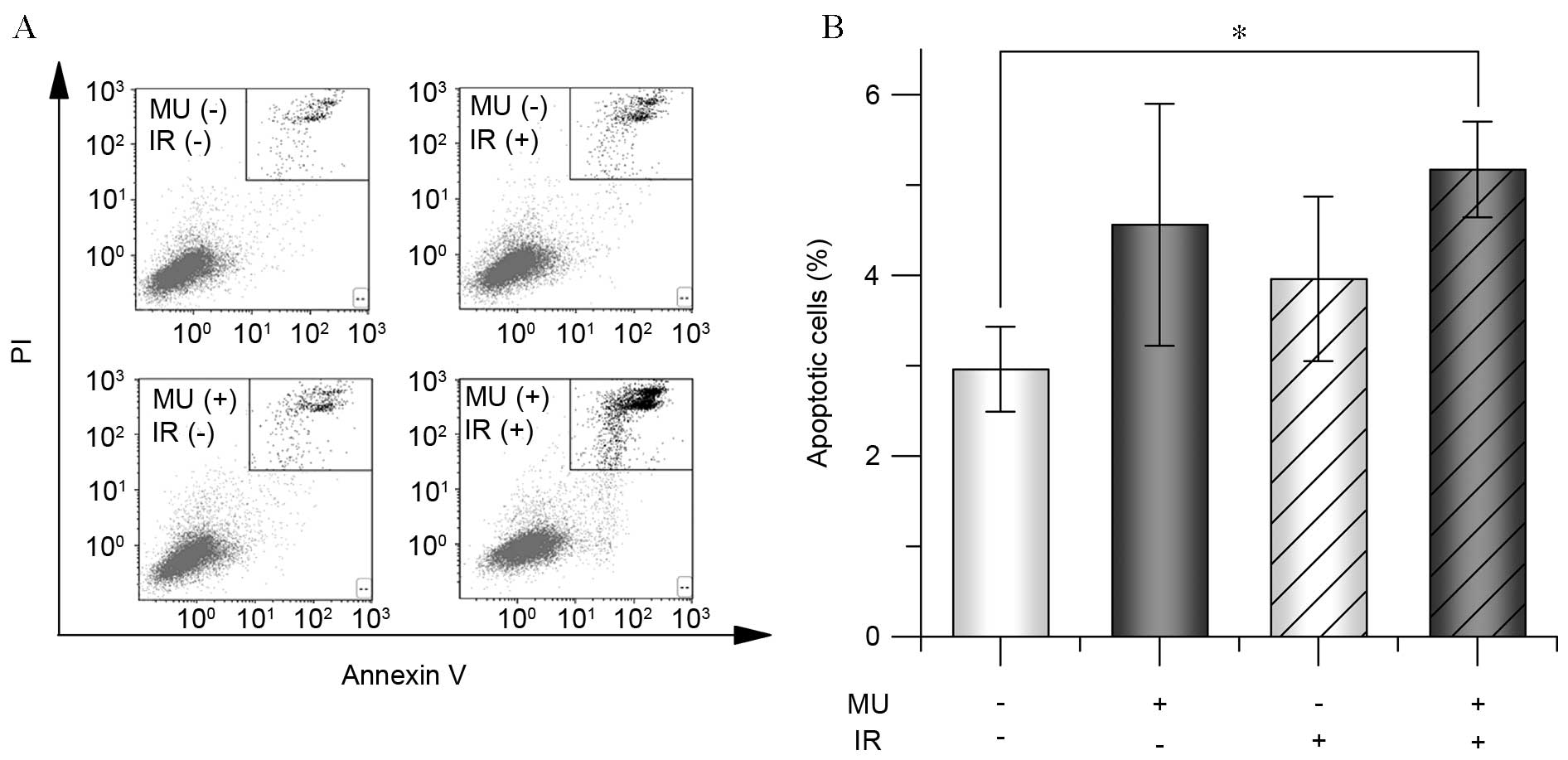
significant increase of the fraction of annexin-V(+), PI(+) cells,
following 2 Gy IR and MU treatment (5.2±0.5%) compared with the
control (3.0±0.5%; Fig. 3),
indicating increased apoptosis. The results from cells treated with
4 Gy were similar to that of cells treated with 2 Gy (data not
shown).
Analysis of cell invasion
potential
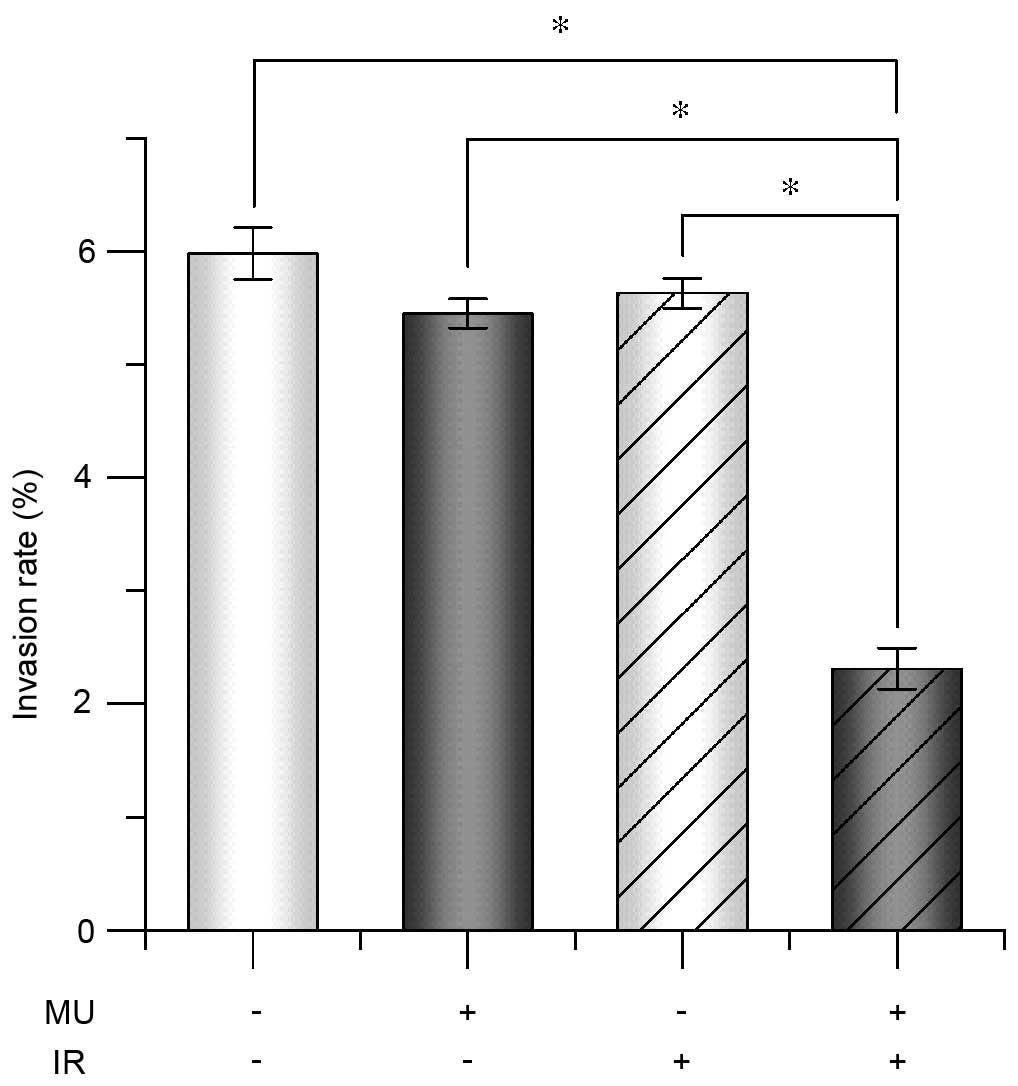
To verify invasion at a cellular level, the
potential invasion rate was quantified. The invasion rate of cells
treated with 2 Gy IR and MU was significantly lower (2.3±0.4%) than
that of the control (6.0±0.6%; Fig.
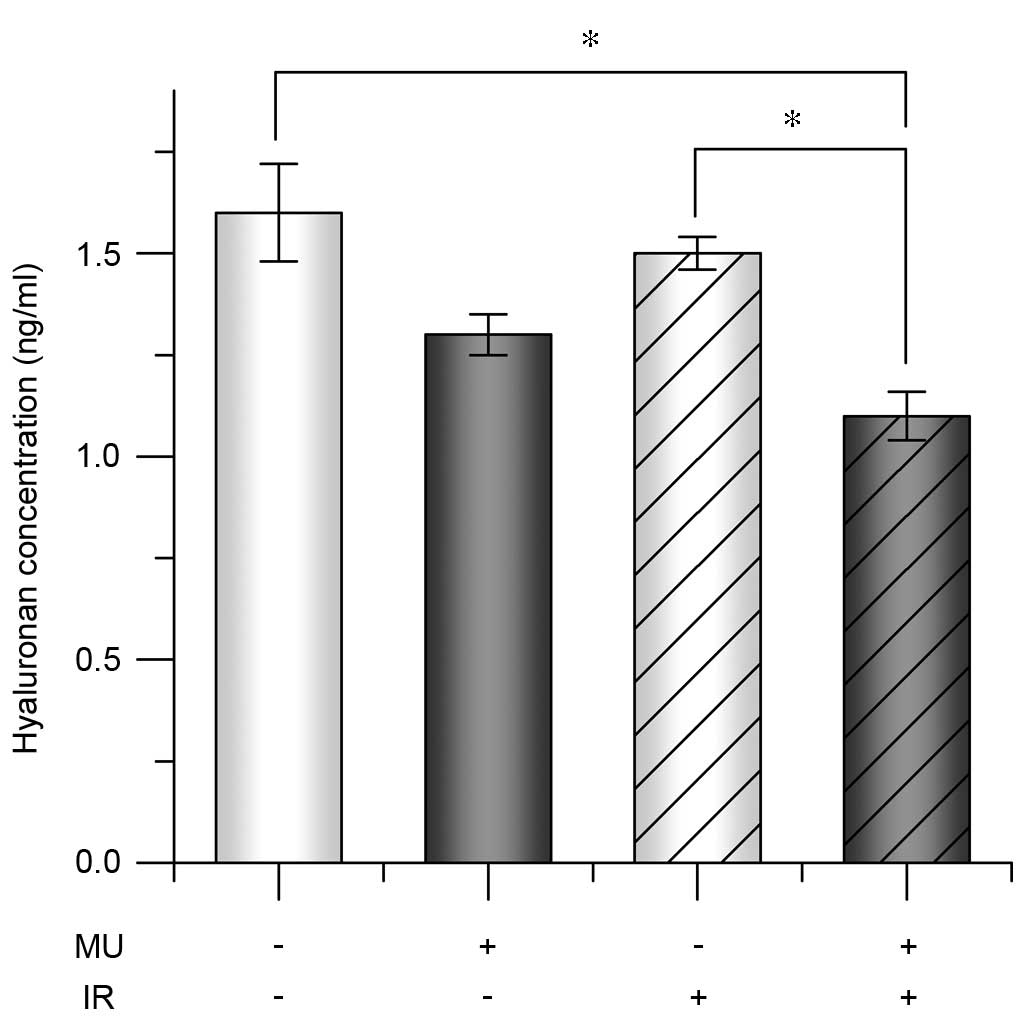
4). To evaluate the extracellular environment under 2 Gy IR
with 100 µM MU, HA concentrations were analyzed in the culture
supernatants. The HA concentration of control HT1080 cells
(1.0×105 cells) was 1.5±0.2×102 ng/ml after
24 h and was similar in cells treated with MU or 2 Gy IR alone (MU,
1.3±0.1×102 ng/ml; 2 Gy IR, 1.4±0.1×102
ng/ml; Fig. 5). However, HA
concentration following 2 Gy IR treatment with MU was significantly
lower (1.1±0.1×102 ng/ml) compared with the control and
other treatments (Fig. 5).
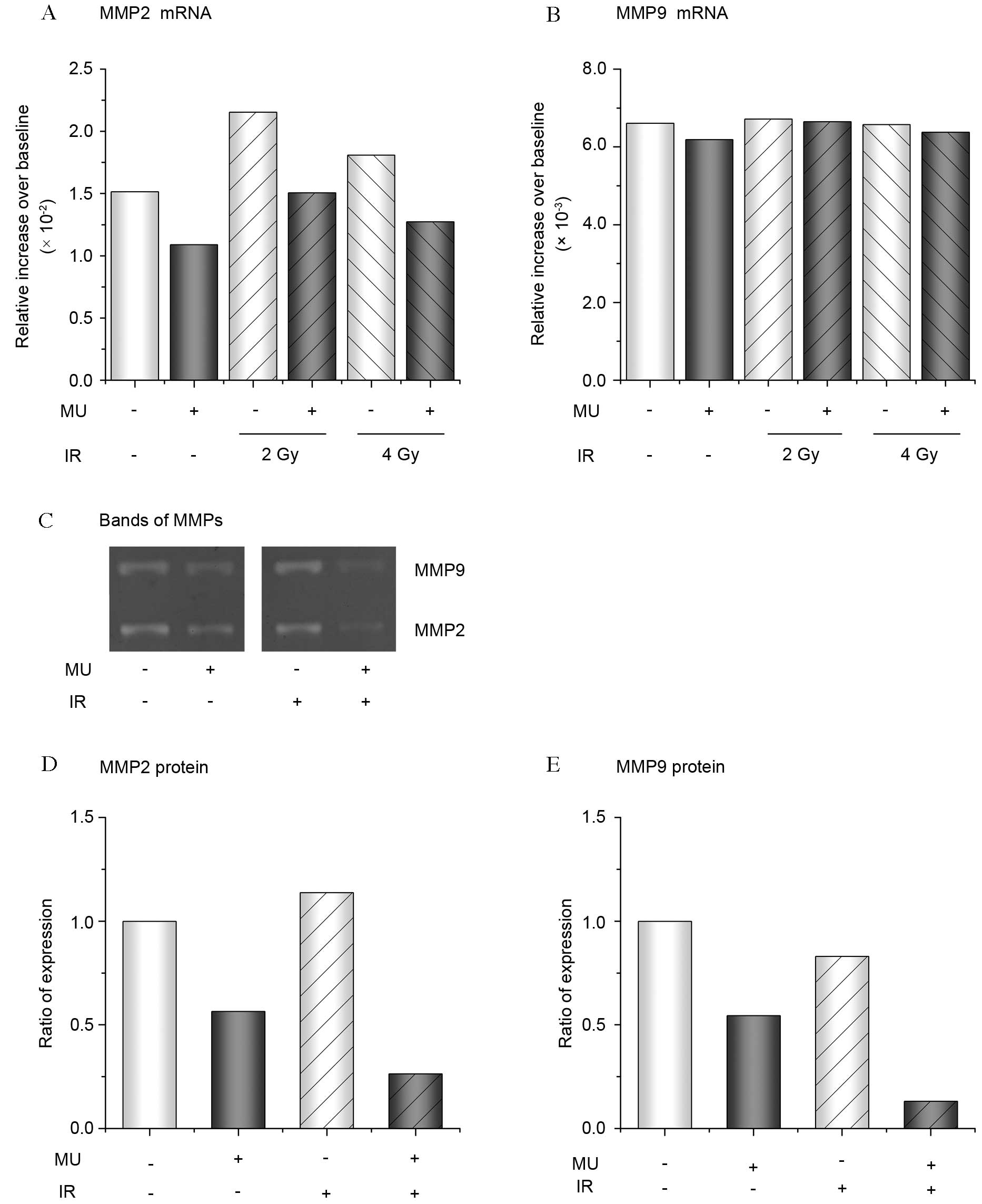
Expression of MMPs
The expression of MMP-2 and −9, an indication of
cell invasion, were analyzed in cells exposed to 2–4 Gy IR with or
without MU. A higher expression of MMP-2 mRNA following 2 and 4 Gy
IR was observed compared with the control (Fig. 6A). However, the addition of MU
suppressed the expression of MMP-2 mRNA (Fig. 6A). A similar expression of MMP-9 mRNA
following treatment with 2 Gy IR and/or MU was observed the control
(Fig. 6B). However, the expression of
MMP protein in the culture supernatant was strongly suppressed
following 2 Gy IR with MU, compared with the control and other
treatments (Fig. 6C-E).
Discussion
Previous studies in prostate and breast cancer cells
have reported that the anti-tumor effect of MU may be due to the
inhibition of cell proliferation and apoptosis induction (5,7).
Furthermore, it has been demonstrated that 1.0 mM MU exerts its
anti-tumor effect on ovarian cancer by suppressing thymidine
phosphorylase expression (11). In
another study, Saito et al (12) indicated that MU leads to growth arrest
and apoptosis mediated by BAX expression and a reduction in HA
synthesis. In the present study, the induction of apoptosis by MU
in HT1080 cells was observed, suggesting that the intracellular
signaling pathway of apoptosis affected by 100 µM MU and is not
dependent on the type of tumor. By contrast, combining 2 Gy IR with
MU in fibroblast cells exerted a stronger anti-cell proliferation
effect that is specific to cancer without being toxic to normal
cells. To the best of our knowledge, this is novel information that
has not yet been reported.
It has been demonstrated that IR produces reactive
oxygen species (ROS) or free radicals, including X-rays and γ-rays
that indirectly and/or directly induce DNA strand breakage and
exert various cytotoxic effects (13,14). Braga
et al (15) reported
previously that HA has antioxidant activity, therefore, it was
suggested that the reduction in HA concentration by MU observed in
the current study increases the accumulation of oxidative stress
and DNA damage by 2 Gy IR.
It is important to control invasion and metastasis
when treating cancer, however, this has not yet been achieved. A
highly potent invasive tumor exhibits aberrant secretion of HA and
overexpression of cluster of differentiation 44 antigen, which acts
as the HA receptor (16). HA is
produced from injured tissue stroma and is rapidly deposited in the
extracellular matrix, where it regulates repair processes through
cross-talk with various inflammatory conditions, including
carcinogenesis (17). Abnormal
secretion of HA has been observed in malignant tumors (18), however, HA is required in normal
tissue, therefore, it is important to clarify the regulating system
for HA secretion. Kim et al (19) have reported that suppression of MMP-9
activity and vascular endothelial growth factor production in
malignant tumor cells reduces tumor metastasis and angiogenic
potency. In addition, Rauhala et al (20) have demonstrated that keratinocytes
exposed to low-dose UVB radiation increased HA synthesis with the
production of ROS. Fibrosarcoma is known as the radioresistant cell
(21), which may be related to the
induction of HA synthesis by radiation.
Previous studies have demonstrated that gene
expression of MMP-2 and −9 is upregulated following IR and is
associated with cellular invasion (22). The results of the current study
regarding mRNA expression following treatment with 2 Gy and 4 Gy
differed in comparison with previous reports. In the current study,
the levels of MMP protein significantly decreased following 2 Gy IR
with MU (Fig. 6C-E). However, direct
comparison of these results may not be possible as the previous
reports do not include clear information regarding dose rate and
radiation energy.
The regulation of ROS and HA synthesis may be
required in fibrosarcoma treatment. The current study presents
evidence that a combination of IR with MU is able to inhibit
invasion potency and this may be a potential cancer treatment for
radioresistant tumors in the case of HA overexpression. Further
elucidation and a biological model analysis of the association
between radiosensitive tumors and HA synthesis is required in the
future.
In conclusion, the present study investigated the
effect of combining MU with external radiotherapy as an anti-tumor
treatment. Cell viability, extracellular HA concentration and
cellular invasion potential in HT1080 cells treated with 2 Gy IR
and MU were analyzed. A decrease in HA concentration, invasion rate
and expression of MMP-2 and −9 following treatment of 2 Gy IR with
MU was observed, along with suppression of clonogenic potential
compared with non-treated cells. These results suggest that a
combination of 2 Gy IR and MU has a synergic effect as an
anti-invasion treatment and significant anti-tumor and -invasion
effects in HT1080 cells and thus may be developed for clinical use
as an inhibitor of distant metastasis in radiation therapy.
Acknowledgements
The present study was supported by the Japan Society
for the Promotion of Science KAKENHI (Tokyo, Japan) Scientific
Research and Young Scientist Grants (grant nos. 24591831 and
25861054), and the Hirosaki University Institutional Research Grant
(Hirosaki, Japan; grant no. 2014).
References
|
1
|
Zagars GK, Ballo MT, Pisters PW, Pollock
RE, Patel SR, Benjamin RS and Evans HL: Prognostic factors for
patients with localized soft-tissue sarcoma treated with
conservation surgery and radiation therapy: An analysis of 1225
patients. Cancer. 97:2530–2543. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hiraga T, Ito S and Nakamura H: Cancer
stem-like cell marker CD44 promotes bone metastases by enhancing
tumorigenicity, cell motility, and hyaluronan production. Cancer
Res. 73:4112–4122. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Duffy MJ, Maguire TM, Hill A, McDermott E
and O'Higgins N: Metalloproteinases: Role in breast carcinogenesis,
invasion and metastasis. Breast Cancer Res. 2:252–257. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Toole BP: Hyaluronan-CD44 Interactions in
cancer: Paradoxes and possibilities. Clin Cancer Res. 15:7462–7468.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lokeshwar VB, Lopez LE, Munoz D, Chi A,
Shirodkar SP, Lokeshwar SD, Escudero DO, Dhir N and Altman N:
Antitumor activity of hyaluronic acid synthesis inhibitor
4-methylumbelliferone in prostate cancer cells. Cancer Res.
70:2613–2623. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Arai E, Nishida Y, Wasa J, Urakawa H, Zhuo
L, Kimata K, Kozawa E, Futamura N and Ishiguro N: Inhibition of
hyaluronan retention by 4-methylumbelliferone suppresses
osteosarcoma cells in vitro and lung metastasis in vivo. Br J
Cancer. 105:1839–1849. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Urakawa H, Nishida Y, Wasa J, Arai E, Zhuo
L, Kimata K, Kozawa E, Futamura N and Ishiguro N: Inhibition of
hyaluronan synthesis in breast cancer cells by
4-methylumbelliferone suppresses tumorigenicity in vitro and
metastatic lesions of bone in vivo. Int J Cancer. 130:454–466.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sano D, Matsumoto F, Valdecanas DR, Zhao
M, Molkentine DP, Takahashi Y, Hanna EY, Papadimitrakopoulou V,
Heymach J, Milas L and Myers JN: Vandetanib restores head and neck
squamous cell carcinoma cells' sensitivity to cisplatin and
radiation in vivo and in vitro. Clin Cancer Res. 17:1815–1827.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chan AT, Leung SF, Ngan RK, Teo PM, Lau
WH, Kwan WH, Hui EP, Yiu HY, Yeo W, Cheung FY, et al: Overall
survival after concurrent cisplatin-radiotherapy compared with
radiotherapy alone in locoregionally advanced nasopharyngeal
carcinoma. J Natl Cancer Inst. 97:536–539. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Byun JW, Lee HS, Song SU, Lee SW, Kim SK,
Kim WC, Lee MH and Choi GS: Combined treatment of murine
fibrosarcoma with chemotherapy (Paclitaxel), radiotherapy, and
intratumoral injection of dendritic cells. Ann Dermatol. 26:53–60.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tamura R, Yokoyama Y, Yoshida H, Imaizumi
T and Mizunuma H: 4-Methylumbelliferone inhibits ovarian cancer
growth by suppressing thymidine phosphorylase expression. J Ovarian
Res. 7:942014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Saito T, Tamura D, Nakamura T, Makita Y,
Ariyama H, Komiyama K, Yoshihara T and Asano R:
4-methylumbelliferone leads to growth arrest and apoptosis in
canine mammary tumor cells. Oncol Rep. 29:335–342. 2013.PubMed/NCBI
|
|
13
|
Bajinskis A, Natarajan AT, Erixon K and
Harms-Ringdahl M: DNA double strand breaks induced by the indirect
effect of radiation are more efficiently repaired by non-homologous
end joining compared to homologous recombination repair. Mutat Res.
756:21–29. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Vignard J, Mirey G and Salles B:
Ionizing-radiation induced DNA double-strand breaks: A direct and
indirect lighting up. Radiother Oncol. 108:362–369. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Braga PC, Dal Sasso M, Lattuada N, Greco
V, Sibilia V, Zucca E, Stucchi L, Ferro E and Ferrucci F:
Antioxidant activity of hyaluronic acid investigated by means of
chemiluminescence of equine neutrophil bursts and electron
paramagnetic resonance spectroscopy. J Vet Pharmacol Ther.
38:48–54. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim Y and Kumar S: CD44-mediated adhesion
to hyaluronic acid contributes to mechanosensing and invasive
motility. Mol Cancer Res. 12:1416–1429. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Briggs A, Rosenberg L, Buie JD, Rizvi H,
Bertagnolli MM and Cho NL: Antitumor effects of hyaluronan
inhibition in desmoid tumors. Carcinogenesis. 36:272–279. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Saito T, Dai T and Asano R: The hyaluronan
synthesis inhibitor 4-methylumbelliferone exhibits antitumor
effects against mesenchymal-like canine mammary tumor cells. Oncol
Lett. 5:1068–1074. 2013.PubMed/NCBI
|
|
19
|
Kim A, Im M, Yim NH and Ma JY: Reduction
of metastatic and angiogenic potency of malignant cancer by
Eupatorium fortunei via suppression of MMP-9 activity and VEGF
production. Sci Rep. 4:69942014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rauhala L, Hämäläinen L, Salonen P, Bart
G, Tammi M, Pasonen-Seppänen S and Tammi R: Low dose ultraviolet B
irradiation increases hyaluronan synthesis in epidermal
keratinocytes via sequential induction of hyaluronan synthases
Has1-3 mediated by p38 and Ca2+/calmodulin-dependent protein kinase
II (CaMKII) signaling. J Biol Chem. 288:17999–18012. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wei K, Kodym R and Jin C: Radioresistant
cell strain of human fibrosarcoma cells obtained after long-term
exposure to x-rays. Radiat Environ Biophys. 37:133–137. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Speake WJ, Dean RA, Kumar A, Morris TM,
Scholefield JH and Watson SA: Radiation induced MMP expression from
rectal cancer is short lived but contributes to in vitro invasion.
Eur J Surg Oncol. 31:869–874. 2005. View Article : Google Scholar : PubMed/NCBI
|