Introduction
In previous decades, cancer has become a common
disease in humans (1,2). Currently, cancer is the second leading
contributor to mortality rates in developing countries and the
leading contributor to mortality rates in developed countries. In
addition, with population growth and aging, and an unhealthy
lifestyle comprising physical inactivity, smoking and ‘westernized’
diets, the burden of cancer is increasing in developing countries
(3–6).
Fortunately, various therapies, including surgical management
(7), radiotherapy (8), chemotherapy (9), traditional Chinese medicine therapy
(10), biotherapy (11), immunotherapy (12), gene therapy (13), thermotherapy (14), photodynamic therapy (15) and interventional therapy (16), have been successfully used to reduce
pain in patients with cancer and prolong life expectancy.
Chemotherapy occupies an important position in
cancer treatment, and it is reported that several components,
including vinca alkaloids, taxanes, camptothecins and
epipodophyllotoxins (17) exhibit
potential antitumor activities. It is important to develop
chemotherapy through the identification of plant-derived components
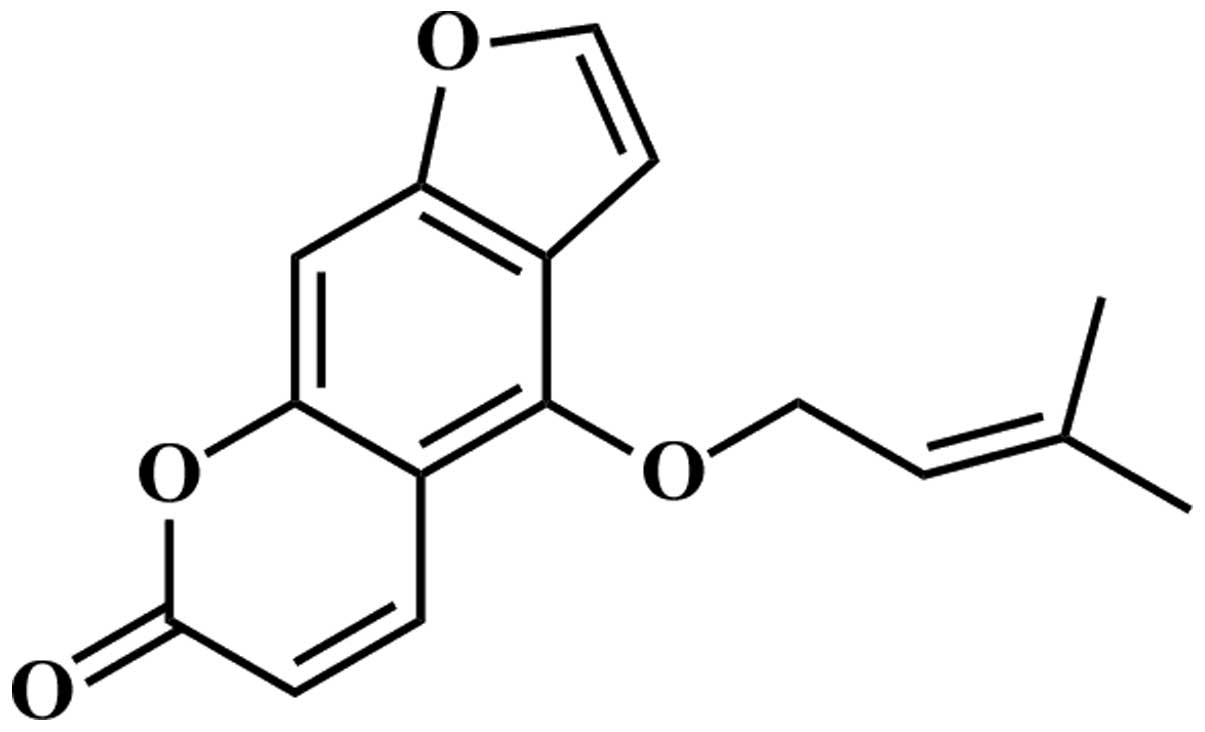
with antitumor activity. Isoimperatorin (Fig. 1), a plant-derived component, exists
widely in the Umbelliferae, and includes Angelica dahurica,
Notopterygium incisum, Angelica pubescens and
Peucedanum praeruptorum. Isoimperatorin has analgesic
(18), antimicrobial (19), vascular relaxing (20) and anticancer activities (21–23).
However, studies by Okuyama et al (21), Zhang (22) and Kim et al (23) focused predominantly on investigating
the antiproliferative activity of isoimperatorin against different
cancer cell lines using MTT or sulforhodamine B techniques. In the
present study, the effects and possible mechanisms of
isoimperatorin on SGC-7901 cells were examined in vitro, and
SGC-7901 cell-induced tumors in vivo were examined by
determining the inhibition rate, apoptotic rate,
mitochondria-mediated apoptosis-associated proteins, tumor volumes
and body weights of nude mice using MTT, flow cytometry, western
blot analysis and xenograft assays.
Materials and methods
Chemicals and reagents
Isoimperatorin was obtained from the National
Institutes for Food and Drug Control (Beijing, China) and was
dissolved and diluted in 0.5% DMSO to obtain different
concentrations for the subsequent assays. RPMI 1640 media and fetal
bovine serum (FBS) were purchased from Invitrogen (Thermo Fisher
Scientific, Inc, Waltham, MA, USA). The MTT cell proliferation and
cytotoxicity assay kit and Annexin V-fluorescein isothiocyanate
(FITC)/propidium iodide (PI) apoptosis detection kit were purchased
from Beyotime Institute of Biotechnology (Shanghai, China) and
Yeasen (Shanghai, China). Primary antibodies for β-actin (catalog
no. sc-47778), Survivin (catalog no. sc-8808), myeloid leukemia
cell-1 (Mcl-1) (catalog no. sc-12756), B cell lymphoma-extra large
(Bcl-xl) (catalog no. sc-8392), B cell lymphoma-2 (Bcl-2) (catalog
no. sc-7382), second mitochondria-derived activator of caspase
(Smac) (catalog no. sc-393118), Bcl-2-associated X factor (Bax)
(catalog no. sc-7480), cleaved (c)-caspase-3 (catalog no. AC033)
and horseradish peroxidase (HRP)-conjugated secondary antibodies
(donkey anti-goat, catalog no. A0181; goat anti-mouse, catalog no.
A0216; and goat anti-rabbit, catalog no. A0208) were purchased from
were purchased from Beyotime Institute of Biotechnology, while
primary antibody for c-caspase-9 (catalog no. 9501) was purchased
from Cell Signaling Technology, Inc. (Danvers, MA, USA). All other
chemicals and reagents used in the present study were analytical
grade reagents.
Animals
Sixteen female nude mice (5–6 weeks old) were
purchased from the SLRC Laboratory Animal Company (Shanghai, China)
and housed in a temperature-controlled vivarium (25°C) with a
relative humidity of 65% and a 12/12-h light-dark cycle. All mice
had free access to water and food. All protocols for treatment of
the animals were performed in strict accordance with the
international ethical guidelines and the National Institutes of
Health Guide concerning the Care and Use of Laboratory Animals
(24). The experiments were performed
with the approval of the Animal Experimentation Ethics Committee of
Yinzhou People's Hospital, School of Medicine, Ningbo University
(Ningbo, China; protocol no. 2013C50026AEEC).
Cell culture
The SGC-7901 cells were purchased from American Type
Culture Collection (Manassas, VA, USA) and cultured in RPMI-1640
medium supplemented with 10% FBS and antibiotics (100 U/ml
streptomycin and 100 U/ml penicillin) at 37°C in 5%
CO2/95% air. When the SGC-7901 cells reached logarithmic
growth phase, the cells were subcultured and the experiments were
performed immediately on the subcultured cells.
MTT reduction assay
The antiproliferative activity of isoimperatorin
against SGC-7901 cells was evaluated using an MTT reduction assay.
The SGC-7901 cells were seeded on 96-well culture plates with
RPMI-1640 medium at a density of 5×103 cells/well. After
24 h of incubation at 37°C in 5% CO2/95% air, the cells
were treated at different concentrations of isoimperatorin (5, 10,
15, 20, 25, 30, 35 and 40 µg/ml) and 0.05% DMSO (control) for 48 h
to examine dose-dependency; or with isoimperatorin at a
concentration of 20 µg/ml and 0.05% DMSO (control) for 12, 24, 36,
48, 60 and 72 h to examine time-dependent effects. Subsequently, 20
µl MTT (5 mg/ml) was separately added into each well, and the cells
were cultured at 37°C in 5% CO2/95% air for another 3 h.
Subsequently, 200 µl DMSO was separately added into each well and
the optical density (OD) of the DMSO solution was measured at 570
nm using a microplate reader (Thermo Fisher Scientific, Inc.). The
inhibition rate of isoimperatorin against the SGC-7901 cells was
determined using the following equation: Inhibition rate (%) =
(ODcontrol - ODtreatment) /
ODcontrol × 100.
Apoptosis assay
Following treatment with isoimperatorin (5, 10 and
20 µg/ml) and 0.05% DMSO (control) for 48 h, the SGC-7901 cells
were collected and washed three times with phosphate
buffered-saline (PBS). Subsequently, the washed SGC-7901 cells
(5×103 cells) were resuspended in 200 µl staining buffer
and stained with 10 µl Annexin V-FITC (20 µg/ml) and 5 µl PI (50
µg/ml), following which, the SGC-7901 cells were quantified using
flow cytometry and analyzed using CellQuest Pro 4.0 acquisition
software (FACS Calibur; BD Biosciences, San Jose, CA, USA).
Western blot analysis
Following treatment with isoimperatorin (5, 10 and
20 µg/ml) and 0.05% DMSO (control) for 48 h, the total proteins of
the SGC-7901 cells were extracted with cell lysis buffer (catalog
no. P0013; Beyotime Institute of Biotechnology), ultrasound and
centrifugation at 12,000 × g for 15 min at 4°C. Protein
concentration was determined using an Enhanced BCA Protein Assay
kit (Applygen Technologies, Inc., Beijing, China). Then total
proteins (40 µg) were separated using 12% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis and transferred onto a
PVDF membrane. Following blocking with 5% fat-free milk, the
membrane was incubated with the anti-β-actin (monoclonal, mouse
anti-human, 1:1,000), anti-Survivin (polyclonal, goat anti-human,
1:1,000), anti-Mcl-1 (monoclonal, mouse anti-human, 1:1,000),
anti-Bcl-xl (monoclonal, mouse anti-human, 1:1,000), anti-Bcl-2
(monoclonal, mouse anti-human, 1:1,000), anti-Smac (monoclonal,
mouse anti-human, 1:1,000), anti-Bax (monoclonal, mouse anti-human,
1:1,000), anti-c-caspase-3 (monoclonal, rabbit anti-human, 1:1,000)
and anti-c-caspase-9 (monoclonal, rabbit anti-human, 1:1,000)
primary antibodies overnight at 4°C. The membrane was then washed
with TBS-Tween (TBST-T) and incubated with the corresponding
HRP-conjugated secondary antibody (monoclonal, donkey anti-goat,
goat anti-mouse or goat anti-rabbit, 1:1,000) in TBS-T for 1 h at
room temperature. Following another rinse, all proteins were
detected using chemiluminescence (Beyo ECL Plus reagent; Beyotime
Institute of Biotechnology). In order to assess protein loading,
β-actin was selected as an internal control.
Antitumor activity of isoimperatorin
in vivo
The SGC-7901 cells (2×106 cells per nude
mouse) were subcutaneously injected into the right flank of nude
mice to establish the tumor xenograft model. When the SGC-7901
cells-induced tumors grew to 2–3 mm in diameter, the nude mice were
randomly divided into control and isoimperatorin groups (n=8)
Animals in the control group received an intraperitoneal injection
of 0.5% DMSO and animals in the isoimperatorin group received an
intraperitoneal injection of 10 mg/kg; the injections were
performed once each day for 20 days. The tumor length and width,
and the body weight of the nude mice were measured on days 0, 5,
10, 15 and 20 using vernier calipers and electronic scales, and the
tumor volumes were calculated using the following formula: Tumor
volume (mm2) = 0.52 × length (mm) × width2
(25). Finally, the nude mice were
sacrificed immediately by decapitation, and then their tumor
tissues were removed and collected for western blot analysis.
Statistical analysis
All data are presented as the mean ± standard
deviation. One-way analysis of variance (Dunnett test) was used to
analyze the differences between two groups with SPSS 22.0 (IBM
SPSS, Armonk, NY, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Cytotoxicity activity of
isoimperatorin against SGC-7901 cells
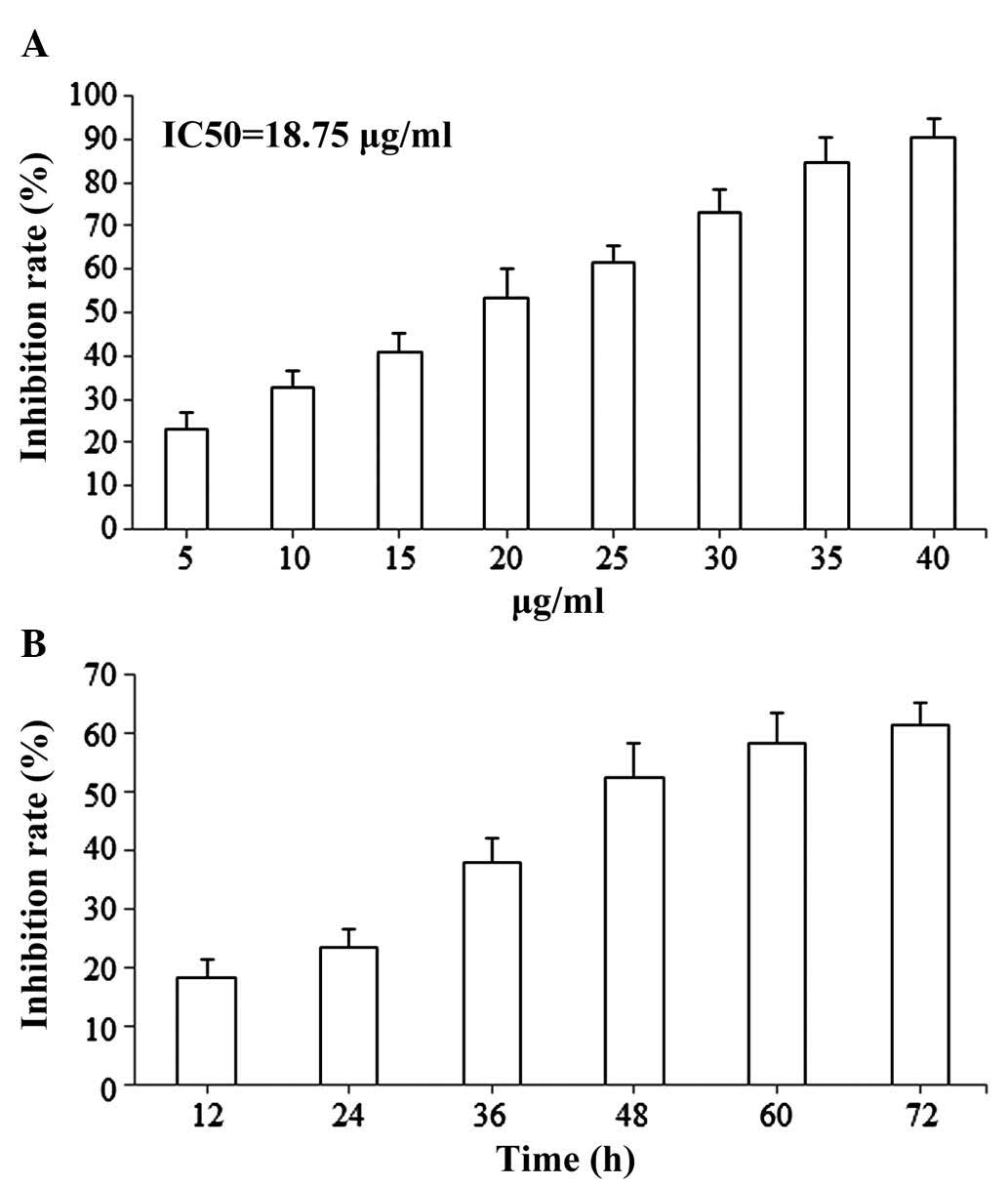
The antiproliferative effects of isoimperatorin on
SGC-7901 cells were evaluated using an MTT reduction assay.
Following treatment with isoimperatorin (5, 10, 15, 20, 25, 30, 35
and 40 µg/ml) for 48 h, proliferation of the SGC-7901 cells was
significantly inhibited in a dose-dependent manner and the
IC50 value was 18.75 µg/ml (Fig. 2A). Following treatment with
isoimperatorin (20 µg/ml) for 12, 24, 36, 48, 60 and 72 h,
proliferation of the SGC-7901 cells was significantly inhibited,
also in a time-dependent manner (Fig.
2B).
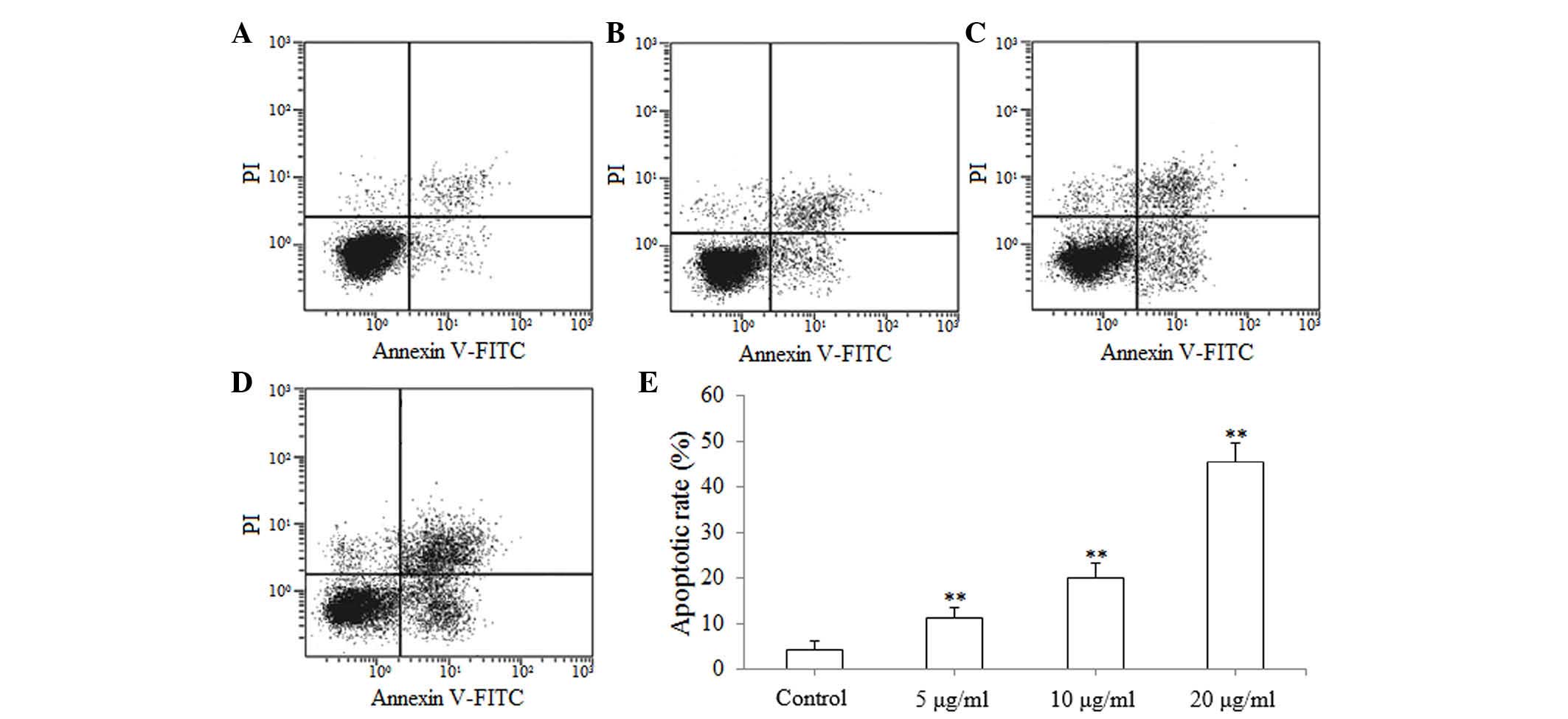
Apoptosis of SGC-7901 cells induced by
isoimperatorin
The results of the MTT reduction assay suggested
that isoimperatorin inhibited the proliferation of SGC-7901 cells.
Subsequently, flow cytometric analysis was used to investigate
whether the antiproliferative activity of isoimperatorin against
SGC-7901 cells was associated with apoptosis. As shown in Fig. 3A-D, following treatment with
isoimperatorin (5, 10 and 20 µg/ml) for 48 h, apoptosis of the
SGC-7901 cells was significantly increased (P<0.01; Fig. 3E), compared with the control group.
The results of the flow cytometric analysis suggested that the
antiproliferative activity of isoimperatorin against SGC-7901 cells
was associated with apoptosis.
Effects of isoimperatorin on the
expression levels of mitochondria-mediated apoptosis-associated
proteins
The results of the flow cytometric analysis
indicated that isoimperatorin induced the apoptosis of SGC-7901
cells, therefore, western blot analysis was subsequently used to
examine the pro-apoptotic mechanisms of isoimperatorin in the
SGC-7901 cells. As shown in Fig. 4,
following treatment with isoimperatorin (5, 10 and 20 µg/ml) for 48
h, the protein expression levels of pro-apoptotic Bax, c-caspase-3
and c-caspase-9 were significantly (P<0.05 or P<0.01)
upregulated, and the protein expression levels of anti-apoptotic
Survivin and Bcl-2 were significantly (P<0.01) downregulated,
compared with the control group. No significant differences in the
Mcl-1, Bcl-xl and Smac apoptosis-associated proteins were
found.
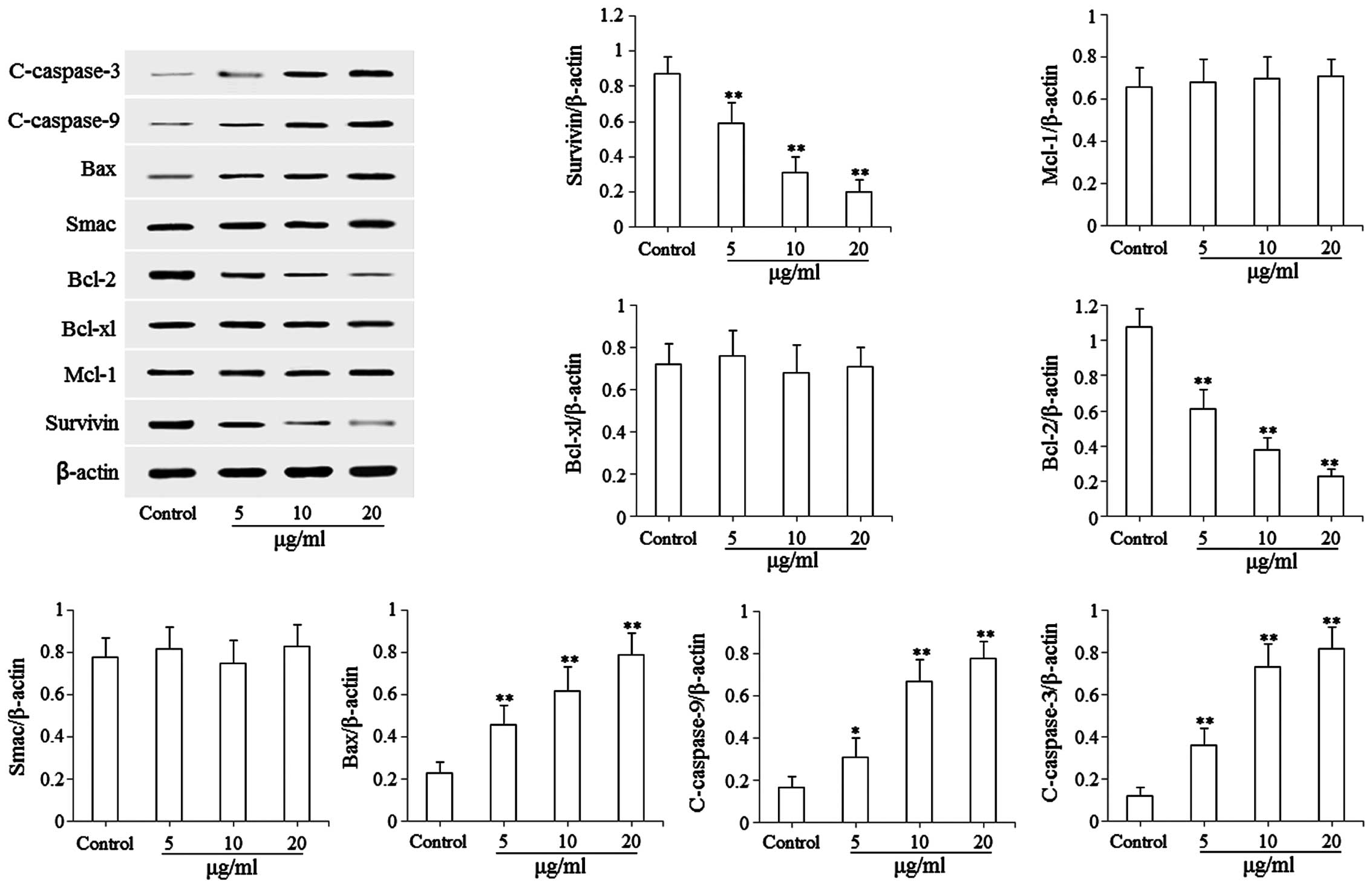 | Figure 4.Effects of isoimperatorin on the
expression levels of mitochondria-mediated apoptosis-associated
proteins. Protein levels of Survivin, Mcl-1, Bcl-xl, Bcl-2, Smac,
Bax, c-caspase-9 and c-caspase-3 were examined. *P<0.05 and
**P<0.01, compared with the control. Mcl-1, myeloid leukemia
cell-1; Bcl-2, B cell lymphoma-2; Bcl-xl, B cell lymphoma-extra
large; Smac, second mitochondria-derived activator of caspase; Bax,
Bcl-2-associated X factor. |
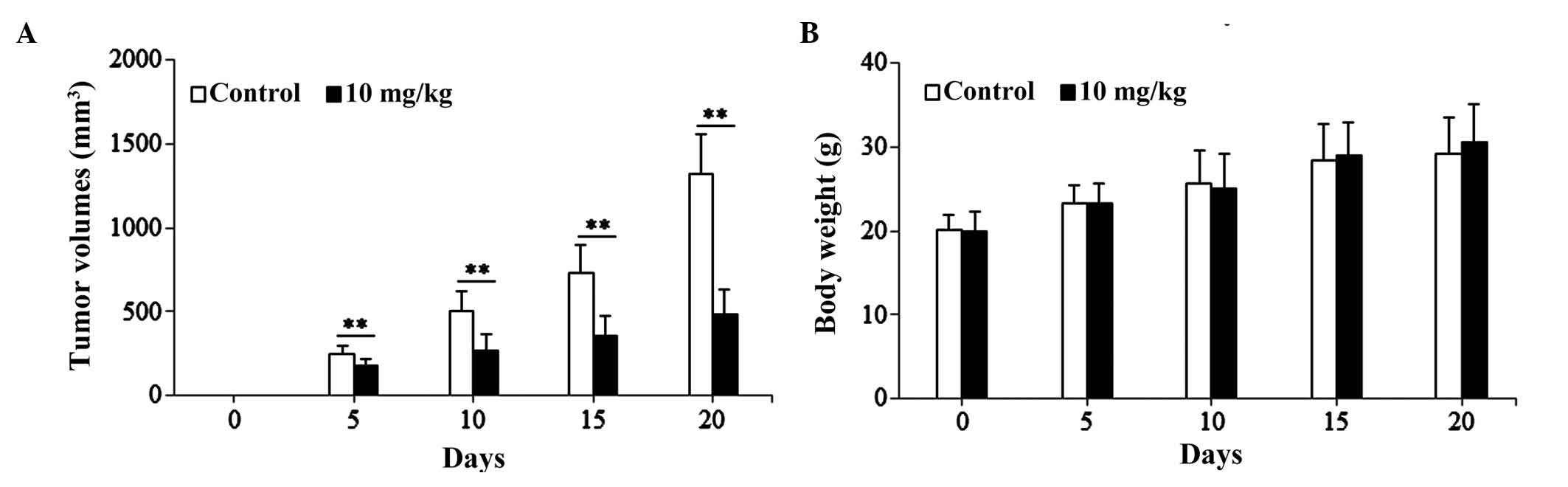
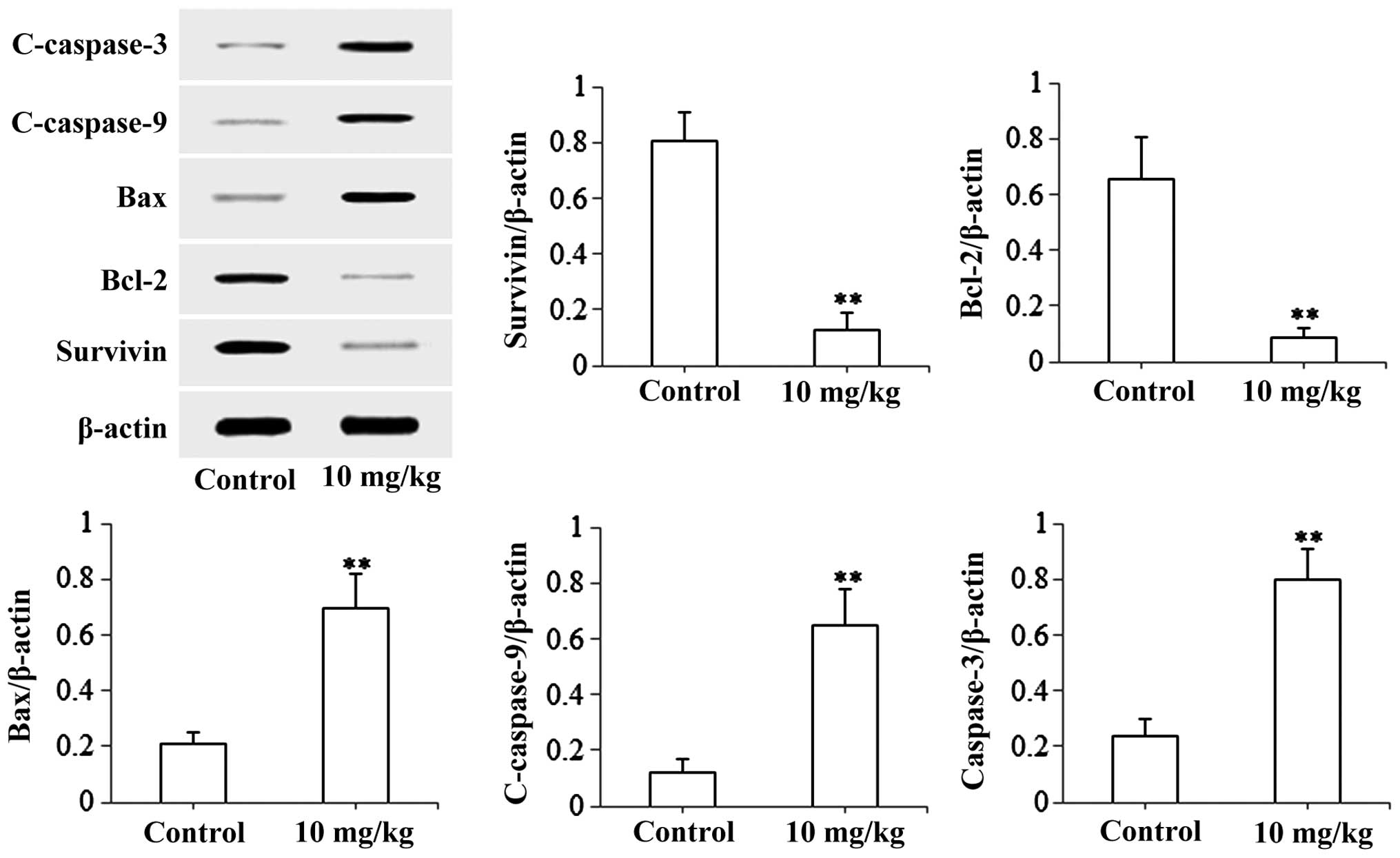
Effects of isoimperatorin on SGC-7901
cells-induced xenograft model
The present study used the SGC-7901 cell-induced
xenograft model to investigate the anti-gastric cancer activity of
isoimperatorin in vivo. As shown in Fig. 5A, following treatment with
isoimperatorin (10 mg/kg/day for 20 days), the SGC-7901
cell-induced tumor growth in the nude mice was significantly
inhibited (P<0.01), compared with that in the control group,
whereas the increase in body weight of the nude mice was not
significantly affected (Fig. 5B).
Additionally, the results of the western blot analysis of tumor
tissues (Fig. 6) suggested that,
following treatment with isoimperatorin (10 mg/kg/day for 20 days),
the protein expression levels of pro-apoptotic Bax, c-caspase-3 and
c-caspase-9 were significantly upregulated (P<0.01) and the
protein expression levels of anti-apoptotic Survivin and Bcl-2 were
significantly downregulated (P<0.01), compared with the control
group.
Discussion
Isoimperatorin has been reported to have anticancer
activity (21–23) and, although previous studies have
focused predominantly on the antiproliferative activities of
isoimperatorin against different cancer cell lines, the
antiproliferative activities of isoimperatorin against SGC-7901
cells and the possible underlying mechanisms remain to be
elucidated. Therefore, the present study investigated the
antiproliferative activity of isoimperatorin against SGC-7901 cells
and the possible underlying mechanisms using an MTT assay, flow
cytometry, western blot analysis and xenograft assays. The results
of the present study provided the first evidence, to the best of
our knowledge, that isoimperatorin inhibited the proliferation of
SGC-7901 cells by inducing apoptosis via the mitochondria-mediated
pathway.
MTT assays are a commonly used method to examine the
effects of anticancer agents on the proliferation of cancer cells
(26,27). In the present study, the results
(Fig. 2A and B) of the MTT assay
indicated that isoimperatorin significantly inhibited the
proliferation of SGC-7901 cells in a dose- and time-dependent
manner. Flow cytometric analysis is a common method used to
investigate whether drugs can induce the apoptosis of cancer cells
(28,29). In the present study, the results of
the flow cytometric analysis (Fig. 3)
indicated that isoimperatorin significantly induced apoptosis of
the SGC-7901 cells. The results of the MTT assay and flow cytometry
indicated that the antiproliferative effect of isoimperatorin on
SGC-7901 cells was associated with apoptosis.
Mitochondria-mediated apoptosis is an important
pathway in the induction of cancer cell apoptosis. The
apoptosis-associated proteins, including Bcl-2, Bcl-xl, Mcl-1,
Survivin, Bax, Smac, caspase-3 and caspase-9, are important in the
mitochondria-mediated apoptotic pathway (30,31). When
mitochondria are stimulated by apoptotic signals induced by
anticancer agents, the expression levels and activation of the
apoptosis-associated proteins are regulated. The associations among
these apoptosis-associated proteins are complex in the
mitochondria-mediated apoptotic pathway (30,31).
Firstly, the apoptotic signals stimulate the expression and release
of Smac and cytochrome c from the mitochondria to the
cytoplasm, however, their release is inhibited by Bcl-2, Bcl-xl and
Mcl-1, whereas Bax inhibits the function of Bcl-2, Bcl-xl and Mcl-1
(32). In the present study,
isoimperatorin significantly upregulated the expression level of
Bax and downregulated the expression level of Bcl-2, without
affecting the expression levels of Bcl-xl and Mcl-1, indicating
that isoimperatorin promoted the release of cytochrome c
from the mitochondria to the cytoplasm. The released cytochrome
c promotes the activation of cytochrome c-dependent
caspase-9 and caspase-3 to generate c-caspase-9 and c-caspase-3
(33). In the present study,
isoimperatorin significantly upregulated the expression levels of
c-caspase-9 and c-caspase-3, indicating that isoimperatorin
promoted the activation of caspase-9 and caspase-3. The activation
of caspase-9 and of caspase-3 are inhibited by the inhibitor of
apoptosis protein (IAP), Survivin; however, Smac eliminates
IAP-induced inhibition (34). In the
present study, isoimperatorin significantly downregulated the
expression level of Survivin without affecting the expression level
of Smac, suggesting that isoimperatorin eliminated IAP-induced
inhibition to promote the activation of caspase-9 and caspase-3.
The apoptosis of cancer cells is induced by effective caspases
(c-caspase-3). The results of the western blot analysis (Fig. 4) suggested that isoimperatorin
significantly induced the apoptosis of SGC-7901 cells in
vitro by regulating the expression levels of
mitochondria-mediated apoptosis-associated proteins. In addition,
the results of the xenograft assay (Figs.
5 and 6) indicated that
isoimperatorin exhibited a significant inhibitory effect on
SGC-7901 cell-induced tumor growth without adversely affecting the
body weight increase of nude mice in vivo by regulating the
expression levels of mitochondria-mediated apoptosis-associated
proteins.
In conclusion, the present study revealed that
isoimperatorin may be able to induce the apoptosis of SGC-7901
cells in vitro and in vivo by regulating the
expression levels of mitochondria-mediated apoptosis-associated
proteins. However, further investigations are required to confirm
the pro-apoptotic activity and mechanisms of isoimperatorin on
SGC-7901 cells.
Acknowledgements
This study was supported by The Science and
Technology Planning Project from Ningbo, China (grant no.
2013C50026).
References
|
1
|
Ries LAG, Melbert D, Krapcho M, Stinchcomb
DG, Howlader N, Horner MJ, Mariotto A, Miller BA, Feuer EJ,
Altekruse SF, et al: SEER cancer statistics review, 1975–2005.
Bethesda MD: National Cancer Institute; pp. 1975–2005. 2008,
https://hero.epa.gov/hero/index.cfm/reference/details/reference_id/730406
|
|
2
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Horton R: GBD 2010: Understanding disease,
injury, and risk. Lancet. 380:2053–2054. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yancik R and Ries LA: Cancer in older
persons: An international issue in an aging world. Semin Oncol.
31:128–136. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hu G, Tuomilehto J, Silventoinen K,
Barengo NC, Peltonen M and Jousilahti P: The effects of physical
activity and body mass index on cardiovascular, cancer and
all-cause mortality among 47 212 middle-aged Finnish men and women.
Int J Obesity (Lond). 29:894–902. 2005. View Article : Google Scholar
|
|
6
|
Moreira DM, Aronson WJ, Terris MK, Kane
CJ, Amling CL, Cooperberg MR, Boffetta P and Freedland SJ:
Cigarette smoking is associated with an in increased risk of
biochemical disease recurrence, metastasis, castration-resistant
prostate cancer, and mortality after radical prostatectomy: Results
from the SEARCH database. Cancer. 120:197–204. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Speer AG, Thursfield VJ, Torn-Broers Y and
Jefford M: Pancreatic cancer: Surgical management and outcomes
after 6 years of follow-up. Med J Aust. 196:511–515. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jia L, Ma S, Hou X, Wang X, Qased AB, Sun
X, Liang N, Li H, Yi H, Kong D, et al: The synergistic effects of
traditional Chinese herbs and radiotherapy for cancer treatment.
Oncol Lett. 5:1439–1447. 2013.PubMed/NCBI
|
|
9
|
Romiti A, Cox MC, Sarcina I, Di Rocco R,
D'Antonio C, Barucca V and Marchetti P: Metronomic chemotherapy for
cancer treatment: A decade of clinical studies. Cancer Chemother
Pharmacol. 72:13–33. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li X, Yang G, Li X, Zhang Y, Yang J, Chang
J, Sun X, Zhou X, Guo Y, Xu Y, et al: Traditional Chinese medicine
in cancer care: A review of controlled clinical studies published
in Chinese. PLoS One. 8:e603382013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gopalakrishnan G, Lepetre S, Maksimenko A,
Mura S, Desmaële D and Couvreur P: Lipid-conjugation of endogenous
neuropeptides: Improved biotherapy against human pancreatic cancer.
Adv Healthc Mater. 4:1015–1022. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Vanneman M and Dranoff G: Combing
immunotherapy and target therapies in cancer treatment. Nat Rev
Cancer. 12:237–251. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Duarte S, Carle G, Faneca H, de Lima MC
and Pierrefite-Carle V: Suicide gene therapy in cancer: Where do we
stand now? Cancer Lett. 324:160–170. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Vrbova B and Vrba J: Microwave
thermotherapy in cancer treatment: Evaluation of homogeneity of SAR
distribution. Prog Electromagn Res. 129:181–195. 2012. View Article : Google Scholar
|
|
15
|
Brown SB, Brown EA and Walker I: The
present and future role of photodynamic therapy in cancer
treatment. Lancet Oncol. 5:497–508. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Niu HX, Du T, Xu ZF, Zhang XK and Wang RG:
Role of wild type p53 and double suicide genes in interventional
therapy of liver cancer in rabbits. Acta Cir Bras. 27:522–528.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nobili S, Lippi D, Witort E, Donnini M,
Bausi L, Mini E and Capaccioli S: Natural compounds for cancer
treatment and prevention. Pharmacol Res. 59:365–378. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang MY, Jia MR, Ma YY and Li XB:
Pharmacological effect of four linear furocoumarins in radix
Angelicae dahuricae. Nat Prod Res Dev. 22:485–489. 2010.
|
|
19
|
Kwon YS, Kobayashi A, Kajiyama S, Kawazu
K, Kanzaki H and Kim CM: Antimicrobial constituents of Angelica
dahurica roots. Phytochemistry. 44:887–889. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li ZK, Liu MQ and Yang HJ: The study of
vasoactive of pungent herbs' chemical constituents in Umbelliferae.
Pharmacol Clin Chin Mat Med. 25:38–40. 2009.
|
|
21
|
Okuyama T, Takata M, Nishino H, Nishino A,
Takayasu J and Lwashima A: Studies on the antitumor-promoting
activity of naturally occurring substances. II. Inhibition of
tumor-promoter-enhanced phospholipid metabolism by umbelliferous
materials. Chem Pharm Bull (Tokyo). 38:1084–1086. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang XX: Studies on the antitumor
constituents of Notopterygium incisum. [dissertation]. Hebei Med
Univ; pp. 1–117. 2009
|
|
23
|
Kim YK, Kim YS and Ryu SY:
Antiproliferative effect of furanocoumarins from the root of
Angelica dahurica on cultured human tumor cell lines. Phytother
Res. 21:288–290. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
National Research Council (US) Committee
for the Update of the Guide for the Care and Use of Laboratory
Animals, . Guide for the Care and Use of Laboratory Animals. 8th.
National Academies Press (US); Washington, DC: 2011, PubMed/NCBI
|
|
25
|
Zhao YL, Zhao LJ, Luo YX, Li X, Zhang YF,
Liu XD, Luo YG and Zhong LL: Synergistic effect of radiation and
traditional Chinese medicine Rhizoma Typhonii ethanol extract
depends on p53 expression in treatment of lewis mouse lung cancer
cells. Afr J Tradit Complement Altern Med. 12:109–114. 2015.
View Article : Google Scholar
|
|
26
|
Acquaviva R, Di Giacomo C, Sorrenti V,
Galvano F, Santangelo R, Cardile V, Gangia S, D'Orazio N, Abraham
NG and Vanella L: Antiproliferation effect of oleuropein in
prostate cell lines. Int J Oncol. 41:31–38. 2012.PubMed/NCBI
|
|
27
|
Lee J, Gupta S, Huang JS, Jayathilaka LP
and Lee BS: HPLC-MTT assay: Anticancer activity of aqueous garlic
extract is from allicin. Anal Biochem. 436:187–189. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li W, Li DY, Wang HD, Zheng ZJ, Hu J and
Li ZZ: Juglans regis hexane extract exerts antitumor effect,
apoptosis induction and cell circle arrest in prostate cancer cells
in vitro. Trop J Pharm Res. 14:399–405. 2015. View Article : Google Scholar
|
|
29
|
Zi FM, He JS, Li Y, Wu C, Yang L, Yang Y,
Wang LJ, He DH, Zhao Y, Wu WJ, et al: Metformin displays
anti-myeloma activity and synergistic effect with dexamethasone in
in vitro and in vivo xenograft models. Cancer Lett. 356:443–453.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shi Y: A structure view of
mitochondria-medated apoptosis. Nat Struct Biol. 8:394–401. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang X: The expanding role of mitochondria
in apoptosis. Genes Dev. 15:2922–2933. 2001.PubMed/NCBI
|
|
32
|
Yang XK, Xu MY, Xu GS, Zhang YL and Xu ZX:
In vitro and in vivo antitumor activity of scutebarbatine A on
human lung carcinoma A549 lines. Molecules. 19:8740–8751. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kim J, Parrish AB, Kurokawa M, Matsuura K,
Freel CD, Andersen JL, Johnson CE and Kornbluth S: Rsk-mediated
phosphorylation and 14-3-3ε binding of Apaf-1 suppresses cytochrome
c-induced apoptosis. EMBO J. 31:1279–1292. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Du C, Fang M, Li Y, Li L and Wang X: Smac,
a mitochondrial protein that promotes cytochrome c-dependent
caspase activation by eliminating IAP inhibition. Cell. 102:33–42.
2000. View Article : Google Scholar : PubMed/NCBI
|