Introduction
Malignant peritoneal mesothelioma (MPM) is a rare
disease that typically demonstrates a poor prognosis, with an
average survival time of 6–12 months (1). The incidence in the United States is
200–400 novel cases annually (2).
Although asbestos exposure is the primary risk factor for the
development of MPM, only 30% of reported cases possess a history of
asbestos exposure (3).
The clinical and morphological distinction between
MPM and serous ovarian carcinoma (OC) may be difficult due to their
overlapping morphological features. This is particularly true when
the latter is of low morphological grade and is associated with
diffuse invasive peritoneal implants, or when high-grade serous
carcinoma has metastasized to the pleura (4). Due to this, a number of studies have
attempted to use a variety of immunohistochemical markers to
distinguish between the two diseases (5,6). However,
few immunostaining markers have proven to be sufficiently specific
or sensitive for either type of cancer. Calretinin, Wilms tumor 1
(WT1), D2-40 and mesothelin are expressed in the majority of
mesotheliomas (high sensitivity); however, these markers may
additionally be expressed in a significant subset of serous OC
cases (low specificity) (5).
Alternative markers, including Ber-EP4, human epididymis protein 4,
cluster of differentiation (CD)15 and B72.3, have been demonstrated
to be expressed more frequently in serous OC compared with
mesothelioma; however, poor sensitivity or specificity of these
markers has limited their use as reliable discriminators (5).
Paired box 8 (PAX8) is a member of the paired box
family of transcription factors, and is significant in
organogenesis of the Müllerian system (7). In the Müllerian system, PAX8 is
expressed in a variety of ovarian tumors, particularly serous
carcinoma. Secretory cells of the normal fallopian tube are
positive for PAX8 expression, and these cells are thought to be the
origin of serous OC in a high proportion of cases (7). Previous studies have suggested that PAX8
immunostaining may be useful for differentiating MPM from serous OC
with high specificity and sensitivity (5,6).
A delayed diagnosis of MPM is common due to the long
interval between initial asbestos exposure and the onset of
symptoms (3). Furthermore, the
symptoms, including abdominal pain, ascites and abdominal
distention without abdominal pain, are non-specific. Therefore,
exact diagnosis of MPM is difficult, and it may appear to present
as primary peritoneal carcinoma or OC (3). In the present study, two cases of MPM
that were distinguished from OC by immunostaining for PAX8 are
discussed.
Case report
Case 1
Patient
A 58-year-old (gravida 2, para 2) woman presented
with abdominal distension. The patient had no history of exposure
to asbestos, and no significant past medical or family history. The
serum cancer antigen 125 (CA125) level was 90 U/ml (normal, <35
U/ml). The sialyl-Tn (STn) antigen level was within normal limits,
and the general examination was also normal.
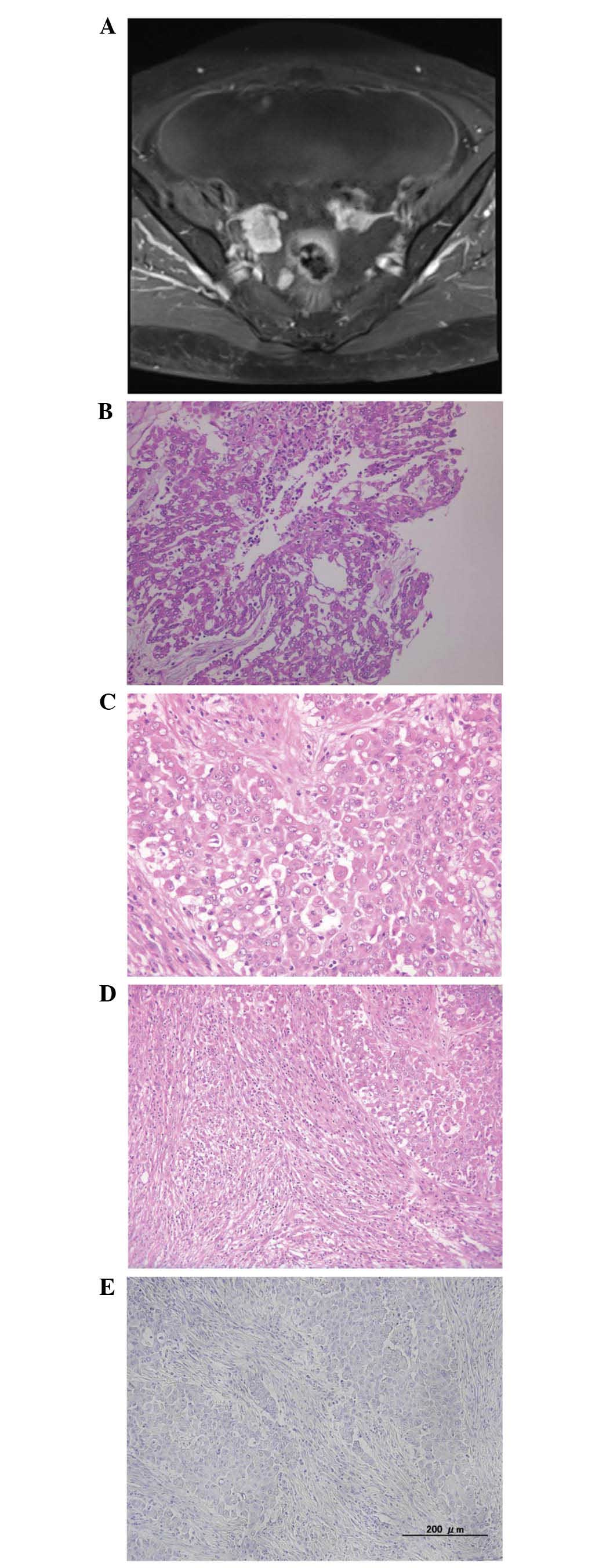
Pelvic magnetic resonance imaging (MRI) revealed
small cysts in both ovaries, and lobular nodules at the surface of
the ovaries (Fig. 1A). MRI
additionally revealed peritonitis carcinomatosa, ascites,
disseminated nodules and metastasis to the omentum.
Contrast-enhanced computed tomography (CT) revealed a number of
small lung nodules, and these findings were considered to represent
metastasis to the lung. No lymphadenopathy was observed. Positron
emission tomography (PET)-CT demonstrated abnormal fludeoxyglucose
(FDG) uptake in the ovarian tumor, disseminated nodules and
omentum. These findings suggested a diagnosis of OC.
A total abdominal hysterectomy (TAH), bilateral
salpingo-oophorectomy (BSO) and omentectomy were performed. At the
conclusion of primary debulking surgery (PDS), the residual tumor
size was <1 cm in diameter. The resected specimens were reviewed
by a pathologist and the mass was subsequently diagnosed as
MPM.
The patient was discharged from hospital on the 14th
postoperative day following an uneventful postoperative period. The
patient was treated with chemotherapy (75 mg/m2
cisplatin and 500 mg/m2 pemetrexed) following PDS and
remains alive without disease progression, 1 year subsequent to the
completion of the first-line chemotherapy.
Pathological findings
Macroscopic examination revealed numerous nodular
lesions in the pelvic cavity. Metastatic findings included
metastasis to the omentum, measuring ~20 cm (omental cake). The
tumor exhibited a number of clusters composed of cuboidal cells
with eosinophilic cytoplasm, forming a tubular, papillary and solid
arrangement (Fig. 1B). The clusters
appeared to include two types of cells (epithelial-like and
sarcomatoid-like cells; Fig. 1C and
D). In the ovarian specimen, a number of clusters that included
epithelial-like cells were observed at the surface of the ovary and
infiltrated into the parenchyma of the ovary. There was no evidence
of malignant cells in the oviduct or the fimbriae.
Immunohistochemical findings
Epithelial-like cells were positive for calretinin
and CAM5.2. Thrombomodulin, D2-40, and CD10 were partially
expressed. These cells were negative for WT1, carcinoembryonic
antigen (CEA), estrogen receptor (ER), progesterone receptor,
Ber-EP4 and PAX8.
Spindle cells were strongly positive for calretinin,
CAM5.2 and vimentin. Thrombomodulin and D2-40 were partially
expressed. These cells were negative for WT1, CEA, Ber-EP4, desmin,
CD10 and PAX8 (Fig. 1E).
Case 2
Patient
A 56-year-old (gravida 1, para 1) woman presented
with a cough. The patient had a history of exposure to asbestos,
and had no significant past medical or family history. Serum CA125,
CA19-9, CEA and STn antigen levels were within normal limits, and
the general examination was additionally normal.
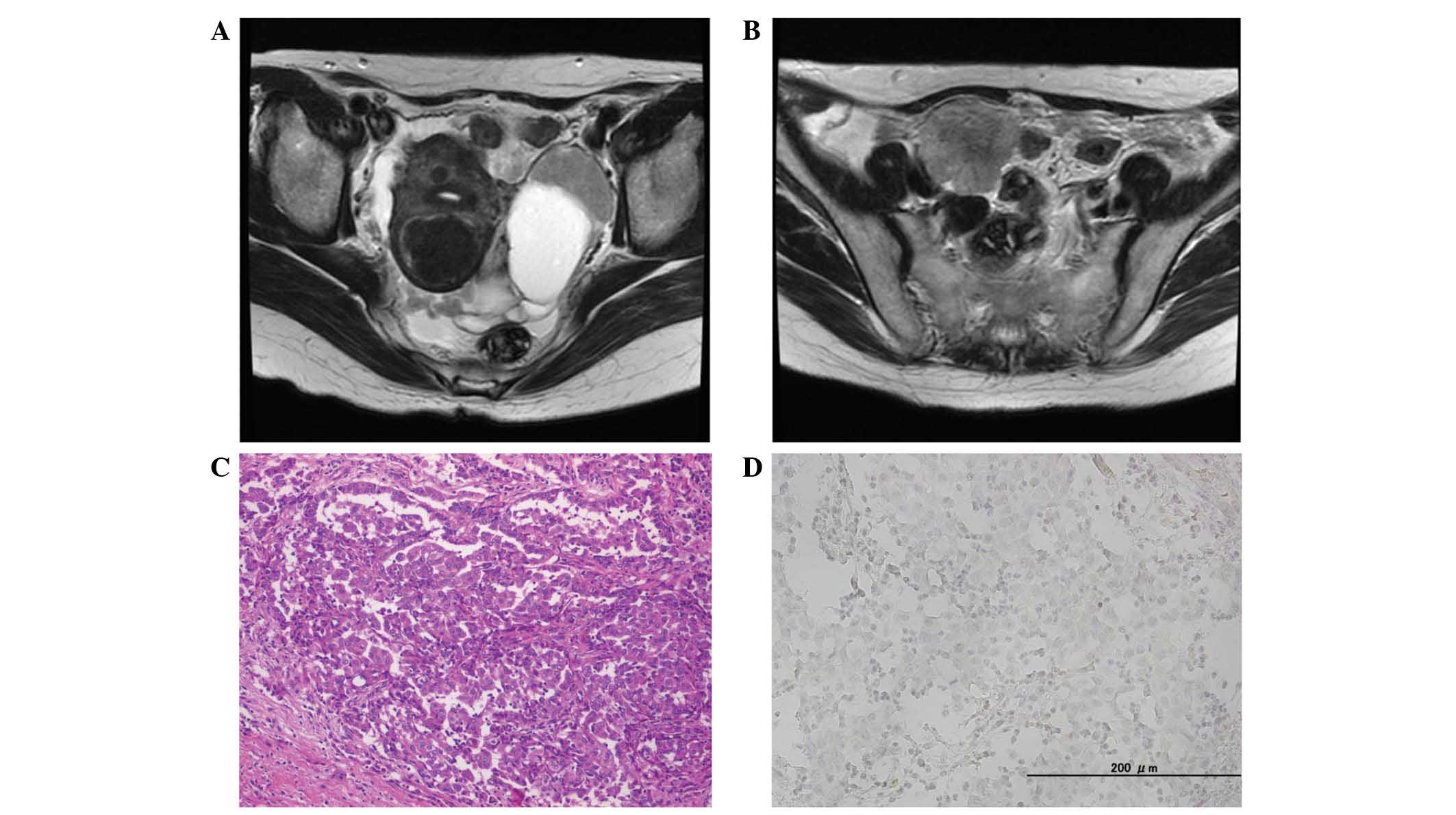
Pelvic MRI revealed a mass with two cysts, measuring
99×42 mm, in the left adnexal region (Fig. 2A), and a solid mass, measuring 46×38
mm in the right adnexal region (Fig.
2B). It additionally revealed peritonitis carcinomatosa,
ascites, disseminated nodules and metastasis to the omentum. No
lymphadenopathy was observed. Contrast-enhanced CT suggested the
possibility of thickened pleura, but there was no indication of
metastasis to the lung. PET-CT revealed abnormal FDG uptake in the
adnexal tumor, but no abnormal uptake in the pleura and lung. These
results suggested a diagnosis of primary OC.
TAH, BSO and omentectomy were performed as the PDS.
At the conclusion of surgery, residual tumors were <1 cm in
diameter. The resected specimens were reviewed by a pathologist and
the mass was subsequently diagnosed as MPM.
The patient was discharged from hospital on day 14
subsequent to surgery, following an uneventful postoperative
period. The patient was treated with chemotherapy (75
mg/m2 cisplatin and 500 mg/m2 pemetrexed)
following surgery, which was well-tolerated. The patient remains
alive without disease progression 3 years subsequent to completion
of first-line chemotherapy.
Pathological findings
Macroscopic examination revealed numerous nodular
lesions in the abdominal cavity, including the ovary, omentum and
ileum. The tumor exhibited a number of clusters composed of
eosinophilic cells, forming a papillary and solid arrangement
(Fig. 2C). Identical findings were
observed in the ovary and ileum specimens.
Immunohistochemical findings
Tumor cells were strongly positive for calretinin,
cytokeratin (CK)7, CK20, D2-40 and CK5/6. CEA was partially
expressed. The cells were negative for WT1, ER, Ber-EP4 and PAX8
expression (Fig. 2D).
Discussion
MPM is a rare malignancy of the peritoneum that
typically remains confined to the abdominal cavity until the
advanced stages of tumor progression. According to the World Health
Organization classification, histological subtypes of MPM include
epithelioid, sarcomatoid and biphasic (mixed epithelioid and
sarcomatoid) (8). Treatment methods
for MPM include cytoreductive surgery and hyperthermic
intraperitoneal chemotherapy; these therapies have resulted in an
improvement in the survival of affected patients, with median
survival times ranging from 29.5 to 94 months (9). All patients provided written informed
consent to be included in the present study.
Patients with MPM do not present with distinctive
symptoms, and the non-specific symptoms commonly observed make
diagnosis and early treatment difficult. MPM is frequently
misdiagnosed as OC. The clinical and morphological distinction
between these malignancies may be difficult due a number of
overlapping morphological features, including papillary structures,
that exist between the two (4).
Imaging is a significant tool for diagnosis.
However, pathological examination of biopsy or resection material
is essential to confirm the diagnosis. In the present cases, it was
not possible to differentiate MPM from OC by imaging alone.
Immunohistochemistry has a significant role in the
distinction between MPM and serous OC. A panel of
immunohistochemical antibodies is used to exclude malignant
mesotheliomas; this panel includes WT1, calretinin, CK5/6 and
BerEP4. An additional panel is typically performed to exclude
adenocarcinoma of unknown origin and includes CK7/CK20, thyroid
transcription factor 1, caudal type homeobox 2, gross cystic
disease fluid protein 15 and WT1 (6).
Although the aforementioned immunomarkers are useful, they have a
number of limitations. Therefore, it is necessary to identify a
marker with high sensitivity and specificity that may be added to
the traditional immunohistochemistry panel of antibodies. PAX8 is a
member of the PAX family of transcription factors, and previous
studies have demonstrated that high levels of PAX8 expression are
specific to serous adenocarcinoma, while all mesotheliomas are
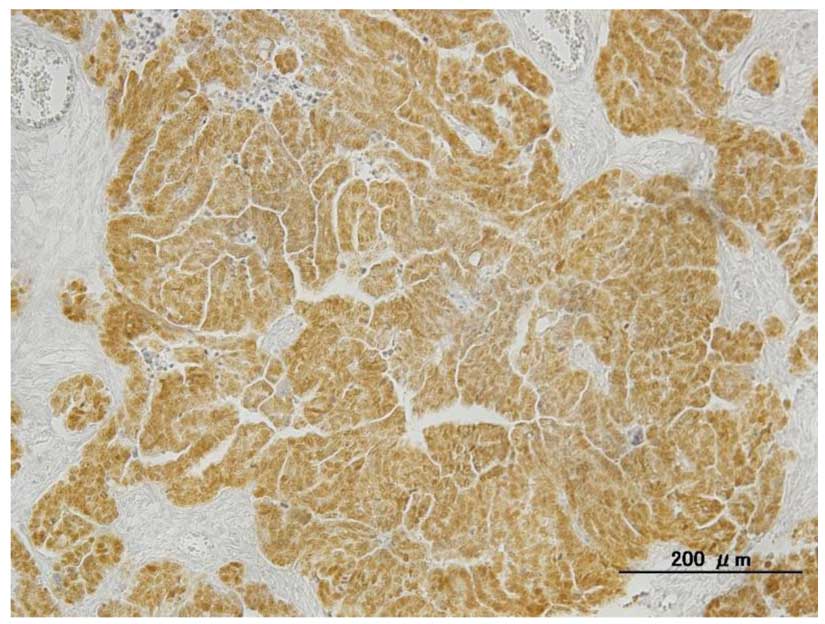
PAX8-negative (5–7). Our group has observed PAX8 nuclear
positivity (Fig. 3) in 65/67 cases of
serous adenocarcinoma (97%) (unpublished data).
In the present two cases, a diagnosis of MPM was
confirmed through immunohistochemical evaluation, which revealed
that both were negative for PAX8. The present immunohistological
analyses were consistent with the aforementioned results regarding
PAX8-negativity in mesotheliomas.
In conclusion, the present cases indicated that PAX8
immunostaining is a useful tool for differentiating MPM from serous
OC, and it is important to consider rare clinical conditions,
including peritoneal mesotheliomas, in patients exhibiting common
and non-specific symptoms, including abdominal pain, ascites and
abdominal distension without abdominal pain. Although MPM is a rare
disease, the possibility of MPM should be considered in patients
presenting with the aforementioned symptoms. In addition, a history
of asbestos exposure is not essential for the disease to occur, and
radiological assessment and traditional immunostaining evaluation
may lead to misdiagnosis as it may be difficult to differentiate
MPM from serous OC. Thus, a thorough comprehensive approach that
includes PAX8 immunostaining is important for achieving a precise
diagnosis and for the correct treatment of patients exhibiting
MPM.
References
|
1
|
Ahmed I, Koulaouzidis I, Iqbal J and Tan
WC: Malignant peritoneal mesothelioma as a rare cause of ascites: A
case report. J Med Case Rep. 2:1212008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Price B and Ware A: Time trend of
mesothelioma incidence in the United States and projection of
future cases: An update based on SEER data for 1973 through 2005.
Crit Rev Toxicol. 39:576–588. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bridda A, Padoan I, Mencarelli R and Frego
M: Peritoneal mesothelioma: A review. MedGenMed.
9:322007.PubMed/NCBI
|
|
4
|
Tangjitgamol S, Warnnissorn M,
Attakettaworn K and Puripat N: Huge peritoneal malignant
mesothelioma mimicking primary ovarian carcinoma. J Med Assoc Thai.
96:107–111. 2013.PubMed/NCBI
|
|
5
|
Laury AR, Hornick JL, Perets R, Krane JF,
Corson J, Drapkin R and Hirsch MS: PAX8 reliably distinguishes
ovarian serous tumors from malignant mesothelioma. Am J Surg
Pathol. 34:627–635. 2010.PubMed/NCBI
|
|
6
|
Ordóñez NG: Value of PAX8, PAX2,
claudin-4, and h-caldesmon immunostaining in distinguishing
peritoneal epithelioid mesotheliomas from serous carcinomas. Mod
Pathol. 26:553–562. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bowen NJ, Logani S, Dickerson EB, Kapa LB,
Akhtar M, Benigno BB and McDonald JF: Emerging roles for PAX8 in
ovarian cancer and endosalpingeal development. Gynecol Oncol.
104:331–337. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lu Z and Chen J: Introduction of WHO
classification of tumours of female reproductive organs, fourth
edition. Zhonghua Bing Li Xue Za Zhi. 42:649–650. 2014.(In
Chinese).
|
|
9
|
Sebbag G, Yan H, Shmookler BM, Chang D and
Sugarbaker PH: Results of treatment of 33 patients with peritoneal
mesothelioma. Br J Surg. 87:1587–1593. 2008. View Article : Google Scholar
|