Introduction
Surgical resection and focal tumor ablation
techniques are the first-line treatments for melanoma and have been
used for decades; the results have been satisfactory in most cases
(1). However, in the majority of
cases, surgery alone is not enough to completely remove or kill all
the tumor cells and recurrence may occur. Therefore, post-surgical
treatment may be as important as the surgery itself. In addition to
radiotherapy and traditional chemotherapy, which are the main
methods currently used to suppress tumor cell activity (2), immunomodulators have potential antitumor
action; previous studies have investigated immunomodulators'
effects on the antitumor immunological response have been
investigated and demonstrated an antitumor effect (3–5).
Panax ginseng is one of the most famous herbal
medicines used in China. It serves an important role in strength
recovery, body nourishing, and health defending in the view of
traditional Chinese medicine. As it has organ-protecting and
spirit-calming effect, panax ginseng is known as ‘herbaceous king’
in China (6). Ginsenoside is the
major compound isolated from panax ginseng and the ginsenoside
family has>30 subtypes. Previous studies have demonstrated that
ginsenoside acts as an immunomodulator in arthritis and other
inflammatory processes (7–9). In addition, ginsenoside was considered
to possess tumor cell-killing functions and to have a suppressive
effect on tumor growth (10–12). Thus, ginsenoside serves a role in
immune modulation and tumor treatment (13,14), which
may indicate its potential triggering effect on antitumor
immunological response. In the present study, a melanoma mouse
model was used to examine ginsenoside Rh2′s triggering effect on
the immunological response.
Materials and methods
Animals, cell lines and medium
Male C57BL6 mice (3–4 weeks old) were purchased from
the laboratory animal research centre of the Fourth Military
Medical University (FMMU), Xi'an, China, and maintained in specific
pathogen-free (SPF) conditions. Animal experimental protocols were
all reviewed and approved by the Ethics Committee of FMMU. The
B16-F10 melanoma cell line was purchased from the American Type
Culture Collection (Manassas, VA, USA). The B16 cell was grown in
RPMI-1640 medium (Hyclone; Thermo Fisher Scientific, Inc., Waltham,
MA, USA) supplemented with 10% fatal calf serum (FBS; Hyclone;
Thermo Fisher Scientific, Inc.), in a humidified incubator
containing 5% CO2. When the cells were ~80% confluent,
the cells were washed with phosphate buffer saline (PBS; pH 7.4) 3
times, then 0.25% trypsin EDTA (Gibco; Thermo Fisher Scientific,
Inc.) was added to passage the cells.
Tumor models
When the B16-F10 melanoma cells reached 90%
confluence, the cells were washed with PBS 3 times, then 0.25%
trypsin EDTA was added for detachment. Following another 3 washes
with PBS, the cells were re-suspended in PBS at the density of
2×107 cells/ml. Under sterile conditions, 50 µl of the
suspension was gently injected into the left back (subcutaneous
tissue) of each mouse. The mice were then contained in SPF
conditions for 5 days, by which point the tumor diameter was ~5–6
mm.
Grouping and ginsenoside
arrangement
The mice were randomly arranged into 4 groups of 80
mice: Tumor group, G-L group, G-H group and Control group. G-L and
G-H refer to a low or high dose of ginsenoside Rh2 injection. For
the tumor group, G-L group and G-H group, the B16-F10 cell line was
injected into the mice as described above. These 3 groups became
tumor bearing groups. For the control group, the same volume of PBS
was injected instead. Ginsenoside Rh2, (National Institute for the
Control of Pharmaceutical and Biological Products, Beijing, China),
was injected into the left back of mice in the G-L and G-H groups.
The dose for the G-H group was 0.5 mg/kg or 0.2 mg/kg for G-L
group, every 2 days after day 5. PBS was injected in the tumor and
control groups at the same time points.
Histological analysis and
immunohistochemistry
Between days 3–12, three mice from each group were
sacrificed and necropsied every 3 days. The tumor sizes were
measured by a caliper and calculated using the formula
[(AxB2) × 0.4]. To evaluate the extent of lymphocyte
infiltration in tumor tissue, the tumors were completely dissected
and analyzed by immunohistochemistry: 6-µm-thick frozen sections of
tumor tissues of the tumor group, G-L group and G-H group were
stained with the rat anti-mouse anti-CD4 (dilution, 1:20; catalog
no., 550280; BD Biosciences, Franklin Lakes, NJ, USA) antibody;
4-µm-thick paraffin sections were stained with the rat anti-mouse
anti-CD8a (dilution, 1:20; catalog no., 550281; BD Biosciences)
antibody. All sections were visualized via a three step staining
procedure in combination with biotinylated polyclonal goat anti-rat
Ig (dilution, 1:500; catalog no., 559286; BD Biosciences) as the
secondary antibody and streptavidin-HRP (BD Biosciences) together
with the diaminobenzidine (Sangon Biotech, Shanghai, China)
detection system. Then the tissue sections were stained with
hematoxylin (ZhuangZhi Biotech, Xi'an, China) for nuclear staining.
The immunohistochemistry results were examined using light
microscopy (Olympus, Tokyo, Japan). Parts of the mice were
maintained for survival analysis.
Cytotoxicity assay
At day 15, 3 mice from each group (tumor group, G-L
group, G-H group and control group) were sacrificed and their
spleens were necropsied. The spleen was ground down and the
lymphocytes were isolated using lymphocyte separation medium
(Sigma-Aldrich, St. Louis, MO, USA) and primarily cultured.
Lymphocytes separated with lymphocyte separation medium were
primarily cultured in RPMI-1640 medium supplemented with 10% FBS as
suspension, and 1 µg/ml penicillin/streptomycin (ZhuangZhi Biotech)
was added in primary culture. All cell flasks were contained in a
humidified incubator containing 5% CO2. Lymphocytes from
the tumor group, G-L group, G-H group and control group were
gathered for cytotoxicity analysis, which was performed using the
CytoTox 96 Non-Radioactive LDH Cytotoxicity Assay (Promega
Corporation, Madison, WI, USA) according to the manufacturer's
protocol.
Adoptive transfers
At day 15, 3 mice from each group were injected with
an additional 2×104 B16-F10 cells with the method
described above. After 2 days, spleen lymphocytes from each group
were isolated and cultured. Then lymphocytes were injected into
recipient naïve mice through tail veins. The recipient naïve mice
receiving lymphocytes from the tumor group, G-L group, G-H group
and control group were named as tumor-trans group, G-L-trans group,
G-H-trans group and control-trans group. These mice were injected
with 1×106 B16-F10 cells 3 days later. Another 15 days
later, these mice were sacrificed and the tumor sizes were
measured. The survival time of the naïve mice were recorded after
B16 challenge for survival analysis.
Statistical analysis
The majority of the experiments were performed in
triplicate and one representative experiment was selected to be
presented. All data are expressed as the mean ± standard deviation.
Data analyses were performed using SPSS software, version 22.0 (IBM
SPSS, Armonk, NY, USA). Significance was determined using Student's
t-test for two groups and one-way ANOVA for multiple comparisons.
The Kaplan-Meier function was calculated for survival and a
log-rank test was used to assess the differences of mice survival.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Antitumor effect of ginsenoside
Rh2
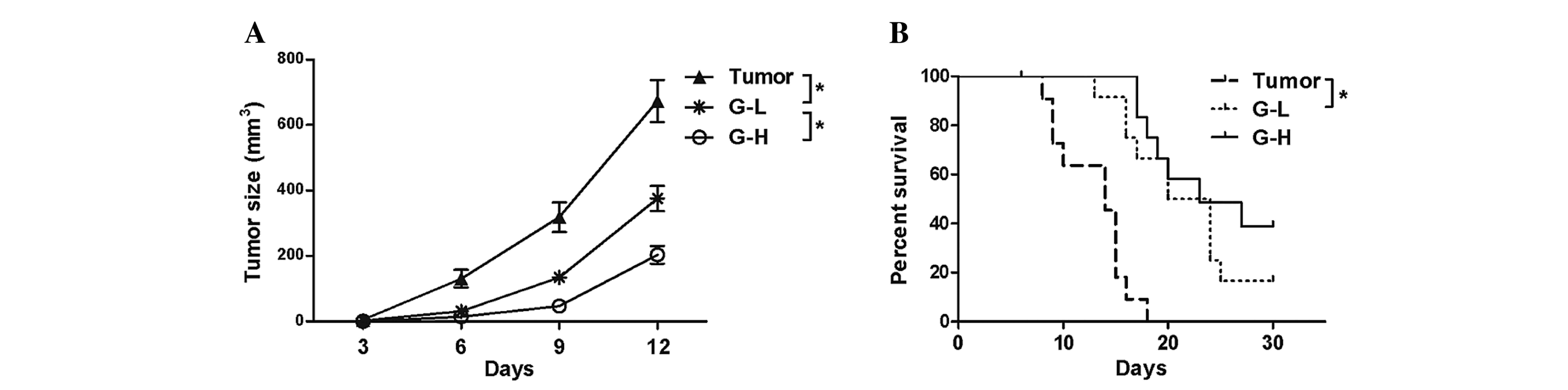
A total of 15 days following B16-F10 cell injection,
tumor sizes from the 3 tumor bearing groups were measured. This
analysis demonstrated that the tumor sizes in the G-L group and G-H
group were reduced compared with the tumor group (Fig. 1A, P<0.05). The survival analysis
revealed that the ginsenoside Rh2 treated groups survived longer
than the untreated tumor group and the effect was dose-dependent
(Fig. 1B, P<0.05), indicating that
ginsenoside Rh2 had served an antitumor role in this mouse
model.
Ginsenoside Rh2 triggers
CD4+ and CD8a+ T-lymphocytes' infiltration in
tumor tissues
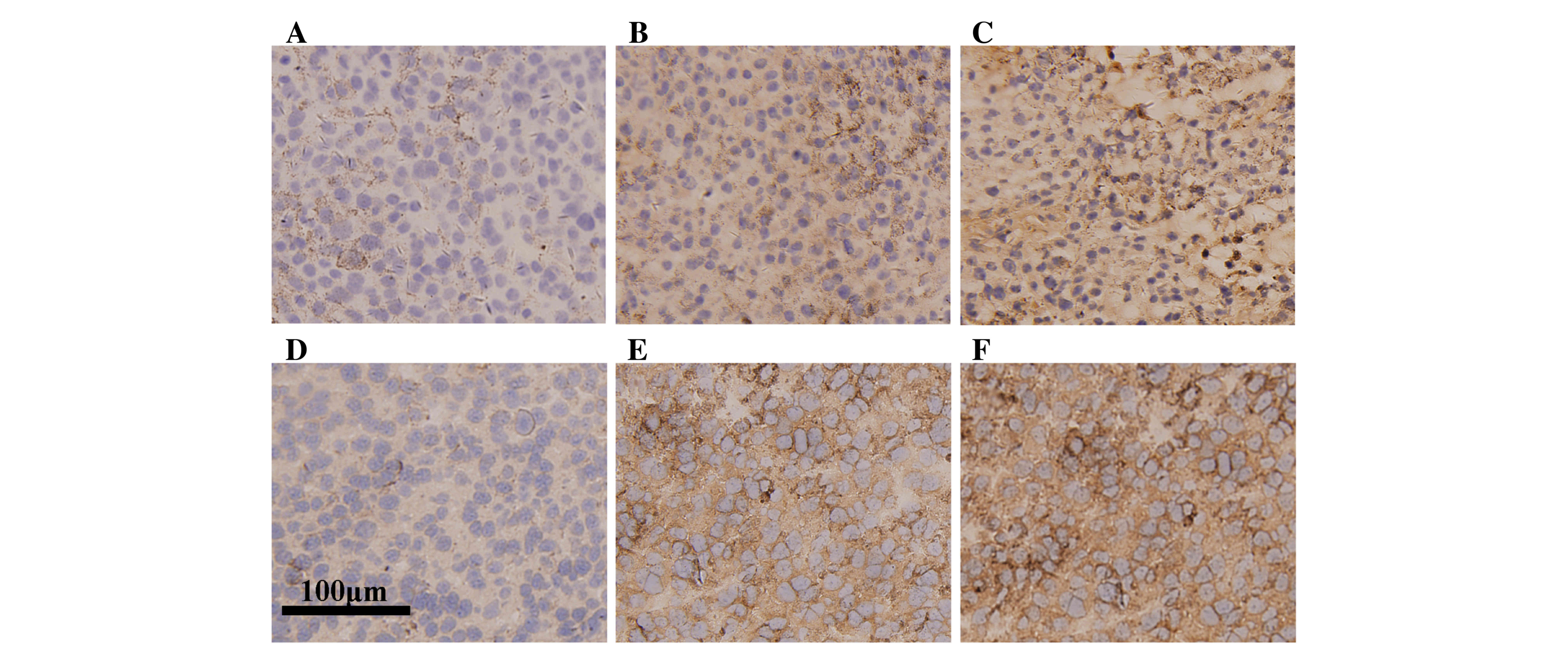
Histologically, the 3 tumor bearing groups exhibited
similar general features in tumor shape, smoothness, hardness and
looked the same at cross section. However immunohistochemical
analysis revealed the tumor group exhibited only sparse
infiltration of CD8a+ lymphocytes (Fig. 2A), compared with an increase in
infiltrative number within the tumors from the G-L and G-H groups
(Fig. 2B and C). In addition, the
staining in G-H group was more intense compared with the G-L group.
Similarly, the tumor group exhibited low infiltration of
CD4+ lymphocytes (Fig.
2D), compared with an increase in infiltrative number within
the tumors from the G-L and G-H groups (Fig. 2E and F). These results indicate that
ginsenoside Rh2 treatment increases lymphocyte infiltration in
tumor tissue, suggesting that the immunological response is
enhanced.
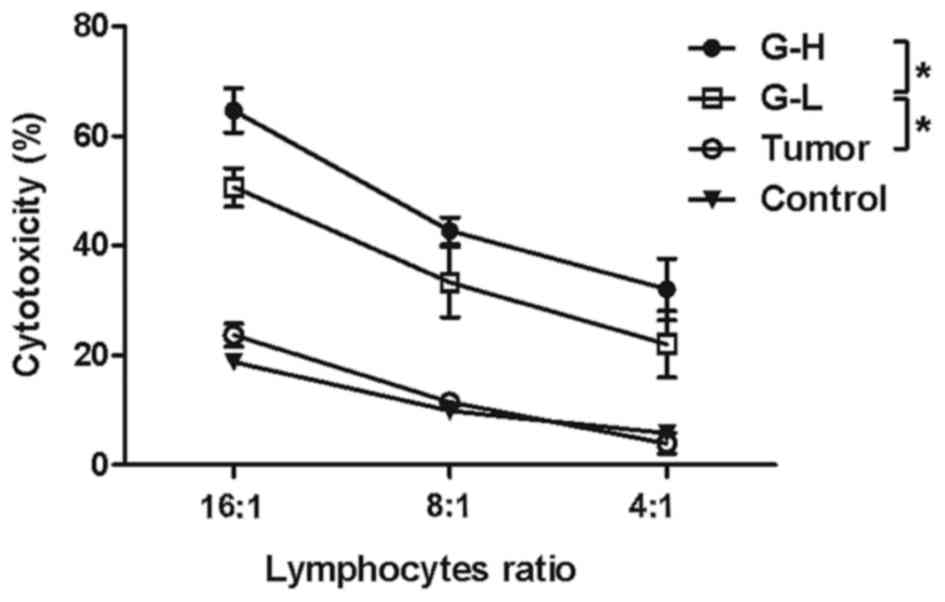
Ginsenoside Rh2 treatment increases
T-lymphocyte cytotoxicity
Immunohistochemistry analysis revealed that a
greater number of CD4+ and CD8a+
T-lymphocytes infiltrated the tumor tissue following ginsenoside
Rh2 injection. To investigate the cytotoxicity of the lymphocytes,
a non-radioactive cytotoxicity assay kit was used to test whether
treatment of ginsenoside Rh2 led to activation of lymphocytes'
killing function. It was observed that ginsenoside Rh2 treatment
did enhance the lymphocytes' cytotoxicity against B16-F10 cells and
this enhancement in cytotoxicity was increased in the G-H group
compared with the G-L group (Fig. 3,
P<0.05).
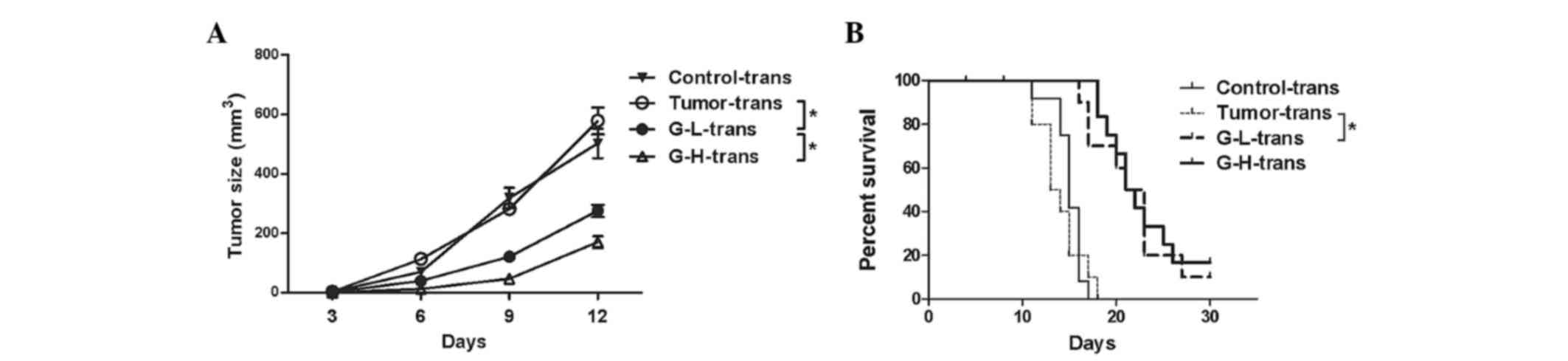
Adoptive transfer of antitumor
immunity in ginsenoside Rh2 arranging groups demonstrated improved
protection
To further demonstrate the antitumor effect of the
ginsenoside Rh2 treated lymphocytes, the present study also
investigated whether this antitumor protection of lymphocytes could
be transferred from ginsenoside Rh2 treated mice to naïve mice.
Delayed tumor growth was observed in recipient naïve mice, which
had received lymphocytes from ginsenoside Rh2 treated groups. The
G-H-trans group exhibited the smallest tumors (Fig. 4A, P<0.05). No delay in tumor growth
was observed in the mice which had received lymphocytes from the
tumor or control group. Survival analysis also indicated a
difference among these groups: Naïve mice which had received
lymphocytes from ginsenoside Rh2 treated groups exhibited improved
survival rates (Fig. 4B, P<0.05).
These results indicate that the antitumor immunity triggered by
ginsenoside Rh2 may be transferred to naïve mice.
Discussion
Surgery resection or tumor ablation techniques are
the primary treatment options for the majority of patients.
However, surgery cannot completely remove the entire tumor tissue
or kill all the malignant cells. Surgery alone will not prevent
recurrence. Therefore, post-surgery therapy is important. Antitumor
immunomodulators have potential as post-surgery treatments
(4). Anti-cytotoxic
T-lymphocyte-associated antigen (CTLA), for example, has been
described to be a tumor rejection promoter, which binds to the
inhibitory T-cell co-receptor CTLA and enhances T-cell functions.
CTLA is located on the surface of T-cells, and acts as an ‘off’
switch when bound to CD80 or CD86 on the surface of
antigen-presenting cells. As a classical immunomodulator, anti-CTLA
has a great antitumor effect on many kinds of tumors (15–17). Apart
from anti-CTLA, IL-2, IFN-gamma, and a number of other
immunomodulators also function in antitumor immunological response
(18).
Ginsenoside is a compound isolated from panax
ginseng, which is popular in China for its nourishing and
protecting effect on human body (19). Ginsenoside Rh2 has been reported to
have an inhibitory effect on prostatic cancer (10), hepatic carcinoma (11), glioblastoma (20) and numerous other malignant tumors
(13,21). In addition to antitumor effects, the
ginsenoside family was also discovered to serve an important role
in immunomodulation. Ginsenoside Rg1 has been demonstrated to
promote immunological response and may enhance T-cell activities
(22,23). Ginsenoside Rh2 and Rd also acted as
immunomodulators in a lot of physiological and pathological
processes (13,24). However, the important relationship
between the antitumor effect and immunological response of
ginsenoside is poorly reported. The present study hypothesized that
ginsenoside Rh2 could enhance antitumor immunological response.
In the present study, ginsenoside Rh2 was injected
into tumor bearing mice. This treatment inhibited the tumor growth
and prolonged mice survival. Also, a greater number of
T-lymphocytes infiltrated the tumor after ginsenoside Rh2
treatment. To investigate the function of the systemic lymphocytes,
cytotoxicity experiments were performed and the lymphocytes from
ginsenoside Rh2 treated groups exhibited enhanced tumor killing
ability. Furthermore, in the adoptive transfer experiment, the
immunity from ginsenoside Rh2 treated mice was successfully
transferred to naïve mice. In the present cytotoxicity experiments,
spleen lymphocytes from ginsenoside Rh2 treated mice were used.
Initially another method was used to trigger an enhanced immune
response using ginsenoside Rh2 treated lymphocytes. Spleen
lymphocytes from the control group were cultured with cell medium
containing ginsenoside Rh2, which one might expect to yield similar
results. However, ginsenoside Rh2 in the cell medium did not
trigger the cytotoxicity of lymphocytes as it did in vivo.
Therefore the triggering effect of ginsenoside Rh2 on immunity may
rely on the microenvironment in vivo, and perhaps
ginsenoside Rh2 does not directly interact with T-lymphocytes
(25). The mechanism between
ginsenoside Rh2 and lymphocytes or antigen presenting cell (APC)
requires further research.
In conclusion, a melanoma mouse model was used to
demonstrate that ginsenoside Rh2 enhanced the antitumor
immunological response Therefore, this Chinese herbal extract may
have potential as an antitumor treatment.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81402574). The
authors would like to thank Miss Xin-Yue Zhao for her assistance in
English language editing.
References
|
1
|
Chu KF and Dupuy DE: Thermal ablation of
tumours: Biological mechanisms and advances in therapy. Nat Rev
Cancer. 14:199–208. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Robinson RD and Knudtson JF: Fertility
preservation in patients receiving chemotherapy or radiotherapy. Mo
Med. 111:434–438. 2014.
|
|
3
|
Killion JJ and Fidler IJ: Therapy of
cancer metastasis by tumoricidal activation of tissue macrophages
using liposome-encapsulated immunomodulators. Pharmacol Ther.
78:141–154. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sheridan C: Industry pursues
co-stimulatory receptor immunomodulators to treat cancer. Nat
Biotechnol. 31:181–183. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Berinstein NL: Enhancing cancer vaccines
with immunomodulators. Vaccine. 25:(Suppl 2). B72–B88. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yun TK: Brief introduction of Panax
ginseng C.A. Meyer. J Korean Med Sci. 16:Suppl. S3–S5. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wu H, Chen J, Wang Q, Jia X, Song S, Yuan
P, Liu K, Liu L, Zhang Y, Zhou A and Wei W: Ginsenoside metabolite
compound K attenuates inflammatory responses of adjuvant-induced
arthritis rats. Immunopharmacol Immunotoxicol. 36:124–129. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu KK, Wang QT, Yang SM, Chen JY, Wu HX
and Wei W: Ginsenoside compound K suppresses the abnormal
activation of T lymphocytes in mice with collagen-induced
arthritis. Acta Pharmacol Sin. 35:599–612. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen J, Wu H, Wang Q, Chang Y, Liu K, Song
S, Yuan P, Fu J, Sun W, Huang Q, et al: Ginsenoside metabolite
compound k alleviates adjuvant-induced arthritis by suppressing T
cell activation. Inflammation. 37:1608–1615. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang Q, Hong B, Wu S and Niu T:
Inhibition of prostatic cancer growth by ginsenoside Rh2. Tumour
Biol. 36:2377–2381. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shi Q, Li J, Feng Z, Zhao L, Luo L, You Z,
Li D, Xia J, Zuo G and Chen D: Effect of ginsenoside Rh2 on the
migratory ability of HepG2 liver carcinoma cells: Recruiting
histone deacetylase and inhibiting activator protein 1
transcription factors. Mol Med Rep. 10:1779–1785. 2014.PubMed/NCBI
|
|
12
|
Tang XP, Tang GD, Fang CY, Liang ZH and
Zhang LY: Effects of ginsenoside Rh2 on growth and migration of
pancreatic cancer cells. World J Gastroenterol. 19:1582–1592. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhou DB, Hu CP, Liang S and Yang HZ:
Effect of ginsenoside Rh2 on immunocompetence of alveolar
macrophages in patients with non-small cell lung cancer. Zhong Nan
Da Xue Xue Bao Yi Xue Ban. 32:868–872. 2007.(In Chinese).
PubMed/NCBI
|
|
14
|
Wu R, Ru Q, Chen L, Ma B and Li C:
Stereospecificity of ginsenoside Rg3 in the promotion of cellular
immunity in hepatoma H22-bearing mice. J Food Sci. 79:H1430–H1435.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Waitz R, Solomon SB, Petre EN, Trumble AE,
Fassò M, Norton L and Allison JP: Potent induction of tumor
immunity by combining tumor cryoablation with anti-CTLA-4 therapy.
Cancer Res. 72:430–439. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li F, Guo Z, Yu H, Zhang X, Si T, Liu C,
Yang X and Qi L: Anti-tumor immunological response induced by
cryoablation and anti-CTLA-4 antibody in an in vivo RM-1 cell
prostate cancer murine model. Neoplasma. 61:659–671. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kvistborg P, Philips D, Kelderman S,
Hageman L, Ottensmeier C, Joseph-Pietras D, Welters MJ, van der
Burg S, Kapiteijn E, Michielin O, et al: Anti-CTLA-4 therapy
broadens the melanoma-reactive CD8+ T cell response. Sci
Transl Med. 6:254ra128. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhao Q, Tong L, He N, Feng G, Leng L, Sun
W, Xu Y, Wang Y, Xiang R and Li Z: IFN-gamma mediates
graft-versus-breast cancer effects via enhancing cytotoxic T
lymphocyte activity. Exp Ther Med. 8:347–354. 2014.PubMed/NCBI
|
|
19
|
Gu Y, Wang GJ, Sun JG, Jia YW, Wang W, Xu
MJ, Lv T, Zheng YT and Sai Y: Pharmacokinetic characterization of
ginsenoside Rh2, an anticancer nutrient from ginseng, in rats and
dogs. Food Chem Toxicol. 47:2257–2268. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li S, Guo W, Gao Y and Liu Y: Ginsenoside
Rh2 inhibits growth of glioblastoma multiforme through mTor. Tumour
Biol. 36:6207–2612. 2015.
|
|
21
|
Li B, Zhao J, Wang CZ, Searle J, He TC,
Yuan CS and Du W: Ginsenoside Rh2 induces apoptosis and
paraptosis-like cell death in colorectal cancer cells through
activation of p53. Cancer Lett. 301:185–192. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lee JH and Han Y: Ginsenoside Rg1 helps
mice resist to disseminated candidiasis by Th1 type differentiation
of CD4+ T cell. Int Immunopharmacol. 6:1424–1430. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lee EJ, Ko E, Lee J, Rho S, Ko S, Shin MK,
Min BI, Hong MC, Kim SY and Bae H: Ginsenoside Rg1 enhances CD4(+)
T-cell activities and modulates Th1/Th2 differentiation. Int
ImmunoPharmacol. 4:235–244. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yang Z, Chen A, Sun H, Ye Y and Fang W:
Ginsenoside Rd elicits Th1 and Th2 immune responses to ovalbumin in
mice. Vaccine. 25:161–169. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang Y, Liu Y, Zhang XY, Xu LH, Ouyang DY,
Liu KP, Pan H, He J and He XH: Ginsenoside Rg1 regulates innate
immune responses in macrophages through differentially modulating
the NF-κB and PI3K/Akt/mTOR pathways. Int Immunopharmacol.
23:77–84. 2014. View Article : Google Scholar : PubMed/NCBI
|