Introduction
Hepatocellular carcinoma (HCC) is a common cancer of
the liver with a typically poor prognosis (1). The current treatment for HCC involves
local ablation, surgical resection, transcatheter arterial
chemoembolization and systemic administration of chemotherapeutic
agents (2,3). Molecular therapy with sorafenib has also
previously been established as a viable therapeutic option for HCC
(4). Sorafenib is a multikinase
inhibitor with a high antitumor efficacy that suppresses cell
proliferation and induces apoptosis in HCC cell lines (5–7). A
limitation of this approach is that HCC cells acquire resistance to
sorafenib (8,9), as it is metabolized by cytochrome P450
(CYP3A4) (10). This has necessitated
the development of alternative therapies for HCC.
Cancer cells have increased glycolysis levels,
requiring more glucose under insufficient oxygen supply (Warburg
effect), compared with surrounding normal tissues (11). Glucose is essential for cell survival
(12,13). Galactose is metabolized to
galactose-1-phosphate with galactokinase, and enters the glycolysis
cycle (14). Arginine is an essential
amino acid that is produced from ornithine by ornithine
carbamoyltransferase in the urea cycle (15). Normal hepatocytes express
galactokinase and ornithine carbamoyltransferase (16,17).
Normal hepatocytes are expected to survive in a medium lacking
glucose and arginine but supplemented with galactose and ornithine
(18,19). In our previous study, a hepatocyte
selection medium (HSM) was developed, which lacks glucose and
arginine but contains galactose and ornithine (20). Primary human hepatocytes are able to
survive in HSM; thus, this medium selects primary human hepatocytes
from a co-culture with human-induced pluripotent stem cells
(21).
Cancer stem cells are characterized as
self-renewing, proliferative, tumorigenic (exhibiting stemness) and
chemoresistant (22). The sphere
formation assay was previously reported to enrich undifferentiated
neural precursor cells (23,24); however, several types of human cancer
cells have been demonstrated to form spheres (25,26). HCC
cells (PLC/PRF/5 cells), human breast cancer cells (MCF7 cells),
glioma cells (U87) and non-small lung cancer cells (A549) form
spheres when cultured in serum-free conditions supplemented with
epidermal growth factor, at a density of 1×103 cells/ml.
Notably, the expression levels of certain stem cell markers
increase, including Oct3/4, CD133, and CD44 (26). These reports suggest that cancer cells
with stem cell character are enriched in a sphere formation
assay.
In the present study, HCC cells were cultured for a
sphere formation assay to obtain stem cell character in HCC cells.
The resultant cells were cultured in HSM to investigate the
potential application to treatment of HCC cells with stem cell
character.
Materials and methods
Cell culture
HLF and PLC/PRF/5 human HCC cells were purchased
from the Riken Cell Bank (RIKEN BioResource Center, Tsukuba,
Japan). HLF cells and PLC/PRF/5 cells were cultured in Dulbecco's
modified Eagle's medium (DMEM; Sigma-Aldrich; Merck Millipore,
Darmstadt, Germany) supplemented with 10% fetal bovine serum (FBS;
Thermo Fisher Scientific, Inc., Waltham, MA, USA). The cell lines
were cultured in 10-cm dishes (Asahi Techno Glass; Funabashi,
Japan) with 5% CO2 at 37°C in a humidified chamber.
Sphere formation assay
HLF and PLC/PRF/5 cells were trypsinized and
cultured in DMEM-F12 (Sigma-Aldrich; Merck Millipore) supplemented
with epidermal growth factor (Wako Pure Chemical Industries, Ltd.,
Osaka, Japan) at 20 ng/ml, basic fibroblast growth factor (Wako
Pure Chemical Industries, Ltd.) at 20 ng/ml, 1% B27 supplement
(Thermo Fisher Scientific, Inc.) and 1% methylcellulose 25 (Wako
Pure Chemical Industries, Ltd.) in Ultra-Low Attachment 6-well
plates (Corning Incorporated, Corning, NY, USA). The cells were
imaged under a microscope (Olympus Corporation, Tokyo, Japan) after
7 and 14 days of incubation.
Preparation of HSM
HSM was prepared from amino acid powders using the
formulation of Leibovitz L-15 medium (Thermo Fisher Scientific,
Inc.), omitting arginine, tyrosine, glucose and sodium pyruvate,
but adding galactose (900 mg/l; Wako Pure Chemical Industries,
Ltd.), ornithine (1 mM; Wako Pure Chemical Industries, Ltd.),
glycerol (5 mM; Wako Pure Chemical Industries, Ltd.) and proline
(260 mM; Wako Pure Chemical Industries, Ltd.). Proline (30 mg/l)
was included in the medium as it is necessary for DNA synthesis
(27). Knockout serum replacement
(Thermo Fisher Scientific, Inc.) at a final concentration of 10%
was used in place of FBS to establish xeno-free conditions.
PRL/PRF/5 cells were subjected to a sphere formation assay. The
spheres were transferred to a tube, trypsinized, and seeded onto
96-well flat-bottom plates (Asahi Techno Glass) at the density of
1,000 cells/well. After a 24-h culture in DMEM, HSM was applied,
and subjected to a cell proliferation assay. PRL/PRF/5 cells
cultured in DMEM in conventional 6-well plates (Asahi Techno Glass)
were used as a control.
Application of sorafenib
PRL/PRF/5 cells were analyzed to determine whether
cell proliferation was suppressed by sorafenib. Following sphere
formation, the cells were seeded to 96-well flat-bottom plates at a
density of 1,000 cells/well and incubated for 24 h in DMEM
supplemented with 10% FBS. Sorafenib (JS Research Chemicals Trading
e.Kfm, Wedel, Germany) was added to the media at concentrations of
0, 1, 3, 10 and 30 µM. PRL/PRF/5 cells cultured in DMEM in
conventional 6-well plates (Asahi Techno Glass) were used as a
control.
Cell proliferation analysis
The cells were cultured for 72 h and subjected to an
MTS assay (Promega Corporation, Madison, WI, USA) according to the
manufacturer's instructions. The cells reduce MTS to a colored
formazan product that has a maximum absorbance at 490 nm. The
absorbance was measured using an iMark Microplate Absorbance Reader
(Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA (5 mg) was isolated using Isogen (Nippon
Gene, Co, Ltd., Tokyo, Japan) and used for the first-strand cDNA
synthesis with SuperScript III and oligo-dT (Thermo Fisher
Scientific, Inc.), according to the manufacturer's instructions.
RT-qPCR was performed with Fast SYBR Green Master Mix (Thermo
Fisher Scientific, Inc.), and the results were analyzed using the
MiniOpticon system (Bio-Rad Laboratories, Inc.). RT-qPCR was
performed for a total of 40 cycles, with 5 sec of denaturation and
5 sec of annealing-extension. The RT-qPCR primer pairs for
ribosomal protein L19 (RPL19) and CYP3A4 were
5′-CGAATGCCAGAGAAGGTCAC-3′ (forward) and 5′-CCATGAGAATCCGCTTGTTT-3′
(reverse) (GenBank, BC000530; expected product size, 157 bp),
5′-TGAGAAATCTGAGGCGGGAAGC-3′ (forward) and
5′-CGATGTTCACTCCAAATGATGTGC-3′ (reverse) (GenBank, J04449; expected
product size, 111 bp), respectively. RPL19 was used as an internal
control, as the target gene was a constitutively expressed
housekeeping gene (28). The primer
sequences were obtained with MacVector 14.0.6 (MacVector Inc.,
Apex, NC, USA).
Statistical analysis
The data were presented as the mean ± standard
deviation. The data were analyzed by one-way analysis of variance
using JMP 10.0.2 software (SAS Institute, Cary, NC, USA). P<0.05
was considered to indicate a statistically significant result.
Results
Sphere formation
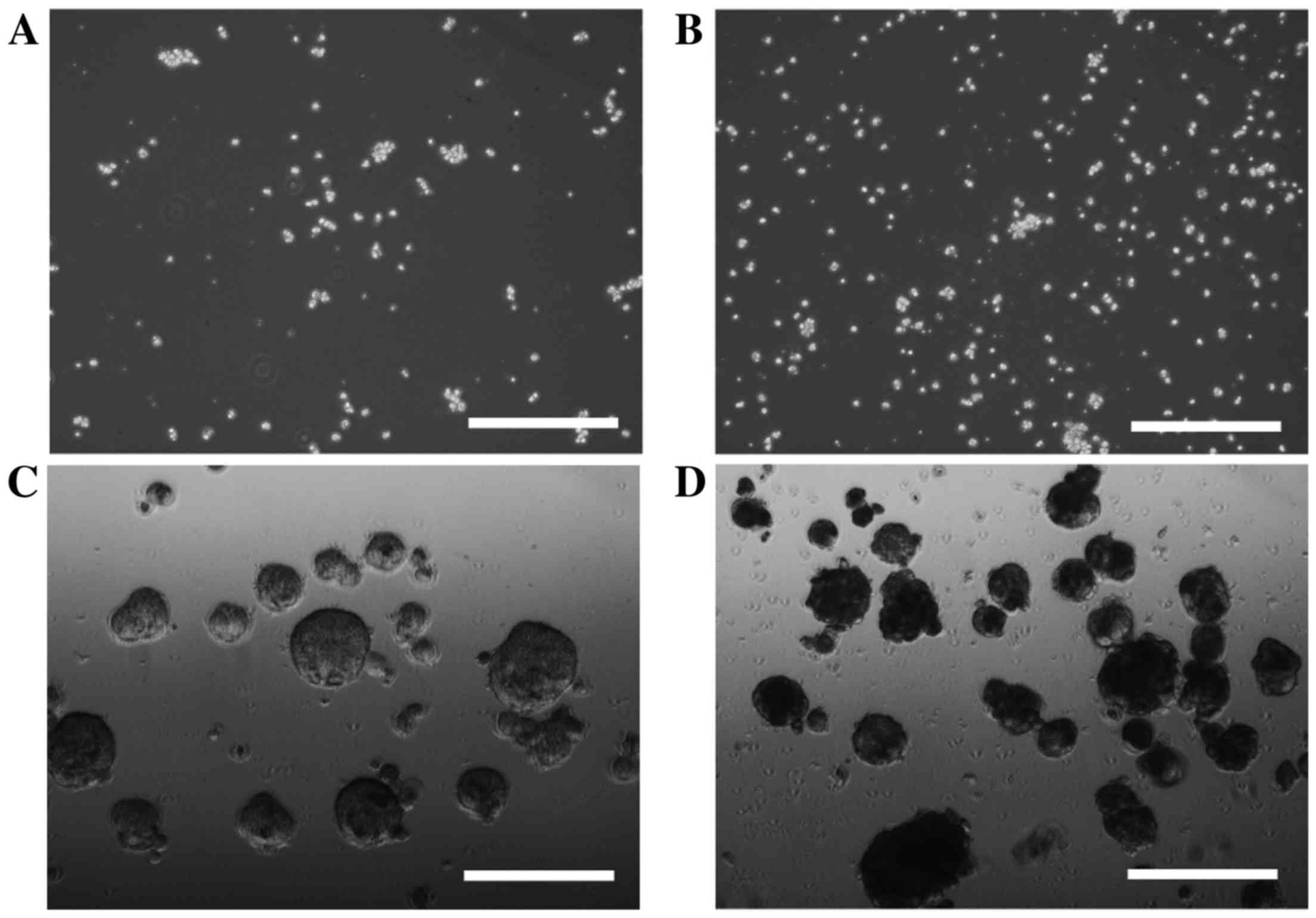
In order to investigate cell sphere formation, HLF
cells and PLC/PRF/5 cells were cultured for sphere formation for 7
or 14 days. HLF cells did not form spheres after 7 days (Fig. 1A) or 14 days (Fig. 1B) of incubation; however, sphere
formation was observed in PLC/PRF/5 cells after 7 days (Fig. 1C) and 14 days (Fig. 1D) of incubation. PRL/PRF/5 cells were
used for further investigation.
Sorafenib
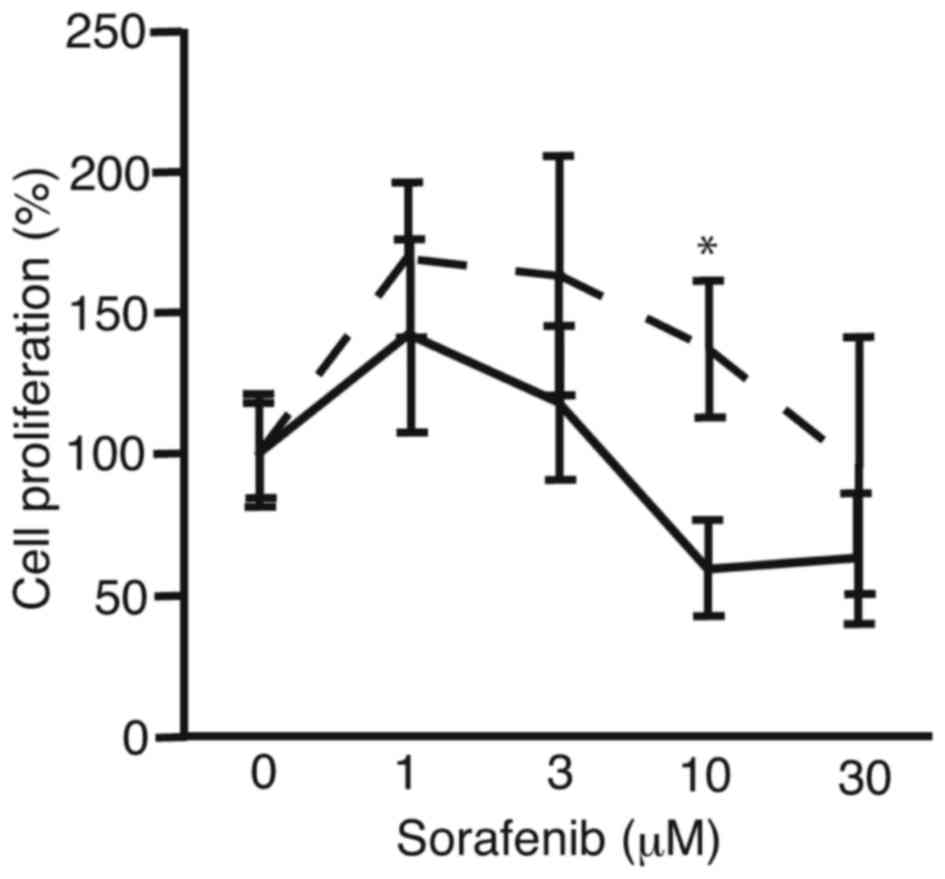
In order to analyze the suppression of proliferation
of PRL/PRF/5 cells after sphere formation, the cells were subjected
to cell proliferation assay (Fig. 2).
PRL/PRF/5 cells cultured in conventional 6-well plates were used
for control. There was a tendency for sorafenib to be more
tolerated by the PRL/PRF/5 cells following sphere formation than
the control cells. At 10 µM, cell proliferation of sphere-formed
cells and control cells were 137±24 and 59±17%, respectively
(P<0.0036). The results suggest the PLC/PRF/5 cells that form
spheres are more resistant to sorafenib compared with cells
cultured in 6-well plates, which did not form spheres.
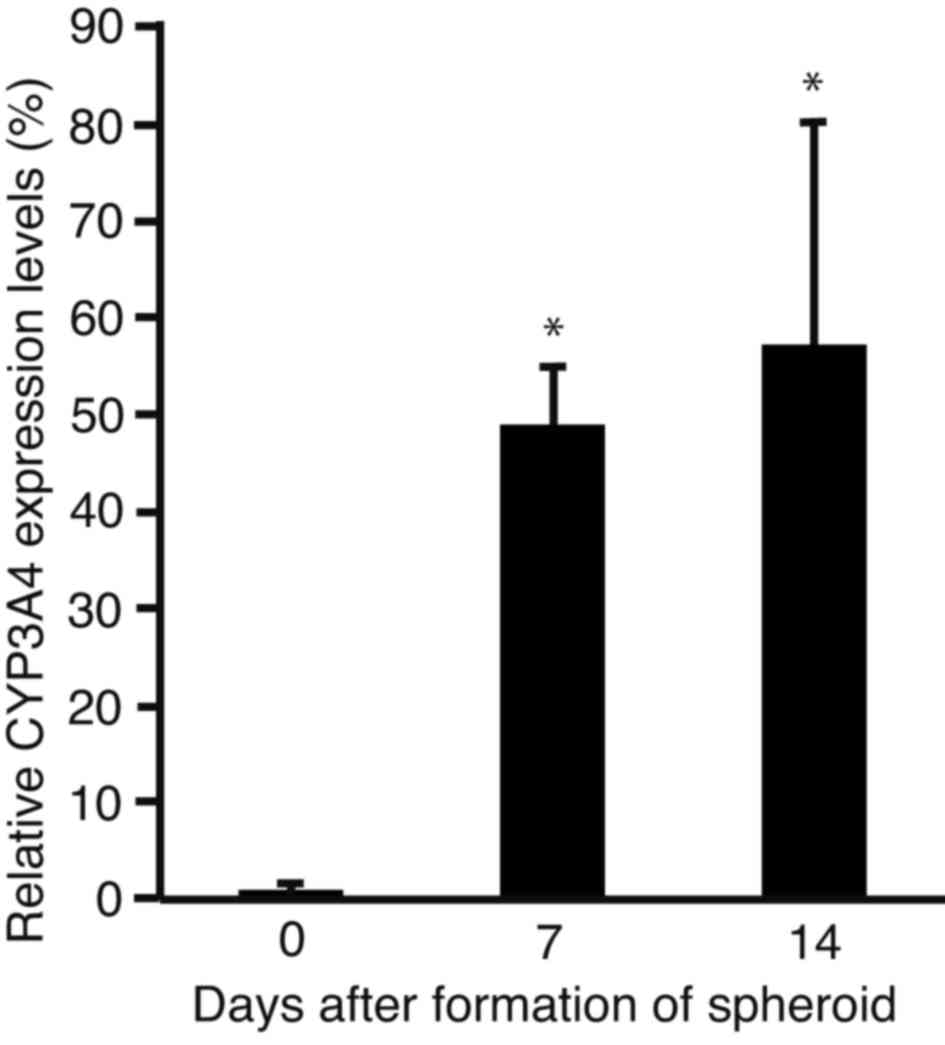
Expression levels of CYP3A4
In order to analyze changes in the expression levels
of CYP3A4, the sphere-forming PLC/PRF/5 cells were subjected to
RT-qPCR (Fig. 3). The expression
levels of CYP3A4 were significantly increased after 7 (P<0.0001)
and 14 days (P<0.001) of incubation, as compared with day 0.
Suppression of cell proliferation with
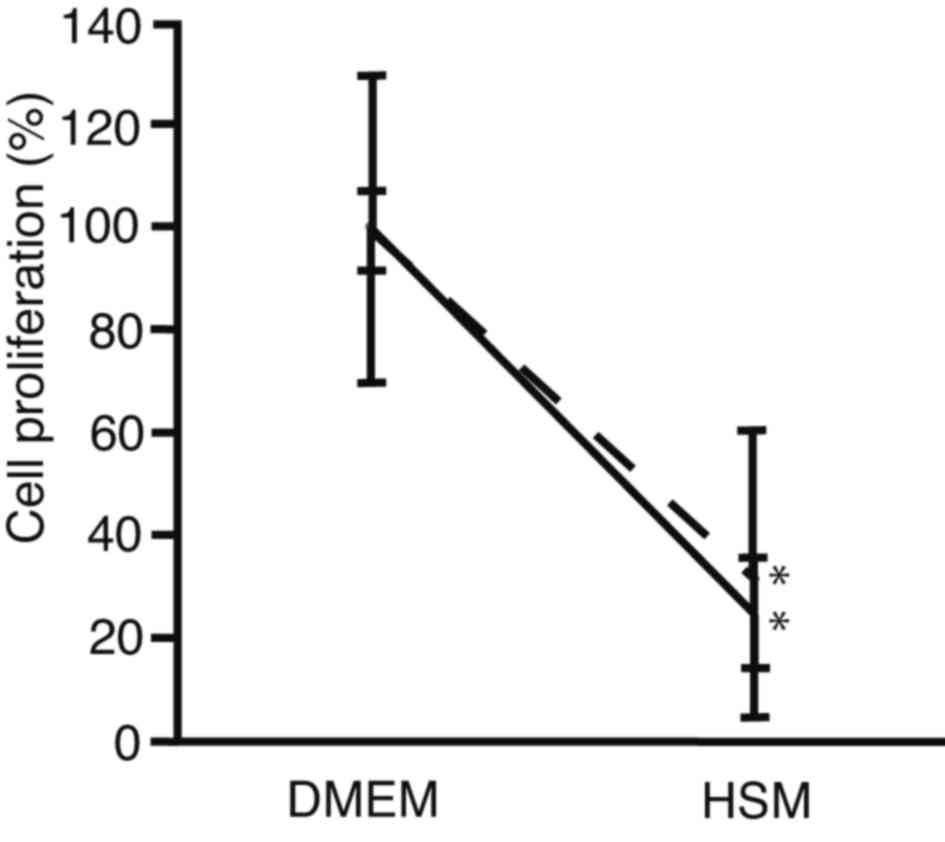
HSM
In order to compare the suppression of cell
proliferation cells between after sphere formation and conventional
culture, PRL/PRF/5 cells were seeded onto 96-well plates. The
medium was changed to HSM, and subjected to cell proliferation
assay after 72 h culture (Fig. 4).
For cells cultured using a conventional method, cell proliferation
decreased to 25±11% in HSM, as compared with DMEM (P<0.0001).
For cells analyzed following sphere formation, it was observed that
cell proliferation decreased to 32±28% in HSM, as compared with
DMEM (P=0.0011). There was no statistically significant difference
in cell proliferation between cells analyzed following sphere
formation and those cultured in HSM (P=0.7100). These results
suggest that HSM suppressed cell proliferation of PRL/PRF/5 cells
following sphere formation at the same levels as conventional
culture. The HSM created in the present study, which did not
contain glucose or arginine, was demonstrated to suppress
proliferation equally in PLC/PRF/5 cell spheres and in control
cells.
Discussion
PLC/PRF/5 cell spheres exhibit stemness and
resistance to fluorouracil, mitomycin C, and sorafenib (26). In the present study, sphere-forming
PLC/PRF/5 cells were seeded onto 96-well plates to evaluate their
sensitivity to sorafenib using a proliferation assay. Suppression
of cell proliferation with sorafenib was decreased in the cells
following sphere formation, as compared with control cells. These
results were consistent with those of a previous report (26). The previous results and the data of
the present study demonstrated that PLC/PRF/5 cells were more
resistance to sorafenib due to their stemness following sphere
formation. The mechanism of resistance was subsequently
investigated.
Sorafenib is metabolized by CYP3A4 (10). In the present study, the expression
levels of CYP3A4 were observed to increase during sphere formation.
The results indicate that PLC/PRF/5 cells acquired resistance to
sorafenib due to the increased expression levels of CYP3A4. Patient
survival would be poor with cancer stem cells due to the resistance
to chemotherapeutic agents, such as sorafenib (29). Cancer stem cells are, therefore, one
of the targets of treatment (30).
Cancer cells require more glucose than normal cells
due to the Warburg effect (11).
Therefore, glucose metabolism is a potential therapeutic target for
the treatment of HCC. The results suggest that the sphere-forming
PLC/PRF/5 cells did not acquire resistance to glucose deprivation.
Glucose metabolism, therefore, represents a potential target for
the treatment of HCC, with a low risk of acquired resistance.
2-Deoxyglucose (2DG) is an analog of glucose that
recapitulates the effect of glucose-deprivation on cancer cells
(31). The anticancer effects of 2DG
have been reported as limited (32);
however, the administration of 2DG in combination with other
anticancer agents may be a potentially effective strategy for the
treatment of certain types of cancer, including HCC. The results
suggest that the deprivation of glucose and arginine may be an
effective approach for the treatment of HCC. Furthermore, the
efficacy of arginase, which metabolizes arginine, has been
previously demonstrated in HCC (33).
The combination of 2DG and arginase must be investigated in further
studies as a potential strategy for the treatment of HCC.
In conclusion, PLC/PRF/5 cells acquired resistance
to sorafenib following sphere formation, suggesting stemness. Cell
proliferation of PRL/PRF/5 cells was suppressed with HSM.
Therefore, the deprivation of glucose and arginine may serve as an
effective potential treatment for HCC.
References
|
1
|
Trojan J, Zangos S and Schnitzbauer AA:
Diagnostics and treatment of hepatocellular carcinoma in 2016:
Standards and developments. Visc Med. 32:116–120. 2016.PubMed/NCBI
|
|
2
|
Lencioni R, Petruzzi P and Crocetti L:
Chemoembolization of hepatocellular carcinoma. Semin Intervent
Radiol. 30:3–11. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kim HY and Park JW: Clinical trials of
combined molecular targeted therapy and locoregional therapy in
hepatocellular carcinoma: Past, present, and future. Liver Cancer.
3:9–17. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Furuse J, Ishii H, Nakachi K, Suzuki E,
Shimizu S and Nakajima K: Phase I study of sorafenib in Japanese
patients with hepatocellular carcinoma. Cancer Sci. 99:159–165.
2008.PubMed/NCBI
|
|
5
|
Wilhelm SM, Carter C, Tang L, Wilkie D,
McNabola A, Rong H, Chen C, Zhang X, Vincent P, McHugh M, et al:
BAY 43–9006 exhibits broad spectrum oral antitumor activity and
targets the RAF/MEK/ERK pathway and receptor tyrosine kinases
involved in tumor progression and angiogenesis. Cancer Res.
64:7099–7109. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu L, Cao Y, Chen C, Zhang X, McNabola A,
Wilkie D, Wilhelm S, Lynch M and Carter C: Sorafenib blocks the
RAF/MEK/ERK pathway, inhibits tumor angiogenesis, and induces tumor
cell apoptosis in hepatocellular carcinoma model PLC/PRF/5. Cancer
Res. 66:11851–11858. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tomizawa M, Shinozaki F, Sugiyama T,
Yamamoto S, Sueishi M and Yoshida T: Sorafenib suppresses the cell
cycle and induces the apoptosis of hepatocellular carcinoma cell
lines in serum-free media. Exp Ther Med. 1:863–866. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Okuyama H, Ikeda M, Kuwahara A, Takahashi
H, Ohno I, Shimizu S, Mitsunaga S, Senda S and Okusaka T:
Prognostic factors in patients with hepatocellular carcinoma
refractory or intolerant to sorafenib. Oncology. 88:241–246. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen J, Jin R, Zhao J, Liu J, Ying H, Yan
H, Zhou S, Liang Y, Huang D, Liang X, et al: Potential molecular,
cellular and microenvironmental mechanism of sorafenib resistance
in hepatocellular carcinoma. Cancer Lett. 367:1–11. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ye L, Yang X, Guo E, Chen W, Lu L, Wang Y,
Peng X, Yan T, Zhou F and Liu Z: Sorafenib metabolism is
significantly altered in the liver tumor tissue of hepatocellular
carcinoma patient. PLoS One. 9:e966642014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Vaitheesvaran B, Xu J, Yee J, Q-Y L, Go
VL, Xiao GG and Lee WN: The Warburg effect: A balance of flux
analysis. Metabolomics. 11:787–796. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Leffert HL and Paul D: Studies on primary
cultures of differentiated fetal liver cells. J Cell Biol.
52:559–568. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Matsumoto K, Yamada K, Kohmura E,
Kinoshita A and Hayakawa T: Role of pyruvate in ischaemia-like
conditions on cultured neurons. Neurol Res. 16:460–464. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Holden HM, Thoden JB, Timson DJ and Reece
RJ: Galactokinase: Structure, function and role in type II
galactosemia. Cell Mol Life Sci. 61:2471–2484. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wheatley DN, Scott L, Lamb J and Smith S:
Single amino acid (arginine) restriction: Growth and death of
cultured HeLa and human diploid fibroblasts. Cell Physiol Biochem.
10:37–55. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ohira RH, Dipple KM, Zhang YH and McCabe
ER: Human and murine glycerol kinase: Influence of exon 18
alternative splicing on function. Biochem Biophys Res Commun.
331:239–246. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ai Y, Jenkins NA, Copeland NG, Gilbert DH,
Bergsma DJ and Stambolian D: Mouse galactokinase: Isolation,
characterization, and location on chromosome 11. Genome Res.
5:53–59. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Phillips JW, Jones ME and Berry MN:
Implications of the simultaneous occurrence of hepatic glycolysis
from glucose and gluconeogenesis from glycerol. Eur J Biochem.
269:792–797. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sumida KD, Crandall SC, Chadha PL and
Qureshi T: Hepatic gluconeogenic capacity from various precursors
in young versus old rats. Metabolism. 51:876–880. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tomizawa M, Toyama Y, Ito C, Toshimori K,
Iwase K, Takiguchi M, Saisho H and Yokosuka O: Hepatoblast-like
cells enriched from mouse embryonic stem cells in medium without
glucose, pyruvate, arginine, and tyrosine. Cell Tissue Res.
333:17–27. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tomizawa M, Shinozaki F, Sugiyama T,
Yamamoto S, Sueishi M and Yoshida T: Survival of primary human
hepatocytes and death of induced pluripotent stem cells in media
lacking glucose and arginine. PLoS One. 8:e718972013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ma S, Chan KW, Hu L, Lee TK, Wo JY, Ng IO,
Zheng BJ and Guan XY: Identification and characterization of
tumorigenic liver cancer stem/progenitor cells. Gastroenterology.
132:2542–2556. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Reynolds BA and Weiss S: Clonal and
population analyses demonstrate that an EGF-responsive mammalian
embryonic CNS precursor is a stem cell. Dev Biol. 175:1–13. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rappa G, Mercapide J, Anzanello F,
Prasmickaite L, Xi Y, Ju J, Fodstad O and Lorico A: Growth of
cancer cell lines under stem cell-like conditions has the potential
to unveil therapeutic targets. Exp Cell Res. 314:2110–2122. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kim J, Jung J, Lee SJ, Lee JS and Park MJ:
Cancer stem-like cells persist in established cell lines through
autocrine activation of EGFR signaling. Oncol Lett. 3:607–612.
2012.PubMed/NCBI
|
|
26
|
Cao L, Zhou Y, Zhai B, Liao J, Xu W, Zhang
R, Li J, Zhang Y, Chen L, Qian H, et al: Sphere-forming cell
subpopulations with cancer stem cell properties in human hepatoma
cell lines. BMC Gastroenterol. 11:712011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nakamura T, Teramoto H, Tomita Y and
Ichihara A: L-proline is an essential amino acid for hepatocyte
growth in culture. Biochem Biophys Res Commun. 122:884–891. 1984.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Davies B and Fried M: The L19 ribosomal
protein gene (RPL19): Gene organization, chromosomal mapping, and
novel promoter region. Genomics. 25:372–380. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Olszewski U, Liedauer R, Ausch C,
Thalhammer T and Hamilton G: Overexpression of CYP3A4 in a COLO 205
Colon Cancer Stem Cell Model in vitro. Cancers (Basel).
3:1467–1479. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Pang RW and Poon RT: Cancer stem cell as a
potential therapeutic target in hepatocellular carcinoma. Curr
Cancer Drug Targets. 12:1081–1094. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Simons AL, Mattson DM, Dornfeld K and
Spitz DR: Glucose deprivation-induced metabolic oxidative stress
and cancer therapy. J Cancer Res Ther. 5:(Suppl 1). S2–S6. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang D, Li J, Wang F, Hu J, Wang S and
Sun Y: 2-Deoxy-D-glucose targeting of glucose metabolism in cancer
cells as a potential therapy. Cancer Lett. 355:176–183. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Phillips MM, Sheaff MT and Szlosarek PW:
Targeting arginine-dependent cancers with arginine-degrading
enzymes: Opportunities and challenges. Cancer Res Treat.
45:251–262. 2013. View Article : Google Scholar : PubMed/NCBI
|