Introduction
Frizzled (Fz)-2 is a receptor of the Wnt signaling
pathway (1,2). Fz-2 is expressed in gastric cancer
tissues, and the proliferation and motility of gastric cancer cells
have been shown to be suppressed by the short-hairpin RNA of Fz-2
(shRNA-Fz2) (3). The introduction of
shRNA-Fz2 is therefore considered a novel target of molecular
therapy, and methods of introducing shRNA-Fz2 must therefore be
developed.
Sonoporation is a method of gene transfer to cells
using ultrasonography (US) (4–7).
Microbubbles grow and collapse when irradiated with US (8,9), and
during sonoporation, H2O2 is produced in the
medium (10). This free radical
production can be measured using the starch-iodide method (11), by which free radicals are quantified
when combined with potassium iodide (KI) (12).
When microbubbles collapse, the cell membrane is
perturbed and a pore is formed, allowing genetic material to enter
the cell via the pore (13,14). Diagnostic US is used to introduce
plasmids and short interfering RNA into cultured cells in this
manner (15–17). Diagnostic US may be a suitable means
of introducing shRNA-Fz2 into gastric cancer tissues. It is
occasionally difficult to visualize gastric cancer with
extracorporeal US (18) and it would
be challenging to introduce therapeutic genes into cancer tissues
when the tissues are not adequately visualized with US. Endoscopy
would thus be the ideal way in which to introduce genetic material
to gastric cancer tissues, since the device also enables the
visualization and monitoring of the cancer.
Endoscopic US (EUS) is indispensable for the
diagnosis and staging of gastric cancer (19). EUS uses an endoscopic device fitted
with US equipment on its tip and the probe is equipped with a
balloon that is filled with water for the examination. The balloon
is placed in direct contact with the area under investigation and
the water in the balloon thus mediates the US between the probe and
the area of examination.
In the present study, cultured gastric cancer cells
were irradiated with EUS in an attempt to introduce shRNA-Fz2 into
the cells.
Materials and methods
Cell culture
The GC cell lines, MKN45 and MKN74, were purchased
from RIKEN Cell Bank (Tsukuba, Japan). The cells were cultured in
Roswell Park Memorial Institute (RPMI)-1640 medium (Sigma-Aldrich;
Merck Millipore, Darmstadt, Germany) supplemented with 10% fetal
bovine serum (FBS; Life Technologies; Thermo Fisher Scientific,
Inc., Waltham, MA, USA). The cell lines were cultured with 5%
carbon dioxide at 37°C in a humidified chamber. At 24 h prior to
the experiments, the cells were split into 96-well
fluoroimmunoassay (FIA) black plates (Greiner Bio-One,
Frickenhausen, Germany), the bottoms of which consist of a
transparent polystyrene film of 190±19 µm in thickness. For
irradiation with EUS or for transfection experiments, the cells
were trypsinized, harvested, seeded into 96-well FIA black plates
(1,000 cells/well) and incubated for 24 h at 37°C in RPMI-1640
supplemented with 10% FBS. The following day, the cultured cells
were subjected to irradiation or transfection.
Irradiation with EUS
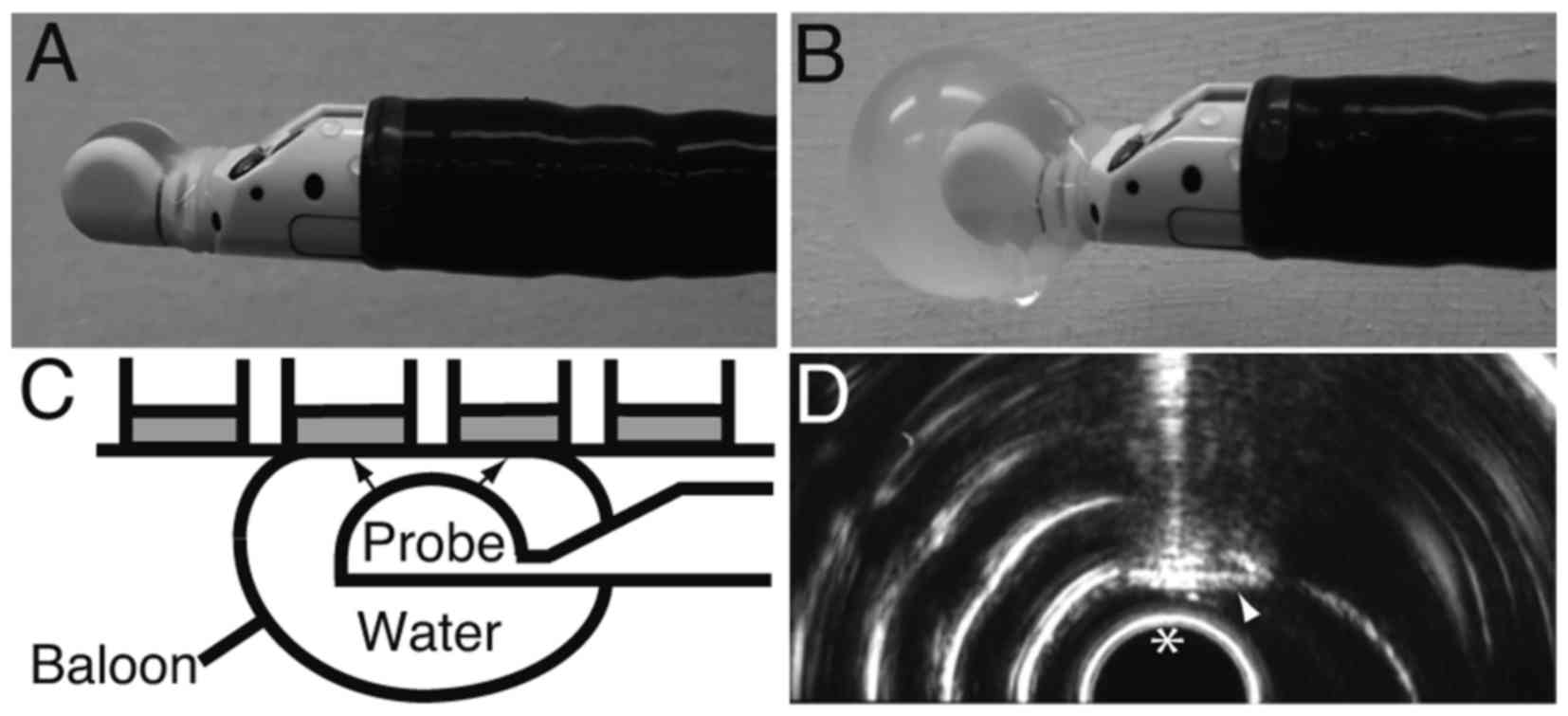
The probe of the EUS device (GF-UCT260; Olympus,
Tokyo, Japan) was covered with a balloon (Fig. 1A). The balloon was filled and enlarged
with water to mediate the US (Fig.
1B). The balloon was then attached to the bottom of a 96-well
FIA black plate, after which ultrasound was irradiated through the
water to the bottom of the wells (Fig.
1C). Plasmid (100 ng) was added to the cells in 25 µl
Opti-Minimal Essential Medium (Opti-MEM) Reduced Serum Media (Life
Technologies; Thermo Fisher Scientific, Inc.), after which the
cell-plasmid mixture was irradiated with EUS for 0.5 or 2 min.
After the irradiation, 25 µl RPMI supplemented with 10% FBS was
added to the cells. The irradiation field was monitored in
real-time using the display of the EUS device (Fig. 1D).
Transfection
Cultured cells were transfected with 100 ng plasmid
using Lipofectamine LTX (Life Technologies; Thermo Fisher
Scientific, Inc.) according to the manufacturer's instructions:
Plasmids for transfection were added to the cells in 25 µl Opti-MEM
and allowed to complex with Lipofectamine LTX. The complex was
mixed with 25 µl RPMI-1640 supplemented with 10% FBS.
Quantification of
H2O2 generation
The generation of H2O2 was
quantified using the starch-iodide method (20). Potassium iodide (100 µl; 0.05 M) and
starch (5 mg/ml) were placed into each well of the 96-well FIA
black plates. Any H2O2 produced by
irradiation with EUS oxidizes I− into I2,
which then reacts with starch to form a purple-colored complex. The
resulting absorbance at 490 nm was analyzed using an iMark
Microplate Absorbance Reader (Bio-Rad Laboratories, Inc., Hercules,
CA, USA).
Luciferase assay
Luciferase assays were performed with
pMetLuc2-control (Promega Corporation, Madison, WI, USA). In the
luciferase assay, Metridia luciferase, the expression of which is
driven by a cytomegalovirus immediate early promoter, is secreted
into the medium. The medium from the gastric cancer cells was
collected 48 h after irradiation with EUS or transfection, and
enzyme activity was assayed using a Ready-To-Glow Secreted
Luciferase Reporter assay (Clontech Laboratories, Inc., Mountain
View, CA, USA) and a Gene Light (GL-200A) luminometer (Microtech
Co., Ltd., Funabashi, Japan). To monitor transfection efficiency,
10 ng of the pSEAP2 control vector (Clontech Laboratories, Inc.)
was used in each well of the 96-well FIA black plates and
transcriptional activity was measured using a secreted embryonic
alkaline phosphatase (SEAP) chemiluminescence kit (Clontech
Laboratories, Inc.) and the Gene Light luminometer according to the
manufacturer's instructions. Luciferase activity was calculated as
the Metridia luciferase activity divided by the SEAP activity.
Cell proliferation analysis
After 72 h of incubation at 37°C following
irradiation with EUS or transfection with shRNA-Fz2, the cells were
subjected to
3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium
inner salt (MTS) assays according to the manufacturer's
instructions (Promega Corporation). The assay is based on the
bio-reduction of MTS by live cells into a colored formazan product
that can be quantified by absorbance at 490 nm. Absorbance was
analyzed at a wavelength of 490 nm using an iMark Microplate
Absorbance Reader (Bio-Rad Laboratories, Inc.).
Statistical analysis
Absorbance was assessed by a one-factor analysis of
variance using JMP5.0 J software (SAS Institute, Cary, NC, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
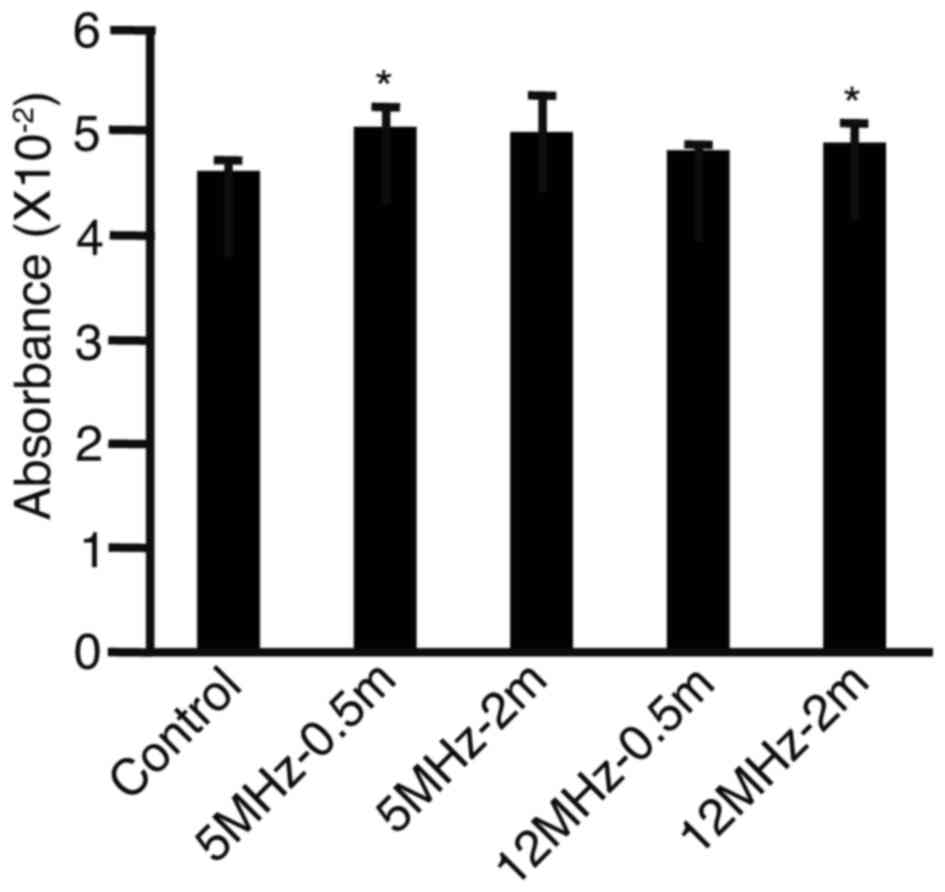
To assess whether free radicals were produced by the
EUS performed in this study, absorbance was measured in cell
cultures after irradiation with EUS or transfection (Fig. 2). Absorbance levels were found to be
higher in wells irradiated for 0.5 min at 5 MHz (P<0.05) and for
2 min at 12 MHz (P<0.05) compared with absorbance levels in the
control cells, suggesting that free radicals may be produced by
irradiation with EUS. Although the absorbance levels were higher
for 0.5 min at 5 MHz and for 2 min at 12 MHz, the differences were
not significant for 2 min at 5 MHz (P=0.0820) or for 0.5 min at 12
MHz (P=0.1220).
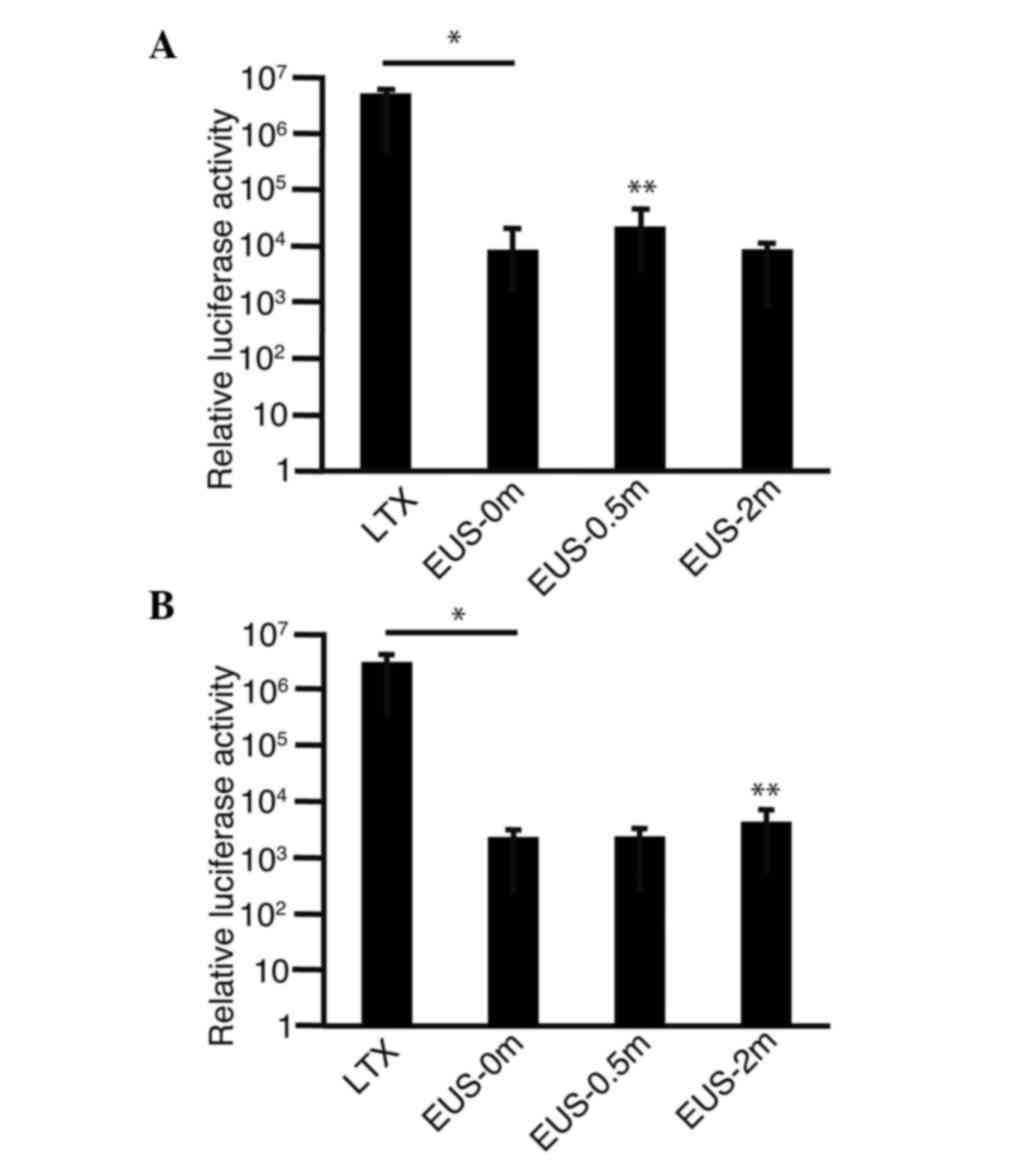
To confirm that plasmids were introduced into the
gastric cancer cells, MKN45 (Fig. 3A)
or MKN74 (Fig. 3B) cells were
combined with pMetLuc2-control in the medium, irradiated with EUS
and subjected to a luciferase assay. Luciferase activity was found
to be significantly higher in transfected cells (LTX) than in the
cells that were untreated (EUS-0m) (P<0.0001). Luciferase
activity was higher in the MKN45 cells subjected to 0.5 min
irradiation (EUS-0.5m) than in the untreated cells (EUS-0m)
(P=0.0329) (Fig. 3A). In the MKN74
cells subjected to 2 min irradiation (EUS-2m), luciferase activity
was found to be higher than that in the untreated cells (EUS-0m)
(P=0.0219) (Fig. 3B). These findings
suggest that plasmids are indeed introduced into the cells by
irradiation with EUS even though the efficiencies were lower than
those achieved with the conventional transfection reagents.
To investigate whether shRNA-Fz2 suppressed the
proliferation of MKN45 (Fig. 4A) and
MKN74 (Fig. 4B) cells, the plasmids
were added into the cell medium and the cells were then irradiated
with EUS. Proliferation was found to be significantly suppressed by
transfection (LTX) in the two cell lines. The proliferation of the
MKN45 cells tended to be suppressed by irradiation with EUS, but
without statistical significance (Fig.
4A). The proliferation of the MKN74 cells was found to be
suppressed by irradiation with 12 MHz for 2 min (P<0.0001)
(Fig. 4B).
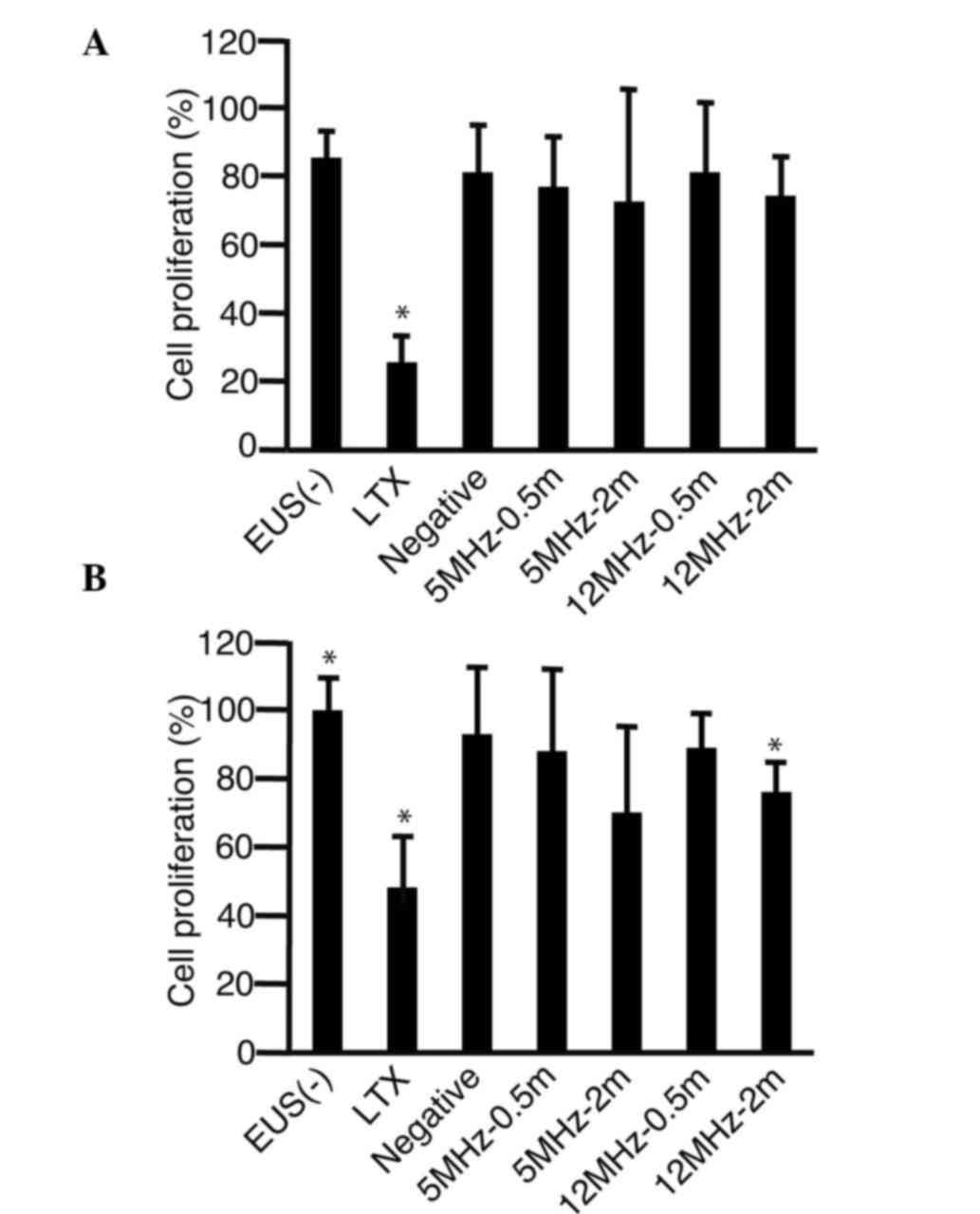 | Figure 4.Cell proliferation assay following
irradiation with EUS. shRNA-Fz2 was added into the medium of the
(A) MKN45 and (B) MKN74 gastric cancer cells. The
3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium
inner salt assay was then performed to analyze cell proliferation
72 h after irradiation with EUS. *P<0.05 compared with the
EUS(−) group (n=3). EUS(−), addition of shRNA-Fz2 without
irradiation with EUS; LTX, shRNA-Fz2 transfection with
Lipofectamine LTX; Negative, addition of negative control of
short-hairpin RNA; 5MH-0.5m, addition of shRNA-Fz2 and 0.5 min
irradiation with 5 MHz; 5MHz-2m, addition of shRNA-Fz2 and 2 min
irradiation with 5 MHz; 12MHz-0.5m, addition of shRNA-Fz2 and 0.5
min irradiation with 12 MHz; 12MHz-2m, addition of shRNA-Fz2 and 2
min irradiation with 12 MHz; shRNA-Fz2, short-hairpin RNA of
frizzled-2; EUS, endoscopic ultrasound. |
Discussion
H2O2 is produced by
irradiation with US. Okada et al irradiated water in 35-mm
dishes and measured H2O2 generation by the
KI-starch method (11). In their
experiments, absorbance values (555 nm) ranged from 0 to 0.35. In
the present experiments, absorbance values at 490 nm ranged from
0.045 to 0.055, suggesting that EUS caused sonoporation (21). The increases in absorbance that were
reported in the present study are not as significant as those
reported by Okada et al, leading us to speculate that
H2O2 is produced at lower levels by
irradiation with EUS than by devices designed specifically for
irradiation experiments (11). The
low levels of H2O2 production may also be a
result of EUS being biologically safe and producing only low levels
of free radicals (22). The standing
wave effects may also have enhanced the production of
H2O2 in previous studies (23): Kinoshita and Hynynen set a water
chamber on the surface of samples to eliminate the standing wave
effects (24).
Therapeutic genes have been introduced into gastric
cancer cells to suppress their proliferation (3,25), and in
the present study, EUS was assessed as a method for the
introduction of such therapeutic genes into cells. A luciferase
assay showed that the reporter plasmids were indeed introduced into
the cells; however, the efficiency of the introduction of the genes
by EUS was lower than that of introduction by transfection. Cell
proliferation tended to be suppressed following irradiation by EUS
after the addition of shRNA-Fz2, although with no statistical
significance except for in MKN74 cells irradiated with 12 MHz for 2
min (Fig. 4). Suppression of cell
proliferation by shRNA-Fz2 was less than that achieved by
conventional transfection methods, possibly due to the lower
efficiency of introduction with irradiation by EUS compared with
transfection.
A limitation of this study is the fact that the
efficiency of the EUS-mediated plasmid introduction was low
compared with the efficiency of plasmid introduction by
conventional transfection reagents. Microbubbles enhance
sonoporation and increase introduction efficiencies of plasmids
(26) and EUS was therefore expected
to achieve more efficient introduction of shRNA-Fz2 than
traditional methods. Further studies would thus include the
application of microbubbles to the culture media of cells to
increase the efficiency of shRNA-Fz2 introduction.
In conclusion, plasmids were successfully introduced
into cultured gastric cancer cells by sonoporation achieved by
irradiation with EUS, as evidenced by the production of
H2O2; however, the efficiency of this plasmid
introduction method was found to be low compared with traditional
transfection methods.
References
|
1
|
Tanaka SS, Kojima Y, Yamaguchi YL,
Nishinakamura R and Tam PP: Impact of WNT signaling on tissue
lineage differentiation in the early mouse embryo. Dev Growth
Differ. 53:843–856. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
MacDonald BT, Tamai K and He X:
Wnt/beta-catenin signaling: Components, mechanisms and diseases.
Dev Cell. 17:9–26. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tomizawa M, Shinozaki F, Motoyoshi Y,
Sugiyama T, Yamamoto S and Ishige N: Gastric cancer cell
proliferation is suppressed by frizzled-2 short hairpin RNA. Int J
Oncol. 46:1018–1024. 2015.PubMed/NCBI
|
|
4
|
Feril LB Jr and Tachibana K: Use of
ultrasound in drug delivery systems: Emphasis on experimental
methodology and mechanisms. Int J Hyperthermia. 28:282–289. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fechheimer M, Boylan JF, Parker S, Sisken
JE, Patel GL and Zimmer SG: Transfection of mammalian cells with
plasmid DNA by scrape loading and sonication loading. Proc Natl
Acad Sci USA. 84:8463–8467. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kim HJ, Greenleaf JF, Kinnick RR, Bronk JT
and Bolander ME: Ultrasound-mediated transfection of mammalian
cells. Hum Gene Ther. 7:1339–1346. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tomizawa M, Ebara M, Saisho H, Sakiyama S
and Tagawa M: Irradiation with ultrasound of low output intensity
increased chemosensitivity of subcutaneous solid tumors to an
anti-cancer agent. Cancer Lett. 173:31–35. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
O'Brien WD Jr: Ultrasound-biophysics
mechanisms. Prog Biophys Mol Biol. 93:212–255. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Newman CM and Bettinger T: Gene therapy
progress and prospects: Ultrasound for gene transfer. Gene Ther.
14:465–475. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang P, Li Y, Wang X, Guo L, Su X and Liu
Q: Membrane damage effect of continuous wave ultrasound on K562
human leukemia cells. J Ultrasound Med. 31:1977–1986. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Okada K, Kudo N, Hassan MA, Kondo T and
Yamamoto K: Threshold curves obtained under various gaseous
conditions for free radical generation by burst ultrasound-Effects
of dissolved gas, microbubbles and gas transport from the air.
Ultrason Sonochem. 16:512–518. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ebrahiminia A, Mokhtari-Dizaji M and
Toliyat T: Correlation between iodide dosimetry and terephthalic
acid dosimetry to evaluate the reactive radical production due to
the acoustic cavitation activity. Ultrason Sonochem. 20:366–372.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Qiu Y, Zhang C, Tu J and Zhang D:
Microbubble-induced sonoporation involved in ultrasound-mediated
DNA transfection in vitro at low acoustic pressures. J Biomech.
45:1339–1345. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kudo N, Okada K and Yamamoto K:
Sonoporation by single-shot pulsed ultrasound with microbubbles
adjacent to cells. Biophys J. 96:4866–4876. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Miller DL and Quddus J: Sonoporation of
monolayer cells by diagnostic ultrasound activation of
contrast-agent gas bodies. Ultrasound Med Biol. 26:661–667. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tomizawa M, Shinozaki F, Sugiyama T,
Yamamoto S, Sueishi M and Yoshida T: Plasmid DNA introduced into
cultured cells with diagnostic ultrasound. Oncol Rep. 27:1360–1364.
2012.PubMed/NCBI
|
|
17
|
Tomizawa M, Shinozaki F, Sugiyama T,
Yamamoto S, Sueishi M and Yoshida T: Short interference RNA
introduced into cultured cells with diagnostic ultrasound. Oncol
Rep. 27:65–68. 2012.PubMed/NCBI
|
|
18
|
Tomizawa M, Shinozaki F, Hasegawa R, Fugo
K, Shirai Y, Ichiki N, Sugiyama T, Yamamoto S, Sueishi M and
Yoshida T: Screening ultrasonography is useful for the diagnosis of
gastric and colorectal cancer. Hepatogastroenterology. 60:517–521.
2013.PubMed/NCBI
|
|
19
|
El Abiad R and Gerke H: Gastric cancer:
Endoscopic diagnosis and staging. Surg Oncol Clin N Am. 21:1–19.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kondo T and Yoshii G: Effect of intensity
of 1.2 MHz ultrasound on change in DNA synthesis of irradiated
mouse L cells. Ultrasound Med Biol. 11:113–119. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tomizawa M, Shinozaki F, Motoyoshi Y,
Sugiyama T, Yamamoto S and Sueishi M: Sonoporation: Gene transfer
using ultrasound. World J Methodol. 3:39–44. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
WFUMB Symposium on safety and
standardisation in medical ultrasound. Issues and recommendations
regarding thermal mechanisms for biological effects of ultrasound.
Hornbaek, denmark, 30 August-1. Ultrasound Med Biol. 18:731–810.
1992.PubMed/NCBI
|
|
23
|
Hassan MA, Buldakov MA, Ogawa R, Zhao QL,
Furusawa Y, Kudo N, Kondo T and Riesz P: Modulation control over
ultrasound-mediated gene delivery: Evaluating the importance of
standing waves. J Control Release. 141:70–76. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kinoshita M and Hynynen K: Key factors
that affect sonoporation efficiency in in vitro settings: The
importance of standing wave in sonoporation. Biochem Biophys Res
Commun. 359:860–865. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang TS, Ding QQ, Guo RH, Shen H, Sun J,
Lu KH, You SH, Ge HM, Shu YQ and Liu P: Expression of livin in
gastric cancer and induction of apoptosis in SGC-7901 cells by
shRNA-mediated silencing of livin gene. Biomed Pharmacother.
64:333–338. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yamaguchi K, Feril LB Jr, Tachibana K,
Takahashi A, Matsuo M, Endo H, Harada Y and Nakayama J:
Ultrasound-mediated interferon β gene transfection inhibits growth
of malignant melanoma. Biochem Biophys Res Commun. 411:137–142.
2011. View Article : Google Scholar : PubMed/NCBI
|