Introduction
Hydrazide-hydrazone derivatives are an important
class of biologically active molecules, which have attracted the
attention of medicinal chemists due to their wide-ranging
pharmacological properties and their potential application as
antitumor, antineoplastic, antiviral and anti-inflammatory agents
(1–6).
In particular, aroylhydrazone complexes of
transition metals are known to provide useful models for the
elucidation of the underlying molecular mechanism of enzyme
inhibition by hydrazine derivatives (7) and for their potential pharmacological
application (8,9). Hydrazone derivatives of isoniazid and
other hydrazides have been reported to exhibit marked antimicrobial
activity (10–14). Furthermore, a number of substituted
hydrazone derivatives have been synthesized and evaluated for their
antitumor activity, with certain promising results having been
reported (15–17).
The aim of the present study was to develop potent
anticancer agents. (E)-N'-(1-(pyridin-2-yl)ethylidene)
nicotinohydrazide (penh), and [Mn(penh)2] (complex 1),
[Co (penh)2] (complex 2), [Cu(penh)2]
(complex 3) and [Cd(penh)2] (complex 4) metal complexes
were synthesized, and their cytotoxicity against human lung cancer
(A549), human gastric cancer (BGC823) and human esophageal cancer
(Eca109) cell lines was investigated.
Materials and methods
Materials
All starting materials were obtained commercially
and used as received. The A549 human lung cancer, BGC823 human
gastric cancer and Eca109 human esophageal cancer cell lines were
obtained from the Cell Culture Center of the Basic Institute of
Medical Sciences (Peking Union Medical College, Beijing, China).
Cell culture reagents were purchased from Gibco (Thermo Fisher
Scientific, Inc., Waltham, MA, USA). An Annexin V/propidium iodide
(PI) double staining kit was purchased from BD Biosciences
(Franklin Lakes, NJ, USA). MTT and dimethyl sulfoxide (DMSO) were
purchased from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany).
Polyvinylidene fluoride (PVDF) membranes and non-fat dried milk
were obtained from EMD Millipore (Billerica, MA, USA). Polyclonal
antibodies against p53 (cat. no. 3036), apoptosis regulator Bax
(Bax) (cat. no. 3032) and apoptosis regulator Bcl-2 (Bcl-2) (cat.
no. 3195) were obtained from BioVision, Inc. (Milpitas, CA, USA).
Anti-GAPDH (cat. no. E7EUT5) polyclonal antibody was purchased from
Abmart, Inc. (Shanghai, China). The alkaline phosphatase
(AP)-conjugated anti-mouse IgG (cat. no. A0258) and AP-conjugated
anti-rabbit IgG (cat. no. A0239) secondary antibodies were obtained
from the Beyotime Institute of Biotechnology (Haimen, China).
Elemental analyses (concentration of each complex,
1.25×10−5 mol/l) were performed in the Microanalytical
Laboratory of the Department of Chemistry of Lanzhou University
(Lanzhou, China). 1H nuclear magnetic resonance (NMR)
spectra were recorded using an ACF300 Fourier-transform NMR
instrument (Bruker Corporation, Billerica, MA, USA) using
tetramethylsilane as an internal reference in
[2H6] DMSO for the ligand. The infrared
spectra (KBr pellet) were recorded using an FTS165
Fourier-transform infrared spectrophotometer (Bio-Rad Laboratories,
Inc., Hercules, CA, USA) between 4,000 and 400 cm−1
(18). Molecular structures were
drawn using ChemDraw® Pro plugin (version 8.0; Cambridge
Soft; PerkinElmer Inc., Waltham, MA, USA).
Compound synthesis
Synthesis of the penh ligand
An ethanol solution (30 ml) containing
salicylaldehyde (1.21 g, 10 mmol) was added dropwise to another
ethanol solution (30 ml) containing the nicotinohydrazide (1.37 g,
10 mmol). Following reflux for 6 h, the mixture was cooled to room
temperature, and the white precipitate solid was collected by
suction filtration and washed with ice-cold ethanol. The penh
ligand was collected following recrystallization using anhydrous
methanol and was dried under a vacuum. The yield was 93.1%.
1H-NMR (C2HCl3, ppm) 7.41–9.15
(8H, m, Ar-H), 2.56 (3H, s, CH3). Infrared (IR) (KBr,
cm−1): ν(OH) 3486, ν(N-N) 3,190, ν(C=O) 1,667, ν(C=N)
1,580. Elemental analysis for penh
(C52H50N19O14): C,
53.61; H, 4.33; N, 22.84 (calculated); C, 53.56; H, 4.51; N, 22.89
(found).
Synthesis of the complexes
Complex 1 was prepared as follows: The penh ligand
(1 mmol, 0.241 g) containing 3 drops of triethylamine was dissolved
in 30 ml anhydrous methanol, MnCl2 (0.5 mmol, 0.063 g)
in anhydrous methanol (10 ml) was then added dropwise with
stirring. After 1 week, single crystals of 1 suitable for X-ray
diffraction were obtained from the reaction solution. Crystals were
separated from the solution by suction filtration, purified by
washing several times with ethanol. The yield was 58.3%. Elemental
analysis for 1
(C26H22MnN8O2): C,
58.54; H, 4.16; N, 21.01 (calculated); C, 58.59; H, 4.22; N, 21.20
(found). IR (KBr, cm−1): ν(OH) 3,461, ν(C=N) 1,517.
The preparation of complex 2 was similar to that of
complex 1, using CoCl2 instead of MnCl2. The
yield was 68.5%. Elemental analysis for complex 2
(C26H22CoN8O2): C,
58.10; H, 4.13; N, 20.85 (calculated); C, 58.25; H, 4.25; N, 21.04
(found). IR (KBr, cm−1): ν(OH) 3,472, ν(C=N) 1,516.
The preparation of complex 3 was similar to that of
complex 1, using CuCl2 instead of MnCl2. The
yield was 66.1%. Elemental analysis for complex 3
(C26H22CuN8O2): C,
57.61; H, 4.09; N, 20.67 (calculated); C, 57.72; H, 4.18; N, 20.77
(found). IR (KBr, cm−1): ν(OH) 3,480, ν(C=N) 1,515.
The preparation of complex 4 was similar to that of
complex 1, using CdCl2 instead of MnCl2. The
yield was 53.5%. Elemental analysis for complex 4
(C26H22CdN8O2): C,
52.85; H, 3.75; N, 18.96 (calculated); C, 52.99; H, 3.86; N, 19.16
(found). IR (KBr, cm−1): ν(OH) 3,481, ν(C=N) 1,516.
X-ray crystallography
The X-ray diffraction measurements for two complexes
were performed on a SMART APEX II charge-coupled device
diffractometer (Bruker Corporation) equipped with graphite
monochromatized Mo-K radiation (λ=0.071073 nm) using φ-ω
scan mode. Semi-empirical absorption correction was applied to the
intensity data using the SADABS program (version 2.03; Bruker AXS,
Inc., Billerica, MA, USA) (19). The
structures were solved and refined by full-matrix least-squares on
F2 using the SHELXTL-97 program (Bruker AXS,
Inc.) (20). All non-H atoms were
refined anisotropically. All H atoms were positioned geometrically
and refined using a riding model (18). Details of the crystal parameters, data
collection and refinement for complexes 1–4 are summarized in
Table I.
 | Table I.Crystal data and structure refinement
for the complexes. |
Table I.
Crystal data and structure refinement
for the complexes.
| Characteristic |
[Mn(penh)2] |
[Co(penh)2] |
[Cu(penh)2] |
[Cd(penh)2] |
|---|
| Empirical
formula |
C26H22N8O2Mn |
C26H22N8O2Co |
C26H22N8O2Cu |
C26H22N8O2Cd |
| Formula mass | 533.46 | 537.45 | 542.06 | 590.92 |
| Temperature, K
(±SD) | 296 (2) | 296 (2) | 296 (2) | 296 (2) |
| Wavelength, nm | 0.071073 | 0.071073 | 0.071073 | 0.071073 |
| Crystal system | Orthorhombic | Orthorhombic | Orthorhombic | Monoclinic |
| Space group | Pbca | Aba2 | Pbca | Cc |
| Z | 8 | 8 | 8 | 4 |
| a, Å | 11.9900 (10) | 12.114 (2) | 11.965 (3) | 20.761 (5) |
| b, Å | 10.3280 (8) | 19.088 (4) | 10.306 (3) | 14.905 (4) |
| c, Å | 39.095 (3) | 10.3482 (19) | 39.234 (11) | 8.1989 (19) |
| β, ° | – | – | – | 105.064 (3) |
| V,
Å3 | 4,841.2 (7) | 2,392.8 (8) | 4,838 (2) | 2,449.9 (10) |
| Dcalc,
g·cm–3 | 1.464 | 1.492 | 1.488 | 1.602 |
| µ,
mm−1 | 0.587 | 0.760 | 0.945 | 0.933 |
| F(000) | 2,200 | 1,108 | 2,232 | 1,192 |
| Independent
reflections (Rint) | 4,261 (0.0815) | 1,535 (0.0528) | 4,261 (0.0755) | 3,969 (0.0129) |
| Final GooF | 1.048 | 1.026 | 1.100 | 1.055 |
|
R1,
wR2 [I>2σ(I)] |
R1 = 0.0641 |
R1 = 0.0439 |
R1 = 0.0680 |
R1 = 0.0254 |
|
|
wR2 = 0.1806 |
wR2 = 0.1069 |
wR2 = 0.1734 |
wR2 = 0.0692 |
|
R1,
wR2 (all data) |
R1 = 0.1145 |
R1 = 0.0643 |
R1 = 0.1043 |
R1 = 0.0269 |
|
|
wR2 = 0.2117 |
wR2 = 0.1203 |
wR2 = 0.1966 |
wR2 = 0.0707 |
Cytotoxic activity of the complexes
To evaluate whether or not complexes 1–4 exhibit a
tumor cell killing ability, the effect of DMSO-soluble complexes
1–4 on A549 (lung cancer), BGC823 (gastric cancer) and Eca109
(esophageal cancer) human solid tumor cell lines was investigated.
All three cell lines were cultured in RPMI-1640 medium supplemented
with 10% fetal bovine serum, 100 units/ml penicillin and 100 µg/ml
streptomycin at 37°C in a humidified atmosphere containing 5%
CO2. The effect of treatment with complexes 1–4 on the
survival of the three human tumor cell lines was assessed using an
MTT assay in distilled water. Cells were seeded in 96-well plates
at 1×104 cells/well. Following overnight growth, the
cells were cultured for 72 h in the presence of 3, 6, 9, 12, 15, 18
and 21 µmol/l complexes 1–4, with cells cultured with the
corresponding uncomplexed penh ligand serving as the control group.
After 72 h incubation, 20 µl MTT was added into each well and cells
were incubated at 37°C for 4 h. Culture medium was removed and 150
µl DMSO was added. The plates were agitated, prior to determination
of the optical density at 570 nm using an ELISA plate reader.
Images of cells were captured using an upright metallurgical
microscope (BX61; magnification, ×20; Olympus Corporation, Tokyo,
Japan). At least three parallel experiments were performed. As
complex 3 exhibited increased cytotoxic activity compared with the
other complexes, particularly for the Eca109 cell line, Eca109
cells and complex 3 were selected for subsequent cell apoptosis
assay and western blot analysis.
Quantification of apoptosis using Annexin V and
PI double staining
Apoptotic rates were determined using flow cytometry
using an Annexin V/PI apoptosis kit. Eca109 cancer cells were
seeded at a density of 1×106 cells/well in 6-well
plates, cultured overnight, and treated with 3, 6 and 9 µmol/l
complex 3 for 48 h at 37°C. After 48 h, the cells were collected by
centrifugation at 200 × g for 5 min at 4°C and washed twice with
ice-cold PBS. Staining was carried out according to the
manufacturer's protocol, and the cells were analyzed using a BD
Acurri™ C6 flow cytometer (BD Biosciences) and analyzed using
CellQuest software (version 6.0; BD Biosciences). Each experiment
was carried out at least three times independently.
Western blot analysis
Eca109 cancer cells were seeded in 6-well plates
overnight. Cells were treated with 4 µmol/l complex 3 for 48 h at
37°C. Total proteins were collected and protein concentration was
determined using a bicinchoninic acid protein assay. Proteins (20
µg) were separated by SDS-PAGE (12% gel), transferred onto PVDF
membranes by electroblotting and probed with antibodies against p53
(1:1,000), Bax (1:1,000), Bcl-2 (1:1,000) and GAPDH (1:2,000)
diluted in 5% BSA (cat. no. 10711454001; Roche Applied Science,
Penzberg, Germany) and blocked using 5% dried skimmed milk
overnight at 4°C. The membranes were incubated with secondary
antibody against IgG labeled with AP and detected using a
Lumi-Phos™ WB enhanced chemiluminescence kit (Thermo Fisher
Scientific, Inc.) at room temperature. Membranes were scanned using
an HP ScanJet G4010 flatbed scanner (HP, Inc., Palo Alto, CA,
USA).
Statistical analysis
Statistical analysis was performed using SAS
software (version 6.12; SAS Institute, Inc., Cary, NC, USA).
Measurement data are presented as the mean ± standard deviation. To
compare the differences between the groups, statistical
significance was determined using a one-way analysis of variance
followed by Dunnett's test comparisons. P<0.05 was considered to
indicate a statistically significant difference.
Results and Discussion
Crystal structures of complexes
1–4
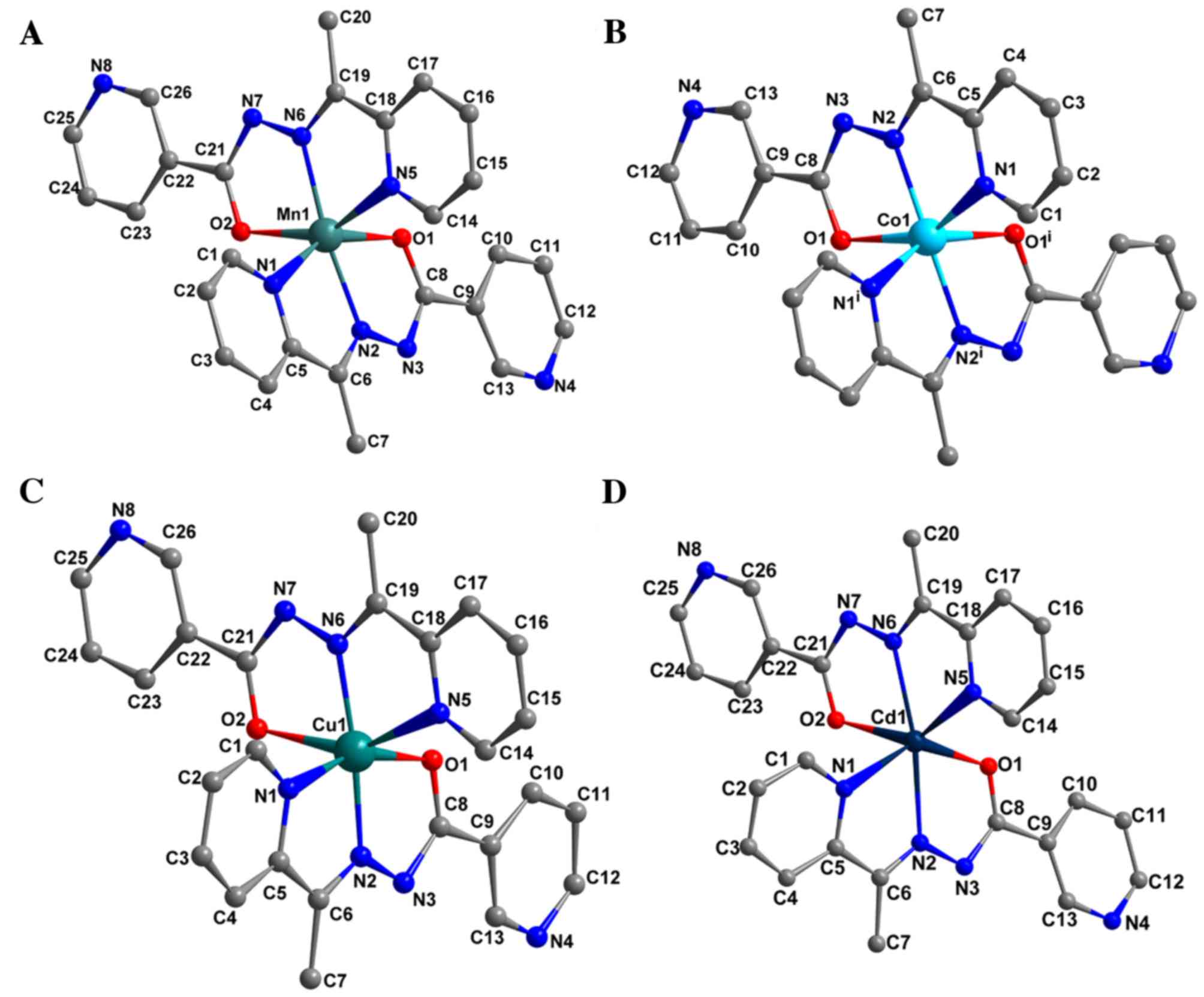
Structures of the four complexes are presented in
Fig. 1. The complexes exhibited
similar structures; however, they were crystallized in distinct
space groups (Table I). In each
complex, the metal center with a distorted octahedron geometry was
identified to be surrounded by two molecules of monoanionic ligand,
which coordinates to the metal ion with an N2O donor
set, namely deprotonated carbonylate-O, azomethine-N and pyridyl-N
atoms. The N2O donor sites of the tridentate ligand
formed four five-membered CN2OM (where M represents the
transition metal) and C2N2M chelate rings
around the metal ion. The carbonyl bond distances of Mn(II),
Ni(II), Cu(II) and Zn(II) complexes were between 1.256 (±0.006) and
1.277 (±0.006) Å, confirming the formation of the M-O bond through
an enolized C-O group (21). In
addition, the distances of the enolized C-O and imine C-N bands in
the complexes were intermediate between single and double bond,
suggesting an extended conjugation in anionic ligand following
complexation. The M-O and M-N bond lengths (Table II) were in the normal range reported
for Mn(II), Co(II), Cu(II) and Cd(II) octahedral complexes
(22). As predicted, there were no
classical hydrogen bonds in any of the complexes.
 | Table II.Selected bond lengths and angles in
the four complexes. |
Table II.
Selected bond lengths and angles in
the four complexes.
| A,
[Mn(penh)2] bonds |
|---|
|
|---|
| Bond | Length, Å |
|---|
| Mn1-N6 | 2.061 (5) |
| Mn1-N2 | 2.065 (5) |
| Mn1-O2 | 2.124 (4) |
| Mn1-O1 | 2.174 (4) |
| Mn1-N1 | 2.193 (5) |
| Mn1-N5 | 2.198 (5) |
|
| B,
[Mn(penh)2] angles |
|
| Angle | Size, ° |
|
| N6-Mn1-N2 | 172.67 (18) |
| N6-Mn1-O2 | 75.45 (17) |
| N2-Mn1-O2 | 107.99 (16) |
| N6-Mn1-O1 | 99.32 (16) |
| N2-Mn1-O1 | 73.88 (16) |
| O2-Mn1-O1 | 99.41 (18) |
| N6-Mn1-N1 | 111.24 (18) |
| N2-Mn1-N1 | 75.32 (18) |
| O2-Mn1-N1 | 93.49 (17) |
| O1-Mn1-N1 | 148.98 (16) |
| N6-Mn1-N5 | 74.57 (18) |
| N2-Mn1-N5 | 102.69 (17) |
| O2-Mn1-N5 | 149.03 (17) |
| O1-Mn1-N5 | 92.90 (16) |
| N1-Mn1-N5 | 90.21 (17) |
|
| C,
[Co(penh)2] bonds |
|
| Bond | Length, Å |
|
| Co1-N2 | 2.032 (4) |
| Co1-O1 | 2.081 (4) |
| Co1-N1 | 2.134 (5) |
|
| D,
[Co(penh)2] angles |
|
| Angle | Size, ° |
|
|
N2-Co1-N2i | 174.71 (4) |
|
N2-Co1-O1i | 101.14 (18) |
| N2-Co1-O1 | 75.57 (18) |
|
O1-Co1-O1i | 104.82 (3) |
| N2-Co1-N1 | 75.40 (2) |
| O1-Co1-N1 | 148.85 (15) |
|
O1-Co1-N1i | 91.61 (18) |
|
N2-Co1-N1i | 108.65 (2) |
|
N1-Co1-N1i | 87.25 (3) |
|
O1-Co1-N1i | 91.61 (18) |
|
N2-Co1-N1i | 108.67 (2) |
|
N1-Co1-N1i | 87.26 (3) |
|
| E,
[Cu(penh)2] bonds |
|
| Bond | Length, Å |
|
| Cu1-N6 | 2.002 (5) |
| Cu1-N2 | 1.948 (5) |
| Cu1-O2 | 2.268 (4) |
| Cu1-O1 | 2.073 (4) |
| Cu1-N1 | 2.079 (5) |
| Cu1-N5 | 2.280 (5) |
|
| F,
[Cu(penh)2] angles |
|
| Angle | Size, ° |
|
| N6-Cu1-N2 | 172.96 (2) |
| N6-Cu1-O2 | 74.07 (17) |
| N2-Cu1-O2 | 98.86 (17) |
| N6-Cu1-O1 | 103.07 (17) |
| N2-Cu1-O1 | 78.03 (18) |
| O2-Cu1-O1 | 97.86 (18) |
| N6-Cu1-N1 | 100.84 (18) |
| N2-Cu1-N1 | 78.62 (19) |
| O2-Cu1-N1 | 91.63 (17) |
| O1-Cu1-N1 | 155.88 (17) |
| N6-Cu1-N5 | 76.37 (19) |
| N2-Cu1-N5 | 110.65 (19) |
| O2-Cu1-N5 | 150.22 (16) |
| O1-Cu1-N5 | 92.22 (18) |
| N1-Cu1-N5 | 90.37 (19) |
|
| G,
[Cd(penh)2] bonds |
|
| Bond | Length, Å |
|
| Cd1-N6 | 2.266 (3) |
| Cd1-N2 | 2.265 (3) |
| Cd1-O2 | 2.291 (3) |
| Cd1-O1 | 2.258 (3) |
| Cd1-N1 | 2.362 (3) |
| Cd1-N5 | 2.356 (3) |
|
| H,
[Cd(penh)2] angles |
|
| Angle | Size, ° |
|
| N6-Cd1-N2 | 169.29 (10) |
| N6-Cd1-O2 | 69.15 (9) |
| N2-Cd1-O2 | 100.32 (9) |
| N6-Cd1-O1 | 113.59 (10) |
| N2-Cd1-O1 | 69.61 (10) |
| O2-Cd1-O1 | 102.07 (11) |
| N6-Cd1-N1 | 106.78 (10) |
| N2-Cd1-N1 | 69.83 (9) |
| O2-Cd1-N1 | 87.61 (11) |
| O1-Cd1-N1 | 139.36 (10) |
|
| H,
[Cd(penh)2] angles |
|
| Angle | Size, ° |
|
| N6-Cd1-N5 | 69.98 (10) |
| N2-Cd1-N5 | 120.21 (10) |
| O2-Cd1-N5 | 138.62 (10) |
| O1-Cd1-N5 | 99.71 (9) |
| N1-Cd1-N5 | 98.14 (10) |
IR spectra of the complexes
The IR spectra of the four complexes were similar.
The ν(C=O) of the uncomplexed penh ligand were at 1,667
cm−1; for complexes, this peak disappeared, indicating
that the amide group was involved in coordination to the metal ion
through the enol form. The band at 1,580 cm−1 for the
uncomplexed penh ligand was assigned to the ν(C=N) stretch, which
shifted to near 1,518 cm−1 for the complexes, indicating
that the N atom of C=N was involved in coordination to the metal
ion. The vibrations ν(N-H) disappeared in the spectra of the four
complexes, indicating that the ligand was deprotonated. The novel
bands near 560 cm−1 for the complexes were assigned to
ν(M-O), and the weaker peaks near 470 cm−1 were assigned
to ν(M-N), respectively (23,24), consistent with the results of X-ray
diffraction analysis.
Antitumor activity of the four
complexes
The four complexes were air-stable for extended
periods and soluble in methanol, dimethylformamide and DMSO,
slightly soluble in water, and insoluble in benzene and diethyl
ether. The stability of the complexes in DMSO were determined by
observing the ultraviolet (UV)-visible spectra at distinct time
intervals for any potential alteration. The Cu(II) complexes
investigated were prepared in DMSO and were freshly diluted in
phosphate buffer (pH 7.4) for experiments. The UV-visible spectra
were subsequently analyzed at various intervals. The results
indicate that the UV-visible spectra remained unaltered in solution
for the entire course of the experiment (72 h) and that the
complexes are stable in solution.
It has been reported that arylhydrazone and its
metal complexes exhibit markedly effective antitumor activities,
possibly due to their NO bidentate systems (24). In vitro cytotoxicity assays
were carried out in the human A549 (human lung cancer), BGC823
(human gastric cancer) and Eca109 (human esophageal cancer) solid
tumor cell lines. Following co-culture with each complex, cell
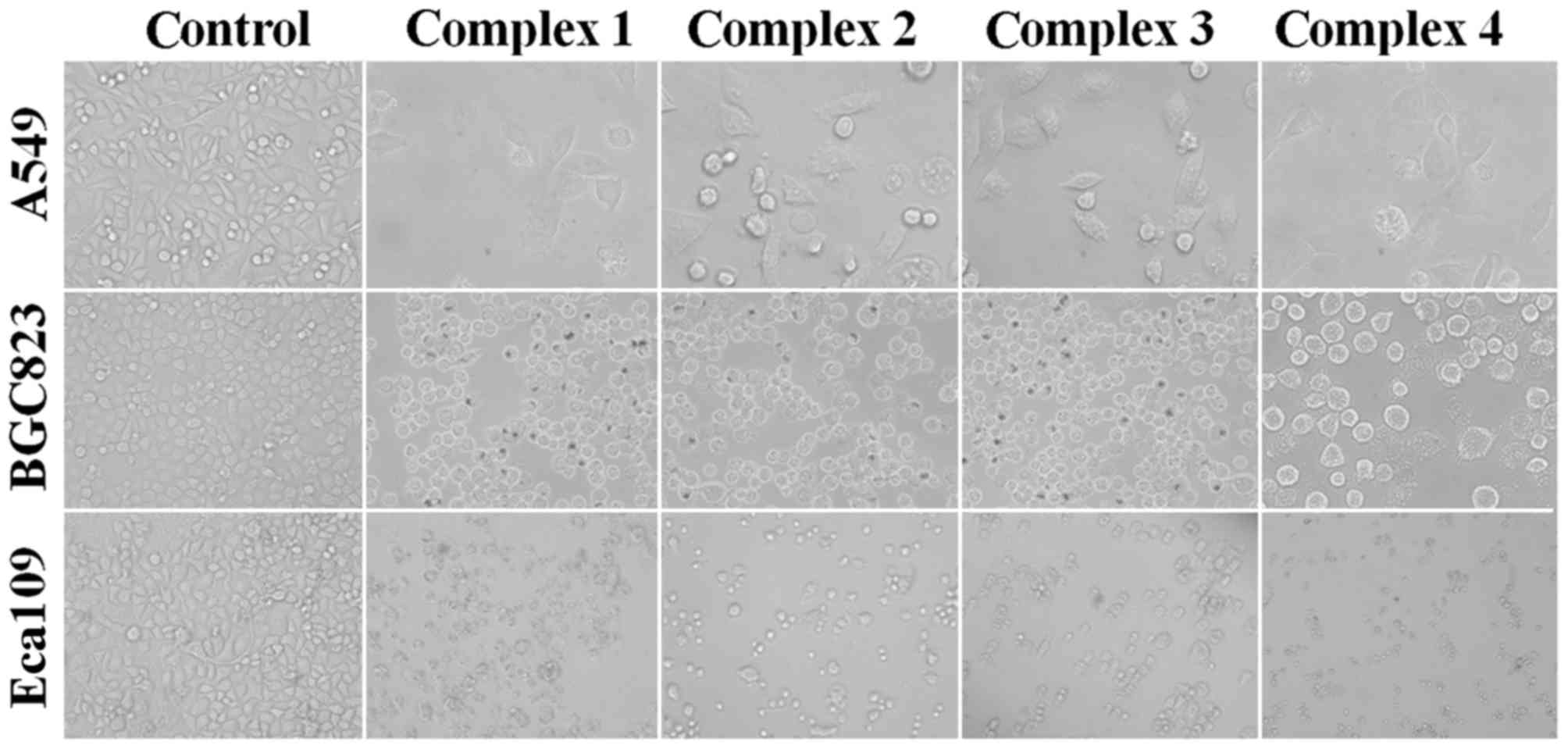
proliferation was markedly inhibited. As presented in Fig. 2, alterations in tumor cell morphology,
including cell shrinkage and cell detachment, were observed. Cell
survival and proliferation were determined using the MTT assay. The
half-maximal inhibitory concentration (IC50) values are
presented in Table III. Complexes
1–4 exhibited significantly decreased IC50 values in
comparison with that of uncomplexed penh ligand for all three cell
lines used (P<0.05). These results demonstrate that the
complexes are able to inhibit tumor cell proliferation, and the
effect was increased compared with the uncomplexed penh ligand,
which suggested that the coordinated transition metal in complexes
1–4 serves a major role in mediating potency of the complexes. The
results of the MTT assay demonstrated that complex 1 and 4
exhibited similar IC50 values for the three human tumor
cell lines, whereas complex 3 exhibited increased cytotoxic
activity compared with the other complexes, particularly for the
Eca109 cell line. Therefore, Eca109 cells and complex 3 were
selected for subsequent cell apoptosis assay and western blot
analysis.
 | Table III.Half-maximal inhibitory concentration
(µmol/l) values for the uncomplexed
(E)-N'-(1-(pyridin-2-yl)ethylidene)nicotinohydrazide ligand and
complexes 1–4 in human lung cancer (A549), human gastric cancer
(BGC823) and human esophageal cancer (Eca109) cell lines. |
Table III.
Half-maximal inhibitory concentration
(µmol/l) values for the uncomplexed
(E)-N'-(1-(pyridin-2-yl)ethylidene)nicotinohydrazide ligand and
complexes 1–4 in human lung cancer (A549), human gastric cancer
(BGC823) and human esophageal cancer (Eca109) cell lines.
| Compound | A549 | BGC823 | Eca109 |
|---|
| Ligand | 156.5 | 196.8 | 165.1 |
| Complex 1 |
12.2 |
18.3 |
9.6 |
| Complex 2 |
8.1 |
11.8 |
10.3 |
| Complex 3 |
7.3 |
9.7 |
6.5 |
| Complex 4 |
13.5 |
15.6 |
11.9 |
Complex 3 induces cell apoptosis
To determine whether the decrease in human tumor
cell growth was attributable to the induction of apoptosis of
cancer cells, Annexin V/PI staining and flow cytometric measurement
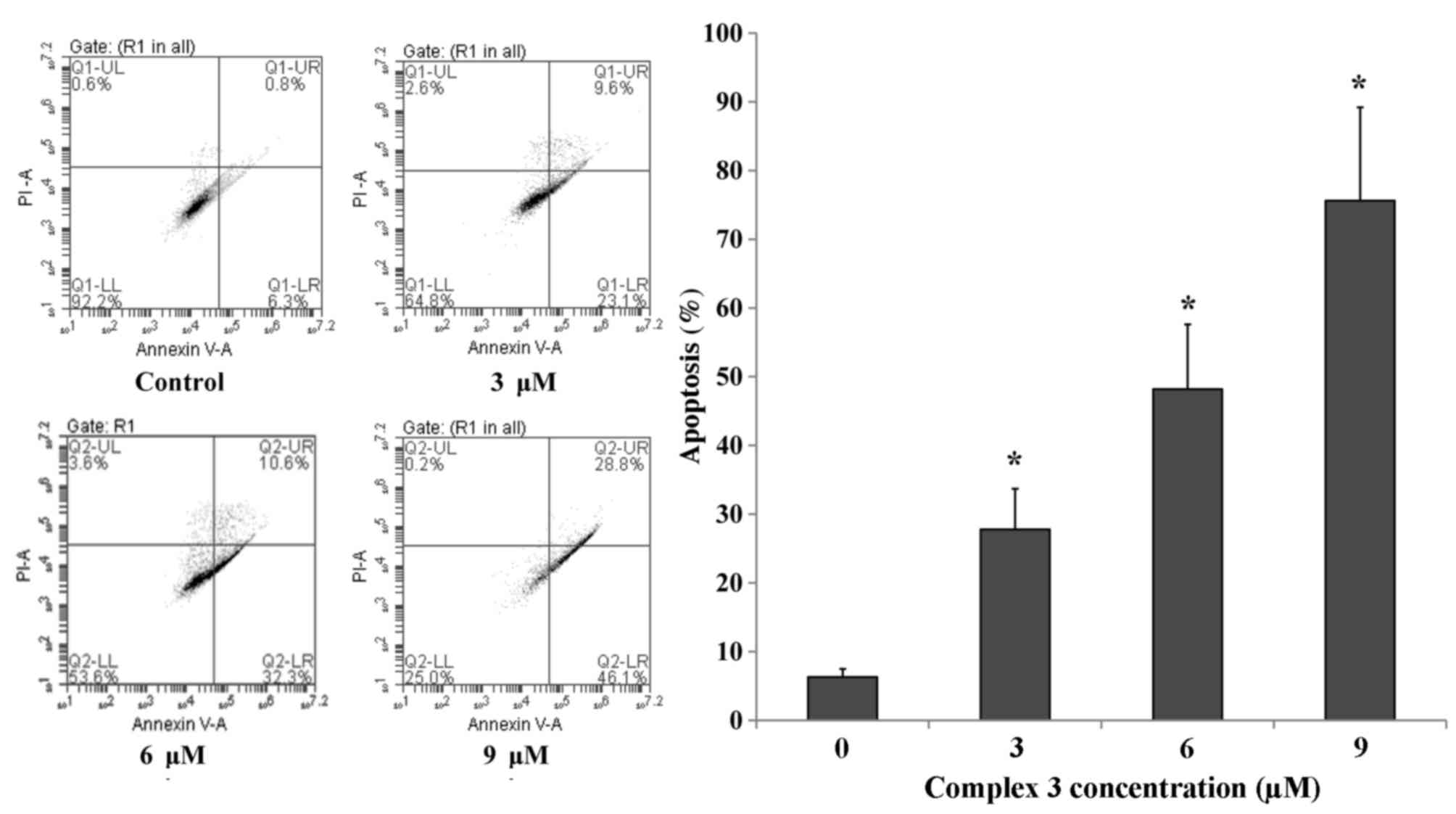
were used to quantify the level of apoptosis. Following incubation
with 3, 6 and 9 µmol/l complex 3 for 48 h, the number of apoptotic
cells of Eca109 was determined. As presented in Fig. 3, the number of apoptotic Eca109 cells
was increased significantly compared with the untreated control in
a dose-dependent manner (P<0.05).
Alterations in apoptotic protein
expression in tumor cells
To investigate the underlying molecular mechanism of
how complexes 1–4 induce apoptosis in cancer cells, levels of
proteins that serve important roles in apoptosis, including p53,
Bax and Bcl-2, were determined. According to previous studies
(25–27), p53 and Bcl-2 family proteins are key
regulators of the apoptotic signaling pathway. Following
stimulation of internal and external factors, the expression level
of protein p53 and Bax is increased in the cells, promoting
apoptosis. By contrast, Bcl-2 inhibits apoptosis by preventing the
release of cytochrome c from mitochondria into the
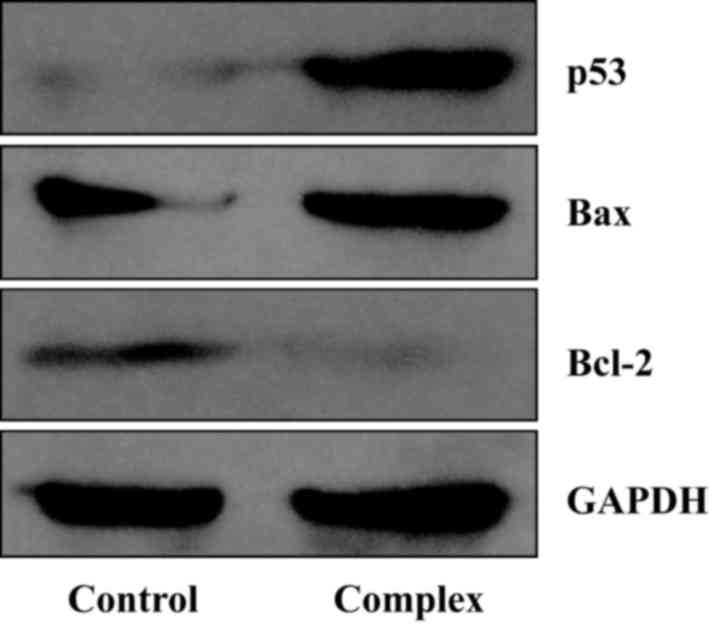
cytoplasm. In the present study, following incubation with 4 µmol/l
complex 3 for 48 h, the levels of p53 and Bax protein were
increased, whereas the expression of Bcl-2 protein was markedly
decreased (Fig. 4).
In the present study, four isostructural divalent
transition metal complexes with the nicotinohydrazone (penh) ligand
were isolated and characterized using elemental analysis, infrared
spectroscopy and single-crystal X-ray diffraction. The results of
the present study indicate that the complexes demonstrate marked
cytotoxic activity against three cancer cell lines (A549 human lung
cancer, BGC823 human gastric cancer and Eca109 human esophageal
cancer), and the IC50 values for the metal complexes
were decreased compared with that of the nicotinohydrazone (penh)
ligand. Furthermore, the complexes were also demonstrated to induce
apoptosis of the cancer cells, as demonstrated using the Annexin
V/PI staining and western blot analysis. Therefore, the results of
the present study suggest that complexes 1–4 have potential
practical application, acting as novel therapeutic reagents in the
treatment of cancer.
Acknowledgements
The present study was supported in part by the
National Natural Science Foundation of China (grant nos. 21404033,
21001040 and 81201908), the Fund of Clinical Science and Technology
of Wuxi (grant no. Q201512), the Foundation of the Social
Development Project of Jiangsu (grant no. BE2015621), the Natural
Science Foundation of Jiangsu (grant no. BK20141122), and the
Medical Science and Technology Development Projects of Nanjing City
(grant no. YKK 14061).
References
|
1
|
Howard RA, Sherwood E, Erck A, Kimball AP
and Bear JL: Hydrophobicity of several rhodium (II) carboxylates
correlated with their biologic activity. J Med Chem. 20:943–946.
1977. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Melnyk P, Leroux V, Sergheraert C and
Grellier P: Design, synthesis and in vitro antimalarial activity of
an acylhydrazone library. Bioorg Med Chem Lett. 16:31–35. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wardakhan WW, El-Sayed NN and Mohareb RM:
Synthesis and anti-tumor evaluation of novel hydrazide and
hydrazide-hydrazone derivatives. Acta Pharm. 63:45–57. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Aranha PE, dos Santos MP, Romera S and
Dockal ER: Synthesis, characterization, and spectroscopic studies
of tetradentate Schiff base chromium (III) complexes. Polyhedron.
26:1373–1382. 2007. View Article : Google Scholar
|
|
5
|
Bernhardt PV, Chin P, Sharpe PC, Wang JY
and Richardson DR: Novel diaroylhydrazine ligands as iron
chelators: Coordination chemistry and biological activity. J Biol
Inorg Chem. 10:761–777. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Suzen S, Tekiner-Gulbas B, Shirinzadeh H,
Uslu D, Gurer-Orhan H, Gumustas M and Ozkan SA: Antioxidant
activity of indole-based melatonin analogues in erythrocytes and
their voltammetric characterization. J Enzyme Inhib Med Chem.
28:1143–1155. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shaabani B, Khandar AA, Mobaiyen H,
Ramazani N, Balula SS and Cunha-Silva L: Novel pseudohalide-bridged
Cu(II) complexes with a hydrazone ligand: Evaluation of
antimicrobial activity. Polyhedron. 80:166–172. 2014. View Article : Google Scholar
|
|
8
|
Drover MW, Tandon SS, Anwar MU, Shuvaev
KV, Dawe LN, Collins JL and Thompson LK: Polynuclear complexes of a
series of hydrazone and hydrazone-oxime ligands-M2 (Fe), M4 (Mn,
Ni, Cu) and Mn (Cu) examples. Polyhedron. 68:94–102. 2014.
View Article : Google Scholar
|
|
9
|
Banerjee S, Sen S, Basak S, Mitra S,
Hughes DL and Desplanches C: Two new pseudohalide-bridged Cu (II)
complexes with a hydrazone ligand: Syntheses, crystal structures
and magnetic studies. Inorganica Chimica Acta. 361:2707–2714. 2008.
View Article : Google Scholar
|
|
10
|
Rollas S and Küçükgüzel SG: Biological
activities of hydrazone derivatives. Molecules. 12:1910–1939. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Narang R, Narasimhan B and Sharma S: A
review on biological activities and chemical synthesis of hydrazide
derivatives. Curr Med Chem. 19:569–612. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Judge V, Narasimhan B and Ahuja M:
Isoniazid: The magic molecule. Med Chem Res. 21:3940–3957. 2012.
View Article : Google Scholar
|
|
13
|
Vicini P, Zani F, Cozzini P and
Doytchinova I: Hydrazones of 1,2-benzisothiazole hydrazides:
Synthesis, antimicrobial activity and QSAR investigations. Eur J
Med Chem. 37:553–564. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kumar P, Narasimhan B, Sharma D, Judge V
and Narang R: Hansch analysis of substituted benzoic acid
benzylidene/furan-2-yl-methylene hydrazides as antimicrobial
agents. Eur J Med Chem. 44:1853–1863. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Altintop MD, Özdemir A, Turan-Zitouni G,
Ilgın S, Atlı Ö, Demirci F and Kaplancıklı ZA: Synthesis and in
vitro evaluation of new nitro-substituted thiazolyl hydrazone
derivatives as anticandidal and anticancer agents. Molecules.
19:14809–14820. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang B, Zhao Y, Zhai X, Wang L, Yang J,
Tan Z and Gong P: Design, synthesis and anticancer activities of
diaryl urea derivatives bearing N-acylhydrazone moiety. Chem Pharm
Bull (Tokyo). 60:1046–1054. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xia Y, Fan CD, Zhao BX, Zhao J, Shin DS
and Miao JY: Synthesis and structure-activity relationships of
novel 1-arylmethyl-3-aryl-1H-pyrazole-5-carbohydrazide hydrazone
derivatives as potential agents against A549 lung cancer cells. Eur
J Med Chem. 43:2347–2353. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jia L, Xu J, Zhao X, Shen S, Zhou T, Xu Z,
Zhu T, Chen R, Ma T, Xie J, et al: Synthesis, characterization, and
antitumor activity of three ternary dinuclear copper (II) complexes
with a reduced Schiff base ligand and diimine coligands in vitro
and in vivo. J Inorg Biochem. 159:107–119. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sheldrick G: SADABS, Program for Empirical
Absorption Correction of Area Detector Data. University of
Göttingen; Göttingen, Germany: 1996
|
|
20
|
Sheldrick G: SHELX-97, Program for the
Solution and Refinement of Crystal Structures. University of
Göttingen; Göttingen, Germany: 1997
|
|
21
|
Singh P, Singh DP and Singh VP: Synthesis,
spectral and single crystal X-ray diffraction studies on Mn(II),
Ni(II), Cu(II) and Zn(II) complexes with 2-hydroxy-benzoic acid
(phenyl-pyridin-2-yl-methylene)-hydrazide. Polyhedron. 81:56–65.
2014. View Article : Google Scholar
|
|
22
|
Krishnamoorthy P, Sathyadevi P, Cowley AH,
Butorac RR and Dharmaraj N: Evaluation of DNA binding, DNA
cleavage, protein binding and in vitro cytotoxic activities of
bivalent transition metal hydrazone complexes. Eur J Med Chem.
46:3376–3387. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lewis FD and Barancyk SV: Lewis acid
catalysis of photochemical reactions. 8. Photodimerization and
cross-cycloaddition of coumarin. J Am Chem Soc. 111:8653–8661.
1989. View Article : Google Scholar
|
|
24
|
Hao ZY, Liu QW, Xu J, Jia L and Li SB:
Synthesis, characterization, antioxidant activities, and
DNA-binding studies of (E)-N'-[1-(pyridin-2-yl)ethylidene]
isonicotinohydrazide and its Pr (III) and Nd (III) complexes. Chem
Pharm Bull (Tokyo). 58:1306–1312. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pflaum J, Schlosser S and Müller M: p53
family and cellular stress responses in cancer. Front Oncol.
4:2852014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mihara M, Erster S, Zaika A, Petrenko O,
Chittenden T, Pancoska P and Moll UM: p53 has a direct apoptogenic
role at the mitochondria. Mol Cell. 11:577–590. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chipuk JE, Kuwana T, Bouchier-Hayes L,
Droin NM, Newmeyer DD, Schuler M and Green DR: Direct activation of
Bax by p53 mediates mitochondrial membrane permeabilization and
apoptosis. Science. 303:1010–1014. 2004. View Article : Google Scholar : PubMed/NCBI
|