Introduction
Colorectal cancer (CRC) or colon cancer, is the
third most frequently diagnosed type of cancer in males, the second
in females and the fourth most frequent cause of cancer mortality
(1,2).
In 2012, it was estimated that 1,360,602 million new cases (women,
614,304; men, 746,298) of CRC were diagnosed (3). The burden of CRC worldwide is expected
to increase by 60% to >2.2 million new cases and 1.1 million
CRC-associated mortalities by 2030 (4). The curative or palliative treatment for
colon cancer is dependent on various factors, including the
patient's health and tumor stage, and curative surgery is typically
recommended if colon cancer is diagnosed early and palliative care
is provided for advanced colon cancer (5,6). The U.S.
National Comprehensive Cancer Network and American Society of
Clinical Oncology have provided guidelines for the recommended
follow-up of colon cancer (7,8) and have identified that surveillance and
detailed follow-up following curative resection of colon cancer may
reduce the 5-year mortality rate and improve overall survival (OS)
and the re-resection rate for recurrent disease (9–11).
However, complications from surgery still occur frequently,
resulting in an average length of hospital stay of 8–12 days
subsequent to a standard colon resection and, therefore, the risk
factors following colectomy for colon cancer require further
investigation (12,13).
The prognosis of patients with colon cancer is
dependent on clinical, pathological and biological factors,
including age (14), gender, Dukes'
stage (15), quantity of blood loss
during surgery (16), lymph node (LN)
invasion, bowel wall penetration, invasive margin character and
type of tumor, and these may be effective prognostic indicators for
predicting the survival rate of patients with colon cancer
following surgery (17–20). In addition, patients with tumor
perforation or incomplete resection have a poor prognosis following
curative resection of Dukes' B colon cancer (21). In previous studies, p53 protein
expression levels have been established to predict the outcome of
Dukes' stage B CRC (22) and the p53
status may provide an effective prognostic factor for patients with
Dukes' B and C colon cancer (23).
The p53 tumor suppressor protein (encoded by TP53) is
involved in DNA damage repair, cell cycle regulation, apoptosis,
aging and cellular senescence and is mutated in ~50% of types of
cancer (24). Additionally, p53
overexpression is associated with a reduced survival rate in
patients with stage III tumors and p53 expression levels are able
to predict CRC biology and clinical behavior (25,26). Using
multivariate analysis, a p53 codon 72 mutation, a mutation hotspot
identified in Egyptian populations, has been suggested to be an
independent prognostic factor for disease-free survival (DFS) in
patients with colon cancer from this geographical region (27). In addition, p53 overexpression was an
independent predictor of tumor recurrence in patients with colon
cancer (28) and increased nuclear
p53 expression levels were associated with the presence of distal
metastasis in stage II colon cancer (29). p53 immunoreactivity was implicated to
be closely associated with clinicopathological variables, including
pathological type, lymphocytic infiltration, tumor grade and the
tumor site in CRC (30). The p53
functional status was demonstrated to provide a prognostic marker
for patients with colon cancer (31);
however, a previous study did not identify a significant
association between p53 and DFS or OS in patients with locally
advanced colon cancer (32). The
prognosis of patients with colon cancer is primarily dependent on
standard clinicopathological factors. In the present study, the
association of p53 expression levels with clinicopathological
features and prognosis was investigated to evaluate their
prognostic value in patients with colon cancer following
surgery.
Materials and methods
Study design and subjects
Between December 2003 and December 2011, 484
patients with primary colon cancer were enrolled onto the present
study at the Department of Gastroenterology, The Affiliated ZhongDa
Hospital of Southeast University (Nanjing, China). The inclusion
criteria were as follows: i) Patients that underwent radical and
palliative surgery for colon cancer treatment; ii) patients with a
pathologically confirmed colon cancer diagnosis; and iii) patients
with complete follow-up data. The exclusion criteria were as
follows: i) Patients who had preoperative chemotherapy or
radiotherapy; and ii) patients with pathologically positive
margins. The patients were composed of 296 males and 188 females
with an average age of 61.94 years (range, 25–88). The age
distribution was categorized as <40 (n=63), 40–60 (n=226) and
>60 years (n=195). There were 58 cases with Dukes' A colon
cancer, 189 cases with Dukes' B colon cancer, 201 cases with Dukes'
C colon cancer and 36 cases with Dukes' D colon cancer according to
the revised Dukes' staging (33). The
types of tissue samples included 210 mass samples, 183 ulceration
samples and 91 invasion samples.
Following approval from the Affiliated ZhongDa
Hospital of Southeast University, written informed consent was
obtained from all patients. Protocols of the present study were
based on the ethical principles of The Declaration of Helsinki for
medical research that includes human patients (34).
Immunohistochemistry
Surgically resected tumor specimens from enrolled
patients were obtained and were formalin-fixed and
paraffin-embedded to create tissue blocks from which 4 µm tissue
sections were cut. Conventional dewaxing and hydration with a
series of graded alcohol was performed. Briefly, the tissue
sections were rinsed with dimethylbenzene twice (10 min/wash),
followed by rinsing with a descending series of graded alcohol
(100, 95, 90, 80 and 70%; 3–5 min/wash) and final 3 min rinse in
distilled water. Subsequent antigen retrieval was performed using a
microwave, followed by the addition of 3% hydrogen peroxide to
block endogenous peroxidase activity. Primary mouse anti-human p53
antibody (DO-7; 1:100; cat. no. MAB-0674; Fuzhou Maixin Biotech
Co., Ltd., Fuzhou, China) was added, and the tissue sections were
incubated at 4°C overnight. Following incubation for 20 min, the
sections were treated with horseradish peroxidase-labeled goat
anti-mouse secondary antibody (1:1,000; cat. no. ab6785; Abcam,
Cambridge, MA, USA) for 30 min at room temperature. The
immunohistochemistry analysis was performed using an SP
ultrasensitive immunostaining kit according to the manufacturer's
protocol, followed by DAB for visualization (both Fuzhou Maixin
Biotech Co., Ltd.). The replacement of the primary antibody with
PBS was used as a negative control. As a positive control, tissue
sections that were positively stained for p53 were included in each
staining run (Fuzhou Maixin Biotech Co., Ltd.). If ≥10% of the
malignant nuclei were stained, the slide was scored as positive
based on the standard scoring for p53 expression status as
described in a previous study (23).
Follow-up of patients with colon
cancer following surgery
All patients were followed up via outpatient review,
telephone calls, letters and other forms of communication. The
follow-up time was measured from the date of surgery and patients
were followed up semiannually during the first 2 to 5 years and
annually thereafter. All follow-ups were completed by December
2013, there was a median follow-up duration of 43 months and the
longest follow-up was 120 months. The survival rate was calculated
from the time of surgery to the date of mortality, or date of final
follow-up.
Statistical analysis
Clinical and pathological data obtained from the
follow-up of patients were coded into a database using Microsoft
Excel 2010 (Microsoft Corporation, Redmond, WA, USA). Statistical
analysis was performed using SPSS software (version 18.0; SPSS
Inc., Chicago, IL, USA). Statistical analysis was performed using
the χ2 test. Survival distributions were estimated using
Kaplan-Meier survival analysis. Estimated probabilities of survival
rate were compared with the log-rank method. To identify
independent prognostic factors, variables that were significantly
associated with the prognostic information from the univariate
analysis were applied in the Cox regression model. P<0.05 was
considered to indicate a statistically significant difference.
Results
Clinical characteristics
The clinical and pathological characteristics of 484
patients with colon cancer are presented in Table I. There were 96 patients (19.38%) with
a disease course length of <1 month, 152 cases (31.40%) with 1–3
months, 65 cases (13.43%) with 3–6 months, 75 cases (15.50%) with
6–12 months and 96 cases (19.84%) with ≥12 months. The median
disease course length was 3–6 months. The tumor location was
categorized as right (cecum, ascending colon and hepatic flexure),
transverse and left (splenic flexure, descending colon and sigmoid
colon), and these accounted for 245 cases (50.62%), 50 cases
(10.33%) and 189 cases (39.05%), respectively. With regard to the
type of surgery that was selected, 403 cases (83.26%) had radical
surgery and 81 cases (16.74%) had palliative surgery for the
treatment of colon cancer. The quantities of perioperative blood
transfusion that were administered to patients were 0 ml (did not
have a blood transfusion) in 295 cases (60.95%), ≤400 ml in 100
cases (20.66%) and 400–800 ml in 89 cases (18.39%). A maximum tumor
diameter of ≤4 cm was observed in 212 cases (43.80%), 4–8 cm in 255
cases (52.69%) and >8 cm in 17 cases (3.51%). Gross types of
colon cancer tissue samples were categorized as fungating type (210
cases, 43.39%), infiltrative type (91 cases, 18.80%) and ulcerative
type (183 cases, 37.81%), respectively. The histological subtypes
were divided into moderately/well-differentiated adenocarcinomas
(383 cases, 79.13%), poorly differentiated adenocarcinoma (43
cases, 8.89%), mucinous adenocarcinoma (49 cases, 10.12%) and
signet-ring cell carcinoma (9 cases, 1.86%).
 | Table I.Clinical and pathological
characteristics from patients with colon cancer. |
Table I.
Clinical and pathological
characteristics from patients with colon cancer.
| Characteristics | Number of patients
(%) |
|---|
| Gender |
|
|
Male | 296 (61.16) |
|
Female | 188 (38.84) |
| Age, years |
|
|
<40 | 63 (13.02) |
|
40–60 | 226 (46.69) |
|
>60 | 195 (40.29) |
| Time course of
disease, months |
|
|
<1 | 96 (19.83) |
|
1–3 | 152 (31.40) |
|
3–6 | 65 (13.43) |
|
6–12 | 75 (15.50) |
|
≥12 | 96 (19.84) |
| Tumor location |
|
| Left
hemicolon | 245 (50.62) |
| Colon
transversum | 50 (10.33) |
| Right
hemicolon | 189 (39.05) |
| Surgery |
|
| Radical
surgery | 403 (83.26) |
|
Palliative surgery | 81 (16.74) |
| Perioperative blood
transfusion, ml |
|
| 0 | 295 (60.95) |
|
≤400 | 100 (20.66) |
|
400–800 | 89 (18.39) |
| Tumor size, cm |
|
| ≤4 | 212 (43.80) |
|
4–8 | 255 (52.69) |
|
>8 | 17 (3.51) |
| Borrmann type |
|
|
Fungating | 210 (43.39) |
|
Infiltrative | 91 (18.80) |
|
Ulcerative | 183 (37.81) |
| Histological
subtypes |
|
|
Moderately/well-differentiated | 383 (79.13) |
|
adenocarcinomas |
|
| Poorly
differentiated adenocarcinoma | 43 (8.89) |
|
Mucinous adenocarcinoma | 49 (10.12) |
|
Signet-ring cell
carcinoma | 9 (1.86) |
| Depth of
invasion |
|
| Mucosa
and submucosa | 24 (4.96) |
|
Muscular coat | 33 (6.82) |
|
Full-thickness | 243 (50.21) |
| Beyond
serosa | 184 (38.02) |
| Dukes' stage |
|
| Dukes'
A | 58 (11.98) |
| Dukes'
B | 189 (39.05) |
| Dukes'
C | 201 (41.53) |
| Dukes'
D | 36 (7.44) |
| Distant
metastasis |
|
|
Positive | 40 (8.26) |
|
Negative | 444 (91.74) |
| Lymph node
metastasis |
|
|
Positive | 228 (47.11) |
|
Negative | 256 (52.89) |
| p53 status |
|
|
Positive | 253 (52.27) |
|
Negative | 231 (47.73) |
Association of p53 expression with
clinical characteristics
As summarized in Table
II, positive nuclear p53 immunoreactivity was identified in 253
patients (52.27%) and tumors from 231 patients (47.73%) were
negative for p53 expression. The p53 expression status (positive or
negative) was significantly different between patient subgroups
when categorized according to age distribution, disease course,
tumor location, maximum tumor diameter, depth of tumor invasion,
Dukes' stage, distant metastasis and LN metastasis (P<0.05).
However, p53 expression levels were not statistically different
between the two genders or the gross tumor type.
 | Table II.The association of p53 expression
levels with clinicopathological characteristics in patients with
colon cancer. |
Table II.
The association of p53 expression
levels with clinicopathological characteristics in patients with
colon cancer.
|
| p53 expression
status |
|
|
|
|---|
|
|
|
|
|
|
|---|
| Clinical pathology
index | Positive | Negative | Positive rate,
% | χ2 | P-value |
|---|
| Gender |
|
|
| 0.056 | 0.812 |
|
Male | 156 | 140 | 52.70 |
|
|
|
Female | 97 | 91 | 51.59 |
|
|
| Age, years |
|
|
| 27.241 | <0.001 |
|
<40 | 28 | 35 | 44.44 |
|
|
|
40–60 | 95 | 131 | 42.04 |
|
|
|
>60 | 130 | 65 | 66.51 |
|
|
| Time course of
disease, months |
|
|
| 11.644 | 0.020 |
|
<1 | 50 | 46 | 52.08 |
|
|
|
1–3 | 92 | 60 | 59.21 |
|
|
|
3–6 | 23 | 42 | 35.38 |
|
|
|
6–12 | 39 | 36 | 52.00 |
|
|
|
≥12 | 49 | 47 | 51.04 |
|
|
| Tumor site |
|
|
| 12.352 | 0.002 |
| Left
hemicolon | 147 | 98 | 60.00 |
|
|
| Colon
transversum | 20 | 30 | 40.00 |
|
|
| Right
hemicolon | 86 | 103 | 45.5 |
|
|
| Tumor size, cm |
|
|
| 29.932 | <0.001 |
| ≤4 | 81 | 131 | 38.21 |
|
|
|
4–8 | 161 | 94 | 63.14 |
|
|
|
>8 | 11 | 6 | 64.71 |
|
|
| Borrmann type |
|
|
| 4.294 | 0.117 |
|
Fungating | 102 | 108 | 48.57 |
|
|
|
Infiltrative | 56 | 35 | 61.54 |
|
|
|
Ulcerative | 95 | 88 | 51.91 |
|
|
| Histological
subtypes |
|
|
| 9.421 | 0.024 |
|
Moderately/well-differentiated
adenocarcinomas | 187 | 196 | 48.83 |
|
|
| Poorly
differentiated adenocarcinoma | 30 | 13 | 69.77 |
|
|
|
Mucinous adenocarcinoma | 30 | 19 | 61.22 |
|
|
|
Signet-ring cell
carcinoma | 6 | 3 | 66.67 |
|
|
| Distant
metastasis |
|
|
| 4.053 | 0.044 |
|
Positive | 27 | 13 | 67.50 |
|
|
|
Negative | 226 | 218 | 50.90 |
|
|
| Lymph node
metastasis |
|
|
| 35.801 | <0.001 |
|
Positive | 152 | 76 | 66.67 |
|
|
|
Negative | 101 | 155 | 39.45 |
|
|
| Dukes' stage |
|
|
| 41.643 | <0.001 |
| Dukes'
A | 17 | 41 | 29.31 |
|
|
Univariate survival rate analysis
The mean OS was 79.78±3.85 months for patients with
colon cancer at the time of the final follow-up. The 3-, 5- and
10-year survival rates were 68.02, 59.70 and 52.27%, respectively.
The univariate analysis (Table III)
revealed that age, course of disease, tumor location, surgery,
perioperative blood transfusion, tumor size, histological subtypes,
LN metastasis, depth of invasion, distant metastasis, Dukes' stage
and p53 protein expression status were significantly associated
with postoperative survival rate in patients with colon cancer
(P<0.05). Statistical associations were not detected between
gender and gross tumor type.
 | Table III.Univariate analysis of associations
between clinicopathological characteristics and follow-up data in
patients with colon cancer. |
Table III.
Univariate analysis of associations
between clinicopathological characteristics and follow-up data in
patients with colon cancer.
|
Characteristics | Number of patients
(%) | Average length of
survival, months | 10-year survival
rate | P-value |
|---|
| Gender |
|
|
| 0.162 |
|
Male | 296 (61.16) | 79.47±3.37 | 49.66 |
|
|
Female | 188 (38.84) | 79.87±3.51 | 56.38 |
|
| Age, years |
|
|
| <0.001 |
|
<40 | 63 (13.02) | 80.96±4.68 | 50.79 |
|
|
40–60 | 226 (46.69) | 79.39±5.97 | 63.27 |
|
|
>60 | 195 (40.29) | 66.41±4.02 | 40.00 |
|
| Time course of
disease, month |
|
|
| 0.039 |
|
<1 | 96 (19.83) | 80.16±2.85 | 48.96 |
|
|
1–3 | 152 (31.40) | 77.88±3.53 | 45.39 |
|
|
3–6 | 65 (13.43) | 80.56±3.42 | 56.92 |
|
|
6–12 | 75 (15.50) | 78.47±3.43 | 66.67 |
|
|
≥12 | 96 (19.83) | 80.12±4.01 | 52.08 |
|
| Tumor location |
|
|
| 0.006 |
| Left
hemicolon | 245 (50.62) | 74.96±5.21 | 46.12 |
|
| Colon
transversum | 50 (10.33) | 85.37±5.67 | 64.00 |
|
| Right
hemicolon | 189 (39.05) | 84.69±3.68 | 59.26 |
|
| Surgery |
|
|
| <0.001 |
| Radical
surgery | 403 (83.26) | 94.18±3.68 | 60.54 |
|
|
Palliative surgery | 81 (16.74) | 26.08±2.82 | 11.11 |
|
| Perioperative blood
transfusion, ml |
|
|
| 0.042 |
| 0 | 295 (60.95) | 81.23±4.66 | 53.89 |
|
|
≤400 | 100 (20.66) | 78.50±6.72 | 55.00 |
|
|
400–800 | 89 (18.39) | 66.18±8.06 | 40.44 |
|
| Tumor size, cm |
|
|
| 0.024 |
| ≤4 | 212 (43.80) | 98.05±7.09 | 70.75 |
|
|
4–8 | 255 (52.69) | 78.50±4.90 | 38.04 |
|
|
>8 | 17 (3.51) | 66.18±7.07 | 35.29 |
|
| Borrmann type |
|
|
| 0.516 |
|
Fungating | 210 (43.39) | 84.59±5.51 | 60.23 |
|
|
Infiltrative | 91 (18.80) | 67.15±3.65 | 49.45 |
|
|
Ulcerative | 183 (37.81) | 79.36±3.05 | 50.27 |
|
| Histological
subtypes |
|
|
| <0.001 |
|
Moderately/well-differentiated
adenocarcinomas | 383 (79.13) | 97.15±6.98 | 56.4 |
|
| Poorly
differentiated adenocarcinoma | 43 (8.89) | 66.70±5.70 | 30.77 |
|
|
Mucinous adenocarcinoma | 49 (10.12) | 86.74±5.98 | 48.82 |
|
|
Signet-ring cell
carcinoma | 9 (1.86) | 22.61±5.22 | 0.00 |
|
| Depth of
invasion |
|
|
| <0.001 |
| Mucosa
and submucosa | 24 (4.96) | 116.72±5.82 | 91.67 |
|
|
Muscular coat/muscularis | 33 (6.82) | 106.21±4.65 | 84.85 |
|
|
Full-thickness | 243 (50.21) | 80.33±4.51 | 60.90 |
|
| Beyond
serosa | 184 (38.02) | 39.59±3.62 | 29.89 |
|
| Dukes' stage |
|
|
| <0.001 |
| Dukes'
A | 58 (11.98) | 116.44±2.84 | 93.1 |
|
| Dukes'
B | 189 (39.05) | 101.86±3.04 | 64.55 |
|
| Dukes'
C | 201 (41.53) | 66.83±7.13 | 36.82 |
|
| Dukes'
D | 36 (7.44) | 29.13±3.98 | 8.33 |
|
| Distant
metastasis |
|
|
|
0.004 |
|
Positive | 40 (8.26) | 39.59±3.29 | 12.50 |
|
|
Negative | 444 (91.74) | 87.58±4.96 | 55.85 |
|
| Lymph node
metastasis |
|
|
| <0.001 |
|
Positive | 228 (47.11) | 44.59±4.74 | 33.77 |
|
|
Negative | 256 (52.89) | 86.74±5.06 | 68.75 |
|
| p53 status |
|
|
| <0.001 |
|
Positive | 253 (52.27) | 37.61±2.61 | 30.83 |
|
|
Negative | 231 (47.73) | 85.18±3.68 | 75.76 |
|
Multivariate survival rate
analysis
In the multivariate analysis, age, surgery,
histological subtypes, tumor size, tumor location, LN metastasis,
distant metastasis, Dukes' stage and p53 expression levels were
identified as independent factors that may influence the survival
rate of patients with colon cancer following surgery (P<0.05;
Table IV; Fig. 1).
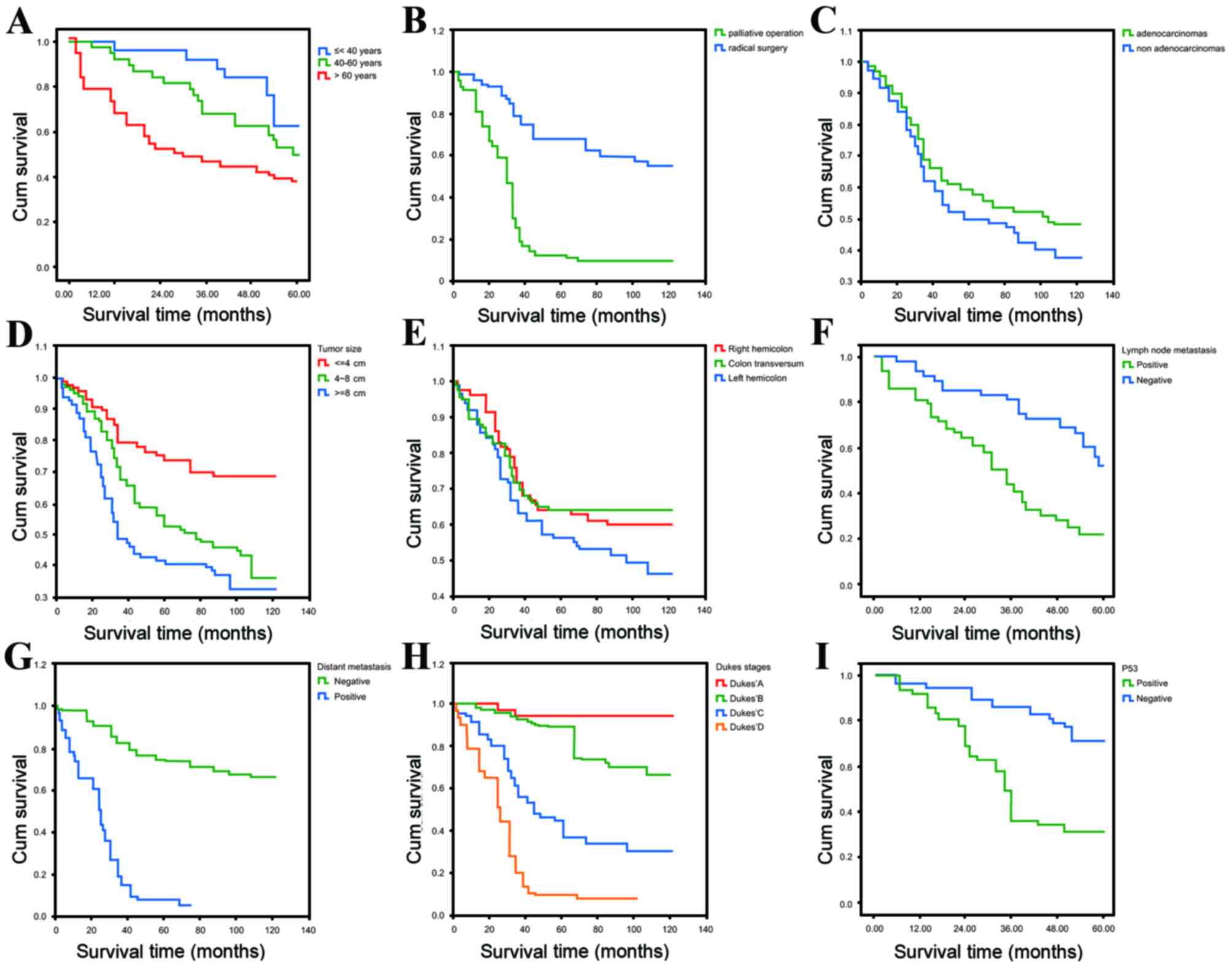 | Figure 1.Kaplan-Meier curves estimating the
10-year survival rate following stratifying patients with colon
cancer according to (A) age (<40, 40–60 and >60 years), (B)
surgery (radical surgery or palliative surgery), (C) histological
subtypes (adenocarcinomas or non-adenocarcinomas), (D) tumor size
(≤4, 4–8 and >8 cm), (E) tumor location (left hemicolon, colon
transversum or right hemicolon), (F) lymph node metastasis
(positive or negative), (G) distant metastasis (positive or
negative), (H) Dukes' stages (Dukes' A, Dukes' B, Dukes' C and
Dukes' D) and (I) p53 status (positive or negative). Cum,
cumulative. |
 | Table IV.Multivariate analysis for overall
survival rate in patients with different prognostic variations of
colon cancer. |
Table IV.
Multivariate analysis for overall
survival rate in patients with different prognostic variations of
colon cancer.
| Characteristic | B | SE | Wald
χ2 | P-value | EXP(B) | 95% CI |
|---|
| Age | 0.022 | 0.055 | 0.158 | 0.011 | 1.522 | 1.018–1.018 |
| Course of
disease | −0.320 | 0.286 | 1.444 | 0.230 | 0.726 | 0.431–1.431 |
| Surgery | 0.789 | 0.257 | 9.424 | 0.002 | 2.200 | 1.330–3.330 |
| Blood
transfusion | −0.028 | 0.091 | 0.082 | 0.775 | 0.974 | 0.818–1.818 |
| Pathological
type | 0.157 | 0.052 | 9.026 | 0.003 | 1.170 | 1.056–1.056 |
| Tumor diameter | 0.149 | 0.063 | 5.487 | 0.019 | 1.160 | 1.025–1.025 |
| Tumor site | 0.087 | 0.035 | 6.022 | 0.014 | 1.091 | 1.018–1.018 |
| Lymph node
metastasis | 0.406 | 0.072 | 31.607 | <0.001 | 1.501 | 1.303–1.303 |
| Distant
metastasis | 0.286 | 0.087 | 10.891 | 0.001 | 1.332 | 1.123–1.123 |
| Dukes' stage | 0.830 | 0.159 | 27.393 | <0.001 | 2.293 | 1.680–3.680 |
| p53 expression | 0.864 | 0.400 | 27.939 | 0.031 | 2.372 | 1.680–3.680 |
Discussion
The present study enrolled 484 patients with primary
colon cancer that had radical and palliative surgery and complete
follow-up data was available. The clinicopathological features and
colon cancer-associated prognostic factors were investigated to
provide a reliable theoretical understanding of influencing factors
in the prognosis of colon cancer, based on long-term follow-up data
that was applicable to the patients in the geographical location of
the present study. There were significant differences in p53
expression levels in various categories, including age
distribution, disease course, tumor location, tumor size, depth of
invasion, Dukes' stage, distant metastasis and LN metastasis, which
suggests that the loss of p53 expression is associated with
aggressive clinicopathological features in patients with colon
cancer. In addition, the age, surgery type, histological subtypes,
tumor size, tumor location, LN metastasis, distant metastasis,
Dukes' stage and p53 expression status were independent factors
that influenced the survival rate of patients with colon cancer
following surgery.
A previous retrospective tumor microarray study also
established that p53 may be a potential biological diagnostic
marker (29) and p53 status has been
demonstrated to be associated with colon cancer prognosis (15). However, a previous study was not able
to demonstrate a significant association between p53 expression
levels and clinical outcomes in patients with colon cancer
(35). Notably, the association
between p53 expression levels and clinicopathological data in
patients with colon cancer was evaluated in a previous study, and
the results suggested that right-sided colon tumors may develop in
a p53-independent manner and, therefore, p53 status in cancer cells
has prognostic value only for left-sided colorectal tumors
(36) However, the association
between p53 expression levels and clinicopathological
characteristics in CRC has yet to be elucidated. A previous study
demonstrated that p53 nuclear expression levels were not associated
with a patient gender, age, tumor location, differentiation, T
stage, N stage or status of lymphatic and vascular vessel invasion
(37). The presence of p53 protein in
tumor cells was not associated with age, gender, nodal status and
tumor stage, but it was previously identified that well to
moderately differentiated tumors exhibited significantly higher
expression levels of p53 compared with poorly differentiated tumors
(38). However, the present study
indicated that the loss of p53 expression in colon cancer may be a
predictor of a more aggressive tumor phenotype and other
clinicopathological characteristics may also require consideration
in conjunction with p53 status to design an effective follow-up
strategy to improve patient survival rate.
Clinicopathological characteristics have been used
as reliable prognostic factors for the clinical outcome of colon
cancer, including age and tumor location, and may predict the
development of metastases, which cause mortality in patients with
colon cancer (39–41). When the length of survival was
stratified by tumor location and analyzed at the end of the
follow-up period, the survival rate in patients with right and left
colon tumors were 53.9 and 51.4 months, respectively (42). With respect to surgery type, advances
in surgical procedures are associate with improved outcomes and
previous studies have identified that surgical treatment of
metastases is an independent prognostic factor for OS (41,43).
Pulmonary metastasis has been demonstrated to occur in patients
with low stage colon tumors and the survival rate following
thoracotomy is dependent on the number of metastases (44). Patients with unilateral metastasis and
Dukes' A primary tumor type exhibit the highest increase in
survival rate following the resection of pulmonary metastasis from
CRC (45). Furthermore, LN metastasis
is a poor prognostic factor for colon cancer and the number of
involved LNs has been implicated in the survival rate of patients
with stage II and stage III colon cancer following surgical
resection, which is consistent with the number of involved LNs
providing an indicator of the care that the patient with colon
cancer requires (17,46). Concordant with this, LN yield (LNY) is
an independent prognostic factor in colon cancer and ≥12 LNs in the
resected specimen obtained from surgery is the current recommended
standard, regardless of age or disease site, and LNY is increased
in right-sided colon cancer and reduces with age (47). It has been established using
multivariate analysis that LN micrometastasis and lymphatic
invasion are independent prognostic factors for CRC (48). The histological pattern may also be
associated with an increased survival rate and patients with grade
I moderately-differentiated tumors exhibit the highest rate of
survival (41,42). Several clinicopathological factors
were not previously identified to be effective independent
predictors of survival rate in patients with colon cancer,
including Dukes' stage, T stage, number of resected nodes and
vascular or lymphatic invasion (49).
In conclusion, the results of the present study
revealed that p53 expression levels are associated with the
clinicopathological features of patients with colon cancer within
the sampled geographical area. Additionally, the age, surgery type,
histological patterns, tumor size, tumor location, LN metastasis,
distant metastasis, Dukes' stage and p53 expression levels are
independent factors that may influence the survival rate of
patients with colon cancer following surgery.
Acknowledgements
The authors would like to acknowledge the helpful
comments on this study received from the reviewers.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Merika E, Saif MW, Katz A, Syrigos K and
Morse M: Review. Colon cancer vaccines: An update. In vivo.
24:607–628. 2010.PubMed/NCBI
|
|
3
|
Lee DH, Keum N and Giovannucci EL:
Colorectal cancer epidemiology in the nurses' health study. Am J
Public Health. 106:1599–1607. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Arnold M, Sierra MS, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global patterns and trends in
colorectal cancer incidence and mortality. Gut pii:
gutjnl-2015-310912. 2016.(Epub ahead of print).
|
|
5
|
Stein A, Atanackovic D and Bokemeyer C:
Current standards and new trends in the primary treatment of
colorectal cancer. Eur J Cancer. 47:(Suppl 3). S312–S314. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cunningham D, Atkin W, Lenz HJ, Lynch HT,
Minsky B, Nordlinger B and Starling N: Colorectal cancer. Lancet.
375:1030–1047. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Desch CE, Benson AB III, Somerfield MR,
Flynn PJ, Krause C, Loprinzi CL, Minsky BD, Pfister DG, Virgo KS
and Petrelli NJ: American Society of Clinical Oncology: Colorectal
cancer surveillance: 2005 update of an American society of clinical
oncology practice guideline. J Clin Oncol. 23:8512–8519. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Engstrom PF, Arnoletti JP, Benson AB III,
Chen YJ, Choti MA, Cooper HS, Covey A, Dilawari RA, Early DS,
Enzinger PC, et al: NCCN clinical practice guidelines in oncology:
Colon cancer. J Natl Compr Canc Netw. 7:778–831. 2009.PubMed/NCBI
|
|
9
|
Jeffery M, Hickey BE and Hider PN:
Follow-up strategies for patients treated for non-metastatic
colorectal cancer. Cochrane Database Syst Rev. CD002200:2007.
View Article : Google Scholar
|
|
10
|
Tjandra JJ and Chan MK: Follow-up after
curative resection of colorectal cancer: A meta-analysis. Dis Colon
Rectum. 50:1783–1799. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Figueredo A, Rumble RB, Maroun J, Earle
CC, Cummings B, McLeod R, Zuraw L and Zwaal C: Gastrointestinal
Cancer Disease Site Group of Cancer Care Ontario's Program in
Evidence-based Care: Follow-up of patients with curatively resected
colorectal cancer: A practice guideline. BMC Cancer. 3:262003.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Longo WE, Virgo KS, Johnson FE, Oprian CA,
Vernava AM, Wade TP, Phelan MA, Henderson WG, Daley J and Khuri SF:
Risk factors for morbidity and mortality after colectomy for colon
cancer. Dis Colon Rectum. 43:83–91. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Debarros M and Steele SR: Perioperative
protocols in colorectal surgery. Clinics Colon Rrectal Surg.
26:139–145. 2013. View Article : Google Scholar
|
|
14
|
Siegel R, Desantis C and Jemal A:
Colorectal cancer statistics, 2014. CA Cancer J Clin. 64:104–117.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nasif WA, Lotfy M, El-Sayed IH, El-Kenawy
Ael M, El-Shahat M and El-Hak NG: Implications of CEA and p53
overexpression in the poor prognosis of colorectal cancer. Med
Oncol. 23:237–244. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Morner ME, Gunnarsson U, Jestin P and
Svanfeldt M: The importance of blood loss during colon cancer
surgery for long-term survival: An epidemiological study based on a
population based register. Ann Surg. 255:1126–1128. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chang GJ, Rodriguez-Bigas MA, Skibber JM
and Moyer VA: Lymph node evaluation and survival after curative
resection of colon cancer: Systematic review. J Natl Cancer Inst.
99:433–441. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gunderson LL, Jessup JM, Sargent DJ,
Greene FL and Stewart AK: Revised TN categorization for colon
cancer based on national survival outcomes data. J Clin Oncol.
28:264–271. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Parnaby CN, Scott NW, Ramsay G, MacKay C,
Samuel L, Murray GI and Loudon MA: Prognostic value of lymph node
ratio and extramural vascular invasion on survival for patients
undergoing curative colon cancer resection. Br J Cancer.
113:212–219. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
García-Solano J, Conesa-Zamora P,
Trujillo-Santos J, Mäkinen MJ and Pérez-Guillermo M: Tumour budding
and other prognostic pathological features at invasive margins in
serrated colorectal adenocarcinoma: A comparative study with
conventional carcinoma. Histopathology. 59:1046–1056. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Saha AK, Smith KJ, Sue-Ling H, Sagar PM,
Burke D and Finan PJ: Prognostic factors for survival after
curative resection of Dukes' B colonic cancer. Colorectal Dis.
13:1390–1394. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bouzourene H, Gervaz P, Cerottini JP,
Benhattar J, Chaubert P, Saraga E, Pampallona S, Bosman FT and
Givel JC: p53 and Ki-ras as prognostic factors for Dukes' stage B
colorectal cancer. Eur J Cancer. 36:1008–1015. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Allegra CJ, Paik S, Colangelo LH, Parr AL,
Kirsch I, Kim G, Klein P, Johnston PG, Wolmark N and Wieand HS:
Prognostic value of thymidylate synthase, Ki-67, and p53 in
patients with Dukes' B and C colon cancer: A national cancer
institute-national surgical adjuvant breast and bowel project
collaborative study. J Clin Oncol. 21:241–250. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lane DP: Cancer. p53, guardian of the
genome. Nature. 358:15–16. 1992. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Perrone G, Vincenzi B, Santini D, Verzì A,
Tonini G, Vetrani A and Rabitti C: Correlation of p53 and bcl-2
expression with vascular endothelial growth factor (VEGF),
microvessel density (MVD) and clinico-pathological features in
colon cancer. Cancer Lett. 208:227–234. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pu XX, Huang GL, Guo HQ, Guo CC, Li H, Ye
S, Ling S, Jiang L, Tian Y and Lin TY: Circulating miR-221 directly
amplified from plasma is a potential diagnostic and prognostic
marker of colorectal cancer and is correlated with p53 expression.
J Gastroenterol Hepatol. 25:1674–1680. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
El-Serafi MM, Bahnassy AA, Ali NM, Eid SM,
Kamel MM, Abdel-Hamid NA and Zekri AR: The prognostic value of
c-Kit, K-ras codon 12, and p53 codon 72 mutations in Egyptian
patients with stage II colorectal cancer. Cancer. 116:4954–4964.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liang JT, Huang KC, Jeng YM, Lee PH, Lai
HS and Hsu HC: Microvessel density, cyclo-oxygenase 2 expression,
K-ras mutation and p53 overexpression in colonic cancer. Br J Surg.
91:355–361. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Resnick MB, Routhier J, Konkin T, Sabo E
and Pricolo VE: Epidermal growth factor receptor, c-MET,
beta-catenin, and p53 expression as prognostic indicators in stage
II colon cancer: A tissue microarray study. Clin Cancer Res.
10:3069–3075. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nasif WA, El-Emshaty HM, Tabll A, Elmasry
SA, Attallah AM and Elhak Gad NA: Immunoreactivity evaluation of
mutant p53 gene product with DNA ploidy pattern in colorectal
carcinoma. Hepatogastroenterology. 51:1001–1006. 2004.PubMed/NCBI
|
|
31
|
Sarasqueta AF, Forte G, Corver WE, de
Miranda NF, Ruano D, van Eijk R, Oosting J, Tollenaar RA, van Wezel
T and Morreau H: Integral analysis of p53 and its value as
prognostic factor in sporadic colon cancer. BMC Cancer. 13:2772013.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Allegra CJ, Parr AL, Wold LE, Mahoney MR,
Sargent DJ, Johnston P, Klein P, Behan K, O'Connell MJ, Levitt R,
et al: Investigation of the prognostic and predictive value of
thymidylate synthase, p53, and Ki-67 in patients with locally
advanced colon cancer. J Clin Oncol. 20:1735–1743. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li M and Gu J: Changing patterns of
colorectal cancer in China over a period of 20 years. World J
Gastroenterol. 11:4685–4688. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Dilmeç F, Özgönül A, Akkafa F, Uzunkoy A
and Kuilenburg ABPV: Determination of ApaI and TaqI Polymorphisms
of VDR gene in a group of Turkish patients with colorectal cancer.
Hernia. 19:21258–21263. 2009.
|
|
35
|
Watanabe T, Wu TT, Catalano PJ, Ueki T,
Satriano R, Haller DG, Benson AB III and Hamilton SR: Molecular
predictors of survival after adjuvant chemotherapy for colon
cancer. N Engl J Med. 344:1196–1206. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Paluszkiewicz P, Berbec H,
Pawlowska-Wakowicz B, Cybulski M and Paszkowska A: p53 protein
accumulation in colorectal cancer tissue has prognostic value only
in left-sided colon tumours. Cancer Detect Prev. 28:252–259. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zeng ZS, Sarkis AS, Zhang ZF, Klimstra DS,
Charytonowicz E, Guillem JG, Cordon-Cardo C and Cohen AM: p53
nuclear overexpression: An independent predictor of survival in
lymph node-positive colorectal cancer patients. J Clin Oncol.
12:2043–2050. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lan YT, Chang SC, Li AF, Lin TC, Chen WS,
Jiang JK, Yang SH, Wang HS and Lin JK: p53 protein accumulation as
a prognostic marker in sporadic colorectal cancer. Int J Colorectal
Dis. 22:499–506. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Veruttipong D, Soliman AS, Gilbert SF,
Blachley TS, Hablas A, Ramadan M, Rozek LS and Seifeldin IA: Age
distribution, polyps and rectal cancer in the Egyptian
population-based cancer registry. World J Gastroenterol.
18:3997–4003. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Fang YJ, Wu XJ, Zhao Q, Li LR, Lu ZH, Ding
PR, Zhang RX, Kong LH, Wang FL, Lin JZ, et al: Hospital-based
colorectal cancer survival trend of different tumor locations from
1960s to 2000s. PLoS One. 8:e735282013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Elferink MA, de Jong KP, Klaase JM,
Siemerink EJ and de Wilt JH: Metachronous metastases from
colorectal cancer: A population-based study in North-East
Netherlands. Int J Colorectal Dis. 30:205–212. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Deliu IC, Georgescu EF and Bezna MC:
Analysis of prognostic factors in colorectal carcinoma. Rev Med
Chir Soc Med Nat Iasi. 118:808–816. 2014.PubMed/NCBI
|
|
43
|
Okuno K: Surgical treatment for digestive
cancer. Current issues- colon cancer. Dig Surg. 24:108–114. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Inoue M, Ohta M, Iuchi K, Matsumura A,
Ideguchi K, Yasumitsu T, Nakagawa K, Fukuhara K, Maeda H, Takeda S,
et al: Benefits of surgery for patients with pulmonary metastases
from colorectal carcinoma. Ann Thorac Surg. 78:238–244. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Inoue M, Ohta M, Iuchi K, Matsumura A,
Ideguchi K, Yasumitsu T, Nakagawa K, Fukuhara K, Maeda H, Takeda S,
et al: Benefits of surgery for patients with pulmonary metastases
from colorectal carcinoma. Ann Thorac Surg. 78:238–244. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kobayashi H, West NP, Takahashi K,
Perrakis A, Weber K, Hohenberger W, Quirke P and Sugihara K:
Quality of surgery for stage III colon cancer: Comparison between
England, Germany, and Japan. Ann Surg Oncol. 21:(Suppl 3).
S398–S404. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ahmadi O, Stringer MD, Black MA and McCall
JL: Influence of age and site of disease on lymph node yield in
colorectal cancer. N Z Med J. 127:31–40. 2014.PubMed/NCBI
|
|
48
|
Ueda Y, Yasuda K, Inomata M, Shiraishi N,
Yokoyama S and Kitano S: Biological predictors of survival in stage
II colorectal cancer. Mol Clin Oncol. 1:643–648. 2013.PubMed/NCBI
|
|
49
|
Galizia G, Ferraraccio F, Lieto E,
Orditura M, Castellano P, Imperatore V, Romano C, Vollaro M,
Agostini B, Pignatelli C and De Vita F: Prognostic value of p27,
p53, and vascular endothelial growth factor in Dukes A and B colon
cancer patients undergoing potentially curative surgery. Dis Colon
Rectum. 47:1904–1914. 2004. View Article : Google Scholar : PubMed/NCBI
|















