Introduction
Steroid sex hormones serve major roles as tumor
promoters by promoting cell cycle progression in several types of
cancer, including hepatocellular carcinoma (HCC) (1–3). The sex
hormone androgen and its associated androgen receptor (AR) are
involved in the development of the lipogenic phenotype of cancer
cells via the induction of proteins and enzymes, including fatty
acid synthase (FASN), required for their autonomous nutrition
(4).
A number of studies have implicated AR, a member of
the nuclear steroid receptor superfamily, in hepatocarcinogenesis
(1,3,5–7). AR is a transcription factor activated
via ligand-dependent and ligand-independent mechanisms (8). Cytochrome P450, transforming growth
factor β1, vascular endothelial growth factor A and heat shock
protein family A member 5 are target genes of ARs in the liver
(3,7,9,10). Crosstalk between ARs and liver X
receptor (nuclear receptor subfamily 1 group H member 2) has also
been implicated in maintaining cholesterol homeostasis (11). It has also been demonstrated that the
activity of ARs is coupled with the upregulation of FASN (4).
Abnormalities in cellular metabolism are closely
associated with HCC occurrence and development (4,12) and it
has been demonstrated that FASN is highly expressed in a number of
cancer types, including HCC (12). In
HCC patients, abnormalities in plasma phospholipid fatty acid
profiles are occasionally observed, which may be attributed to the
alteration of intrinsic fatty acid metabolism caused by the cancer
itself (13). Dietary fatty acids may
be implicated in the risk of developing non-viral
hepatitis-associated HCC (14);
however, there is data to refute this hypothesis (13).
Fatty acid metabolism also serves a role in human
carcinogenesis and is associated with a poor prognosis in HCC
patients. The present study aimed to investigate the effects of AR
expression on fatty acid metabolism-associated gene regulation in
human HCC cell lines.
Materials and methods
Cell culture
The human HCC cell lines HepG2 and Huh7, as
described previously (3), were
purchased from the Japanese Collection of Research Bioresources
Cell Bank (Ibaraki, Japan). The cells were maintained in Dulbecco's
modified Eagle's medium supplemented with 10% fetal calf serum
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) and grown at 37°C
with 5% CO2.
Transfection and RNA extraction
A total of 4×105 cells per well were
placed in 6-well plates 24 h prior to transfection. The cells were
transfected with 0.4 µg human AR protein-expressing plasmid
(pSG5-AR) or 0.4 µg control vectors, which were kindly provided by
Professor J.T. Isaacs of The Johns Hopkins University School of
Medicine (Baltimore, MD, USA) (15),
using Effectene Transfection Reagent (Qiagen GmbH, Hilden, Germany)
according to the manufacturer's protocol. At 48 h
post-transfection, cellular RNA was collected using RNeasy (Qiagen
GmbH), and RNA was stored at −80°C until use. RNA quality was
measured by a NanoDrop spectrophotometer (Thermo Fisher Scientific,
Inc., Waltham, MA, USA).
Complementary DNA (cDNA) synthesis and
quantiative polymerase chain reaction (qPCR) arrays
cDNA synthesis was performed by an RT2
First Strand kit (Qiagen GmbH) using 1 µg of RNA per reaction. The
cDNA synthesis reaction was incubated at 42°C for 15 min and then
halted by heating at 95°C for 5 min. A human fatty acid metabolism
PCR array (no. PAHS-007Z; Qiagen GmbH) was carried out according to
the manufacturer's protocol. Briefly, amplification of cDNA was
monitored with SYBR-Green by quantitative PCR analysis. PCR was
performed in 25 µl of ROX PCR Master mix (Qiagen GmbH) containing
primers and 1 µl of the reverse-transcription reaction mixture,
using a 7300 Real-Time PCR system (Applied Biosystems; Thermo
Fisher Scientific, Inc.) according to the manufacturer's protocol.
The PCR reaction was performed as follows: 95°C for 1 min, followed
by 40 cycles of 95°C for 5 sec and 60°C for 30 sec. The data
analysis was performed using the comparative threshold cycle method
(16) and analyzed using PCR Array
Data Analysis Software v2.1 (http://www.sabiosciences.com/pcrarraydataanalysis.php).
Western blot analysis
Cell lysates were prepared 48 h post transfection in
1X SDS sample buffer (Wako Pure Chemical Industries, Ltd., Osaka,
Japan). Protein concentration was determined using the Bradford
method. Cell lysate proteins (5 µg/well) were separated by 5–20%
SDS-PAGE and transferred onto a nitrocellulose membrane (ATTO
Corp., Tokyo, Japan). AR expression was analyzed by western
blotting by incubating membranes with rabbit polyclonal antibodies
against AR (no. sc-816; dilution, 1:1,000) or GAPDH (no. sc-25778;
dilution, 1:2,000) (both from Santa Cruz Biotechnology Inc.,
Dallas, TX, USA) at 4°C for 16 h. Membranes were then incubated
with anti-rabbit IgG HRP-linked secondary antibody (no. 7074;
dilution, 1:3,500; Cell Signaling Technology, Inc., Danvers, MA,
USA) at room temperature for 1 h. Proteins were visualized using an
enhanced chemiluminescence western blotting substrate (GE
Healthcare Life Sciences, Chalfont, UK) and scanned with an image
analyzer (LAS-4000) and Image Gauge software (version 3.1) (both
from Fujifilm, Tokyo, Japan). The relative quantity of protein was
determined by normalizing to GAPDH.
Statistical analysis
For statistical analysis, two-tailed Student's
t-tests were performed with DA Stats software version PAF01644
(NIFTY Corp.; Fujitsu). P<0.05 was considered to indicate a
statistically significant difference.
Results
Overexpression of AR in human HCC
cells
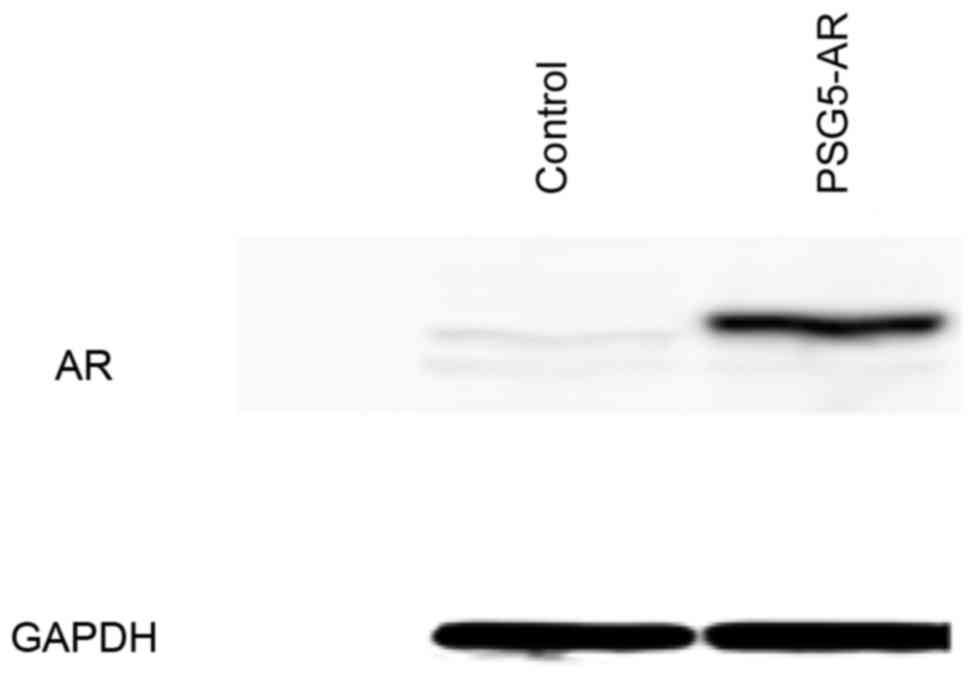
Overexpression of AR was induced in HepG2 and Huh7
cells to validate hypotheses of the effect of AR on the regulation
of fatty acid metabolism-associated gene expression. In HepG2 cells
transiently transfected with pSG5-AR, overexpression of AR was
confirmed by western blot analysis (Fig.
1), and AR overexpression in Huh7 cells transiently transfected
with pSG5-AR was demonstrated by western blotting in a previous
study (3).
Downregulated fatty acid
metabolism-associated genes in AR-overexpressing HepG2 cells
Fatty acid metabolism-associated gene expression
profiles were examined using a qPCR-based array. Expression levels
between HepG2 cells transiently overexpressing AR and control
vector-transfected HepG2 cells were compared. In HepG2 cells, AR
overexpression significantly downregulated the expression of 35
fatty acid metabolism-associated genes, including 6 acyl-coenzyme A
(CoA) dehydrogenases [acyl-CoA dehydrogenase family member
(ACAD)10, acyl-CoA dehydrogenase, very long chain, acyl-CoA
dehydrogenase, C-2 to C-3 short chain (ACADS), ACAD9, ACADS
branched (B) and enoyl-CoA hydratase and 3-hydroxyacyl CoA
dehydrogenase (EHHADH)], 2 acyl-CoA oxidases [acyl-CoA oxidase
(ACOX) 1 and ACOX3], 4 acyl-CoA synthetases [acyl-CoA synthetase
long-chain family member (ACSL)1, acyl-CoA synthetase bubblegum
family member (ACSBG)2, ACSBG1 and ACSL4], 4 acyl-CoA thioesterases
[acyl-CoA thioesterase (ACOT)8, ACOT1, ACOT2 and ACOT7], 4
carnitine transferases [carnitine palmitoyltransferase (CPT)2,
carnitine O-octanoyltranserase, carnitine O-acetyltransferase,
CPT1B], 5 other fatty acid metabolism-associated genes [aldehyde
dehydrogenase 2 family (mitochondrial) (ALDH2), 2,4-dienoyl CoA
reductase 2, peroxisomal, enoyl CoA hydratase, short chain, 1,
mitochondrial, enoyl-CoA δ isomerase 2 and methylmalonyl CoA
mutase], 2 genes of fatty acid transport [CPT1A and solute carrier
family 27 (fatty acid transporter), member 4], 4 genes of fatty
acid biosynthesis regulation [protein kinase, cAMP-dependent,
catalytic, α (PRKACA), protein kinase, AMP-activated, α 2 catalytic
subunit, protein kinase, AMP-activated, γ 1 non-catalytic subunit
and protein kinase, AMP-activated, α 1 catalytic subunit], 1 gene
of ketogenesis and ketone body metabolism (3-hydroxybutyrate
dehydrogenase, type 1) and 3 genes of triacylglycerol metabolism
[glycerol-3-phosphatase dehydrogenase 1 (soluble), lipase,
hormone-sensitive and glycerol kinase; Table I].
 | Table I.Fatty acid metabolism-associated genes
are significantly downregulated in androgen receptor-overexpressing
HepG2 cells examined by polymerase chain reaction arrays. |
Table I.
Fatty acid metabolism-associated genes
are significantly downregulated in androgen receptor-overexpressing
HepG2 cells examined by polymerase chain reaction arrays.
| Symbol | Description | Fold change (vs.
control) | P-value |
|---|
| GPD1 |
Glycerol-3-phosphatase dehydrogenase 1
(soluble) | 0.40 | 0.0259 |
| ACAD10 | Acyl-CoA
dehydrogenase family, member 10 | 0.44 | 0.0135 |
| LIPE | Lipase,
hormone-sensitive | 0.50 | 0.0099 |
| ACADVL | Acyl-CoA
dehydrogenase, very long chain | 0.54 | 0.0018 |
| CPT2 | Carnitine
palmitoyltransferase 2 | 0.54 | 0.0181 |
| CROT | Carnitine
O-octanoyltranserase | 0.54 | 0.0069 |
| CPT1A | Carnitine
palmitoyltransferase 1A (liver) | 0.57 | 0.0039 |
| ACSL1 | Acyl-CoA synthetase
long-chain family member 1 | 0.57 | 0.0375 |
| ACSBG2 | Acyl-CoA synthetase
bubblegum family member 2 | 0.58 | 0.0096 |
| ACADS | Acyl-CoA
dehydrogenase, C-2 to C-3 short chain | 0.58 | 0.0448 |
| ACAD9 | Acyl-CoA
dehydrogenase family, member 9 | 0.59 | 0.0177 |
| ACADSB | Acyl-CoA
dehydrogenase, short/branched chain | 0.60 | 0.0475 |
| ACOX1 | Acyl-CoA oxidase 1,
palmitoyl | 0.60 | 0.0020 |
| ACOX3 | Acyl-CoA oxidase 3,
pristanoyl | 0.60 | 0.0199 |
| PRKACA | Protein kinase,
cAMP-dependent, catalytic, α | 0.61 | 0.0012 |
| PRKAA2 | Protein kinase,
AMP-activated, α 2 catalytic subunit | 0.61 | 0.0347 |
| SLC27A4 | Solute carrier
family 27 (fatty acid transporter), member 4 | 0.61 | 0.0428 |
| BDH1 | 3-hydroxybutyrate
dehydrogenase, type 1 | 0.61 | 0.0130 |
| ACSBG1 | Acyl-CoA synthetase
bubblegum family member 1 | 0.61 | 0.0233 |
| EHHADH | Enoyl-CoA,
hydratase/3-hydroxyacyl CoA dehydrogenase | 0.62 | 0.0212 |
| ACSL4 | Acyl-CoA synthetase
long-chain family member 4 | 0.63 | 0.0341 |
| ACOT8 | Acyl-CoA
thioesterase 8 | 0.63 | 0.0191 |
| ACOT1 | Acyl-CoA
thioesterase 1 | 0.64 | 0.0017 |
| ALDH2 | Aldehyde
dehydrogenase 2 family (mitochondrial) | 0.64 | 0.0088 |
| PRKAG1 | Protein kinase,
AMP-activated, γ 1 non-catalytic subunit | 0.64 | 0.0321 |
| CRAT | Carnitine
O-acetyltransferase | 0.66 | 0.0002 |
| DECR2 | 2,4-dienoyl CoA
reductase 2, peroxisomal | 0.66 | 0.0412 |
| ECHS1 | Enoyl CoA
hydratase, short chain, 1, mitochondrial | 0.69 | 0.0368 |
| ECI2 | Enoyl-CoA δ
isomerase 2 | 0.70 | 0.0347 |
| GK | Glycerol
kinase | 0.72 | 0.0049 |
| ACOT2 | Acyl-CoA
thioesterase 2 | 0.73 | 0.0252 |
| CPT1B | Carnitine
palmitoyltransferase 1B (muscle) | 0.73 | 0.0038 |
| PRKAA1 | Protein kinase,
AMP-activated, α 1 catalytic subunit | 0.76 | 0.0409 |
| MUT | Methylmalonyl CoA
mutase | 0.81 | 0.0453 |
| ACOT7 | Acyl-CoA
thioesterase 7 | 0.81 | 0.0098 |
Upregulated fatty acid
metabolism-associated genes in AR-overexpressing HepG2 cells
In HepG2 cells, AR significantly upregulated the
expression of 6 fatty acid metabolism-associated genes (Table II), including 3 fatty acid transports
[Solute carrier family (SLC) 27A5, SLC27A2 and fatty acid binding
protein 4, adipocyte], 1 fatty acid biosynthesis regulator [protein
kinase, AMP-activated, γ 2 non-catalytic subunit (PRKAG2)], 1
acyl-CoA thioesterase (ACOT9) and 1 other fatty acid
metabolism-associated gene [pyrophosphatase (inorganic) 1
(PPA1)].
 | Table II.Fatty acid metabolism-associated
genes are significantly upregulated in androgen
receptor-overexpressing HepG2 cells examined by polymerase chain
reaction arrays. |
Table II.
Fatty acid metabolism-associated
genes are significantly upregulated in androgen
receptor-overexpressing HepG2 cells examined by polymerase chain
reaction arrays.
| Symbol | Description | Fold change (vs.
control) | P-value |
|---|
| FABP4 | Fatty acid binding
protein 4, adipocyte | 2.25 | 0.0015 |
| SLC27A2 | Solute carrier
family 27 (fatty acid transporter), member 2 | 1.51 | 0.0167 |
| SLC27A5 | Solute carrier
family 27 (fatty acid transporter), member 5 | 1.40 | 0.0156 |
| ACOT9 | Acyl-CoA
thioesterase 9 | 1.25 | 0.0216 |
| PPA1 | Pyrophosphatase
(inorganic) 1 | 1.23 | 0.0162 |
| PRKAG2 | Protein kinase,
AMP-activated, γ 2 non-catalytic subunit | 1.20 | 0.0331 |
Upregulated and downregulated fatty
acid metabolism-associated genes in AR-overexpressing Huh7
cells
In Huh7 cells, AR overexpression significantly
downregulated the expression of the following 11 fatty acid
metabolism-associated genes: 4 acyl-CoA synthetases [acyl-CoA
synthetase medium-chain family member (ACSM) 2A, ACSL6, ACSL3 and
ACSM3], 3 acyl-CoA thioesterases (ACOT7, ACOT9 and ACOT6), 1
acyl-CoA oxidase (ACOX3), 1 fatty acid transporter (SLC27A2), 1
fatty acid biosynthesis regulator (PRKAG2) and 1 regulator of
ketogenesis and ketone body metabolism (3-hydroxybutyrate
dehydrogenase, type 2; Table III).
However, AR overexpression did not significantly upregulate the
expression of any fatty acid metabolism-associated genes in Huh7
cells.
 | Table III.Fatty acid metabolism-associated
genes are significantly downregulated in androgen
receptor-overexpressing Huh7 cells. |
Table III.
Fatty acid metabolism-associated
genes are significantly downregulated in androgen
receptor-overexpressing Huh7 cells.
| Symbol | Description | Fold change (vs.
control) | P-value |
|---|
| ACOX3 | Acyl-CoA oxidase 3,
pristanoyl | 0.62 | 0.0180 |
| PRKAG2 | Protein kinase,
AMP-activated, γ 2 non-catalytic subunit | 0.65 | 0.0402 |
| ACSM3 | Acyl-CoA synthetase
medium-chain family member 3 | 0.66 | 0.0249 |
| SLC27A2 | Solute carrier
family 27 (fatty acid transporter), member 2 | 0.69 | 0.0387 |
| BDH2 | 3-hydroxybutyrate
dehydrogenase, type 2 | 0.69 | 0.0015 |
| ACSL3 | Acyl-CoA synthetase
long-chain family member 3 | 0.87 | 0.0127 |
| ACOT6 | Acyl-CoA
thioesterase 6 | 0.88 | 0.0020 |
| ACSL6 | Acyl-CoA synthetase
long-chain family member 6 | 0.89 | 0.0052 |
| ACOT9 | Acyl-CoA
thioesterase 9 | 0.91 | 0.0434 |
| ACOT7 | Acyl-CoA
thioesterase 7 | 0.92 | 0.0480 |
| ACSM2A | Acyl-CoA synthetase
medium-chain family member 2A | 0.93 | 0.0018 |
Discussion
The present study demonstrated that overexpression
of AR alters fatty acid metabolism-associated gene expression in
human HCC cell lines. Previous studies (17,18)
demonstrated that in patients with HCC, ACADSB and EHHADH
expression are downregulated in tissue isolated from cirrhotic
livers compared with control tissues from healthy patients.
Acyl-CoA oxidase deficient-mice spontaneously develop HCC (19). Li et al (20) reported that cluster of differentiation
(CD) 147 expression significantly contributed to the reprogramming
of fatty acid metabolism in HCC cells, and that CD147 downregulated
CPT1A and ACOX1 by activating the p38 mitogen-activated protein
kinase signaling pathway to inhibit fatty acid β-oxidation. A
previous study in phosphatase and tensin homolog null nonalcoholic
steatohepatitis liver tissue isolated from mice revealed that ACSL1
expression is decreased, and is almost non-existent in tumors
isolated from these livers (21). A
recent study (22) demonstrated that
the presence of a specific mutation in ALDH2 (E487 K) led to
increased protein turnover and promoted murine
hepatocarcinogenesis. The presence of the DnaJ heat shock protein
family (Hsp40) member B1-PRKACA chimeric transcript in
fibrolamellar HCC suggests that this genetic alteration contributes
to tumor pathogenesis (23).
Therefore, the present study supports previous reports (17–23)
(Table I).
Sakabe et al (24) reported that PRKAG2 enhances the effect
of interferon (IFN)-α/fluorouracil (5-FU) and serves as a
prognostic marker for IFN-α/5-FU therapy in HCC. The present study
demonstrated that AR significantly upregulated the expression of
PRKAG2 (Table II). ACOT7 has been
implicated in the regulation of neuronal fatty acid metabolism to
prevent neurotoxicity (25), and
ACOX3 is expressed at particularly low levels in the liver
(26). Notably, ACOT7 and ACOX3 were
downregulated by the overexpression of AR in HepG2 and Huh7 cells
(Tables I and III). These results support the results of
a previous study by Bolton et al (27) that implicated AR in the regulation of
cellular lipid metabolism.
In general, cancer cells synthesize a high amount of
fatty acids via increased expression, and subsequent activity, of
lipogenic enzymes to fulfill their high energy requirements and to
maintain cell membranes for growth (4,28,29). In patients with HCC, the alterations
in lipid and lipoprotein metabolism can be clearly observed
(30).
AR has been demonstrated to be associated with
hepato-carcinogenesis and HCC development (1,3,5–7,9,10). The
present study revealed that AR could serve a role in
hepatocarcinogenesis via the regulation of hepatocellular fatty
acid metabolism. Hepatitis C virus (HCV) augments AR-mediated
signaling (7) and replicates in
hepatocytes using lipid droplets, which are used for the storage of
neutral lipids (31). In a previous
study, eradication of HCV using an interferon-free antiviral
regimen resulted in rapid changes in the metabolic signaling
pathway in the liver, which suggested that there was a direct
effect of HCV replication on lipid homeostasis (32); however, further studies are required
to determine potential indirect effects of HCV on lipid
homeostasis. Hepatitis B virus (HBV) has also been revealed to
augment AR-mediated signaling (5,6) and to
induce hepatic steatosis by influencing fatty acid metabolism
(33). HBV serves a more minor role
in hepatic steatosis compared with HCV (34).
In conclusion, improving the understanding of the
association between AR and fatty acid metabolism in
hepatocarcinogenesis and the development of HCC may aid in the
creation of novel therapeutic and prevention strategies.
Acknowledgements
The authors would like to thank Professor J.T.
Isaacs (The Johns Hopkins University School of Medicine, Baltimore,
MD, USA) for providing the plasmid pSG5-AR. The present work was
supported by grants from the Ministry of Health, Labour and Welfare
of Japan. This study was partially supported by the Research
Program on Hepatitis from the Japan Agency for Medical Research and
Development (AMED).
References
|
1
|
Nagasue N, Chang YC, Hayashi T, Galizia G,
Kohno H, Nakamura T and Yukaya H: Androgen receptor in
hepatocellular carcinoma as a prognostic factor after hepatic
resection. Ann Surg. 209:424–427. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Okitsu K, Kanda T, Imazeki F, Yonemitsu Y,
Ray RB, Chang C and Yokosuka O: Involvement of interleukin-6 and
androgen receptor signaling in pancreatic cancer. Genes Cancer.
1:859–867. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jiang X, Kanda T, Nakamoto S, Miyamura T,
Wu S and Yokosuka O: Involvement of androgen receptor and
glucose-regulated protein 78 kDa in human hepatocarcinogenesis. Exp
Cell Res. 323:326–336. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rochefort H and Chalbos D: The role of sex
steroid receptors on lipogenesis in breast and prostate
carcinogenesis: A viewpoint. Horm Cancer. 1:63–70. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chiu CM, Yeh SH, Chen PJ, Kuo TJ, Chang
CJ, Chen PJ, Yang WJ and Chen DS: Hepatitis B virus X protein
enhances androgen receptor-responsive gene expression depending on
androgen level. Proc Natl Acad Sci USA. 104:2571–2578. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zheng Y, Chen WL, Ma WL, Chang C and Ou
JH: Enhancement of gene transactivation activity of androgen
receptor by hepatitis B virus X protein. Virology. 363:454–461.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kanda T, Steele R, Ray R and Ray RB:
Hepatitis C virus core protein augments androgen receptor-mediated
signaling. J Virol. 82:11066–11072. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ueda T, Bruchovsky N and Sadar MD:
Activation of the androgen receptor N-terminal domain by
interleukin-6 via MAPK and STAT3 signal transduction pathways. J
Biol Chem. 277:7076–7085. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gehlhaus M, Schmitt N, Volk B and Meyer
RP: Antiepileptic drugs affect neuronal androgen signaling via a
cytochrome P450-dependent pathway. J Pharmacol Exp Ther.
322:550–559. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yoon G, Kim JY, Choi YK, Won YS and Lim
IK: Direct activation of TGF-beta1 transcription by androgen and
androgen receptor complex in Huh7 human hepatoma cells and its
tumor in nude mice. J Cell Biochem. 97:393–411. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Krycer JR and Brown AJ: Cross-talk between
the androgen receptor and the liver X receptor: Implications for
cholesterol homeostasis. J Biol Chem. 286:20637–20647. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hao Q, Li T, Zhang X, Gao P, Qiao P, Li S
and Geng Z: Expression and roles of fatty acid synthase in
hepatocellular carcinoma. Oncol Rep. 32:2471–2476. 2014.PubMed/NCBI
|
|
13
|
Qiu JF, Zhang KL, Zhang XJ, Hu YJ, Li P,
Shang CZ and Wan JB: Abnormalities in plasma phospholipid fatty
acid profiles of patients with hepatocellular carcinoma. Lipids.
50:977–985. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Koh WP, Dan YY, Goh GB, Jin A, Wang R and
Yuan JM: Dietary fatty acids and risk of hepatocellular carcinoma
in the Singapore Chinese health study. Liver Int. 36:893–901. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Litvinov IV, Chang C and Isaacs JT:
Molecular characterization of the commonly used human androgen
receptor expression vector, pSG5-AR. Prostate. 58:319–324. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Schröder PC, Segura V, Riezu JI, Sangro B,
Mato JM, Prieto J, Santamaría E and Corrales FJ: A signature of six
genes highlights defects on cell growth and specific metabolic
pathways in murine and human hepatocellular carcinoma. Funct Integr
Genomics. 11:419–429. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Suto K, Kajihara-Kano H, Yokoyama Y,
Hayakari M, Kimura J, Kumano T, Takahata T, Kudo H and Tsuchida S:
Decreased expression of the peroxisomal bifunctional enzyme and
carbonyl reductase in human hepatocellular carcinomas. J Cancer Res
Clin Oncol. 125:83–88. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Meyer K, Lee JS, Dyck PA, Cao WQ, Rao MS,
Thorgeirsson SS and Reddy JK: Molecular profiling of hepatocellular
carcinomas developing spontaneously in acyl-CoA oxidase deficient
mice: Comparison with liver tumors induced in wild-type mice by a
peroxisome proliferator and a genotoxic carcinogen. Carcinogenesis.
24:975–984. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li J, Huang Q, Long X, Zhang J, Huang X,
Aa J, Yang H, Chen Z and Xing J: CD147 reprograms fatty acid
metabolism in hepatocellular carcinoma cells through
Akt/mTOR/SREBP1c and P38/PPARα pathways. J Hepatol. 63:1378–1389.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Muir K, Hazim A, He Y, Peyressatre M, Kim
DY, Song X and Beretta L: Proteomic and lipidomic signatures of
lipid metabolism in NASH-associated hepatocellular carcinoma.
Cancer Res. 73:4722–4731. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jin S, Chen J, Chen L, Histen G, Lin Z,
Gross S, Hixon J, Chen Y, Kung C, Chen Y, et al: ALDH2(E487K)
mutation increases protein turnover and promotes murine
hepatocarcinogenesis. Proc Natl Acad Sci USA. 112:9088–9093. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Honeyman JN, Simon EP, Robine N,
Chiaroni-Clarke R, Darcy DG, Lim II, Gleason CE, Murphy JM,
Rosenberg BR, Teegan L, et al: Detection of a recurrent
DNAJB1-PRKACA chimeric transcript in fibrolamellar hepatocellular
carcinoma. Science. 343:1010–1014. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sakabe T, Tsuchiya H, Kanki K, Azumi J,
Gonda K, Mizuta Y, Yamada D, Wada H, Shomori K, Nagano H and Shiota
G: Identification of the genes chemosensitizing hepatocellular
carcinoma cells to interferon-α/5-fluorouracil and their clinical
significance. PLoS One. 8:e561972013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ellis JM, Wong GW and Wolfgang MJ: Acyl
coenzyme A thioesterase 7 regulates neuronal fatty acid metabolism
to prevent neurotoxicity. Mol Cell Biol. 33:1869–1882. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zha S, Ferdinandusse S, Hicks JL, Denis S,
Dunn TA, Wanders RJ, Luo J, De Marzo AM and Isaacs WB: Peroxisomal
branched chain fatty acid beta-oxidation pathway is upregulated in
prostate cancer. Prostate. 63:316–323. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bolton EC, So AY, Chaivorapol C, Haqq CM,
Li H and Yamamoto KR: Cell- and gene-specific regulation of primary
target genes by the androgen receptor. Genes Dev. 21:2005–2017.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Baron A, Migita T, Tang D and Loda M:
Fatty acid synthase: A metabolic oncogene in prostate cancer? J
Cell Biochem. 91:47–53. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mounier C, Bouraoui L and Rassart E:
Lipogenesis in cancer progression (review). Int J Oncol.
45:485–492. 2014.PubMed/NCBI
|
|
30
|
Jiang J, Nilsson-Ehle P and Xu N:
Influence of liver cancer on lipid and lipoprotein metabolism.
Lipids Health Dis. 5:42006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Miyanari Y, Atsuzawa K, Usuda N, Watashi
K, Hishiki T, Zayas M, Bartenschlager R, Wakita T, Hijikata M and
Shimotohno K: The lipid droplet is an important organelle for
hepatitis C virus production. Nat Cell Biol. 9:1089–1097. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Meissner EG, Lee YJ, Osinusi A, Sims Z,
Qin J, Sturdevant D, McHutchison J, Subramanian M, Sampson M,
Naggie S, et al: Effect of sofosbuvir and ribavirin treatment on
peripheral and hepatic lipid metabolism in chronic hepatitis C
virus, genotype 1-infected patients. Hepatology. 61:790–801. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wu YL, Peng XE, Zhu YB, Yan XL, Chen WN
and Lin X: Hepatitis B virus X protein induces hepatic steatosis by
enhancing the expression of liver fatty acid binding protein. J
Virol. 90:1729–1740. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Haga Y, Kanda T, Sasaki R, Nakamura M,
Nakamoto S and Yokosuka O: Nonalcoholic fatty liver disease and
hepatic cirrhosis: Comparison with viral hepatitis-associated
steatosis. World J Gastroenterol. 21:12989–12995. 2015. View Article : Google Scholar : PubMed/NCBI
|