Introduction
Malignant melanoma is one of the most lethal and
aggressive types of human malignancy, and is characterized by
suppressed tumor cell apoptosis and high levels of invasion
(1). Malignant melanoma is commonly
treated with a combination of therapies including surgical removal,
chemotherapy and radiotherapy. However, the reported long-term
survival rate of melanoma remains low due to the ability of this
malignancy to develop resistance to common chemotherapies and
rapidly metastasize (2).
The alkylating agent temozolomide (TMZ) is one of
the most effective single chemotherapeutic agents for patients with
malignant melanoma (3), but
resistance develops quickly and frequently (3). There has been relatively little progress
in terms of developing novel pharmacological agents to treat
metastatic melanoma over previous years. Cancer cells develop
resistance to numerous pharmaceuticals through a variety of
mechanisms, including decreased drug uptake, increased drug efflux,
intracellular drug inactivation, repair of drug-induced damage and
resistance to drug-induced apoptosis (4,5). Gene
therapy is one of the treatment strategies that have been proposed
to circumvent drug resistance (6).
Interleukin-24 (IL-24) is a promising candidate for
cancer gene therapy (7). A notable
characteristic of IL-24 is that it preferentially inhibits growth
and induces apoptosis in a variety of cancer cells without harming
normal cells (8). Additionally, IL-24
exhibits potent ‘anti-tumor bystander’ activity, inhibits tumor
angiogenesis, synergizes with chemotherapy, radiation and
monoclonal antibody therapies, and exhibits immune modulatory
activity (9). The synergistic effects
of IL-24 and TMZ have been documented using a replication-defective
adenovirus expressing IL-24 (Ad-IL-24) (10). However, replication-deficient
adenoviruses such as Ad-IL-24 are generally considered inadequate
to efficiently infect tumor cells (11).
Conditionally replicating adenoviruses exhibit an
advantage over replication-deficient adenovirus vectors and have
been revealed to be effective for the treatment of malignant tumors
(12). Conditionally replicating
adenoviruses exhibit a dual mechanism of action in tumor cells, as
they act as an oncolytic transgene delivery system that selectively
replicates in and lyses tumor cells, and additionally amplify the
expression and function of therapeutic genes in the tumor
microenvironment (13,14). Conditionally replicating adenoviruses
may be generated by deleting a viral element that is necessary for
virus replication in normal cells but is dispensable in tumor cells
(15). An example of this approach is
deleting the E1B-55 gene from the adenovirus ZD55 (11,16).
Conditionally replicating adenoviruses may also be used as
potential vectors to express high levels of therapeutic genes in
tumor cells (17). Therefore, the
present study hypothesized that the combination of an E1B-55
gene-deleted conditionally replicating adenovirus expressing IL-24
and TMZ may synergistically provide an enhanced antitumor effect
against malignant melanoma by inducing apoptosis.
Materials and methods
Cell culture and reagents
The human melanoma A375 and M14 cell lines were
purchased from Nanjing KeyGen Biotech Co., Ltd. (Nanjing, China).
HEK293 cells were purchased from Shanghai Cell Collection
(Shanghai, China). The cells were cultured in Dulbecco's modified
Eagle's medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) supplemented with 10% heat-inactivated fetal bovine serum
(Gibco; Thermo Fisher Scientific, Inc.), 4 mM glutamine, 50 U/ml
penicillin and 50 µg/ml streptomycin at 37°C in a humidified
atmosphere with 5% CO2. The cells were routinely
screened to verify they were free of mycoplasma contamination, and
were used for experiments in the logarithmic phase of growth.
TMZ, also known as 3,4-dihydro-3-methyl-4-oxoimidazo
(5,1-d)-as-tetrazine-8-carboxamide (Schering-Plough Corporation,
Kenilworth, NJ, USA) was dissolved in PBS and used at
concentrations ranging between 0 and 800 µMol/l, as indicated. The
experiments used 25, 50, 100, 200 and 400 µMol/l TMZ.
Constructing the IL-24-expressing
E1B-55-deleted adenovirus
The recombinant adenoviruses used in the present
study have been previously described, which included ZD55 carrying
IL-24 (ZD55-IL-24), ZD55 carrying enhanced green fluorescence
protein (ZD55-EGFP), and adenovirus carrying IL-24 (Ad-IL-24)
(18). The expression cassette
containing the IL-24 gene under the control of the H1 promoter was
produced by polymerase chain reaction (PCR) amplification of
Ad-IL-24 and inserted into the pZD55 construct (18). The resulting product, pZD55-IL-24,
lacked E1B-55 expression and produced IL-24. The plasmid,
pZD55-IL-24, was co-transfected into HEK293 cells with the plasmid
pBHGE3 (Microbix Biosystems, Toronto, Canada) to produce a
recombinant conditionally replicating adenovirus, ZD55-IL-24.
The viral genome was extracted from the purified
stock of ZD55-IL-24 using a QIAamp DNA blood mini kit (Qiagen GmbH,
Hilden, Germany). The IL-24 gene was verified by conventional PCR
using the following primers: IL-24 forward, 5-GAA TTC GAT ATC TCT
AGAC-3′ and reverse, 5-ATA GAT ATC TCA GAG CTT GTA-3. The
thermocycling conditions were 94°C for 5 min, followed by 30 cycles
at 94°C for 1 min, 50°C for 30 sec, 72°C for 1 min and a final
extension at 72°C for 10 min. The amplification product was
visualized by electrophoresis on a 1% agarose gel containing
ethidium bromide. All viruses were plaque screened, propagated in
HEK293 cells and purified by CsCl gradient ultracentrifugation
following a previously described method (18). Viral concentration was determined by
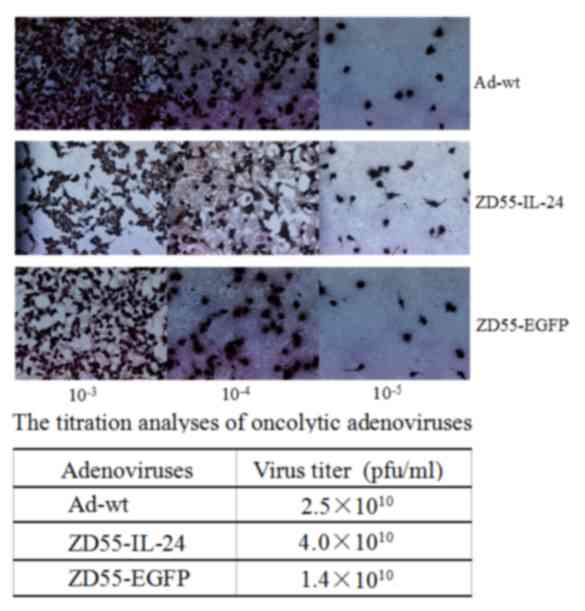
absorbance measurements at 260 nm, and plaque-forming unit (pfu)
titer was estimated by plaque assay as previously described
(19) on HEK293 cells (Fig. 1). The PFU titer of ZD55-IL-24 was
4×1010 PFU/ml.
Western blot analysis
The cells were harvested from the plates and lysed
in radioimmunoprecipitation (RIPA) buffer (pH 7.4) containing
phenylmethylsulfonyl fluoride at a ratio of 100:1 (Beyotime
Institute of Biotechnology, Haimen, China). Protein concentration
was determined using a Bradford assay. The cell aliquots were
separated using SDS-PAGE (12% gel) with 50 µg protein loaded/lane.
The proteins were then transferred to nitrocellulose membranes and
incubated overnight at 4°C with the primary antibodies (all 1:1,000
dilution). The following rabbit polyclonal primary antibodies were
used: Anti-caspase-3 (cat. no. AB42437), anti-B-cell lymphoma-2
(Bcl-2; cat. no. AB40415), anti-myeloid cell leukemia-1 (Mcl-1;
cat. no. AB38113), anti-Bcl-2-like protein 4 (Bax; cat. no.
AB40636), anti-phosphorylated γ-H2AX (cat. no. AB41808),
anti-nuclear factor (NF)-κB (cat. no. AB41808), anti-p53 (cat. no.
AB38007) (all AbSci, Vancouver, WA, USA), anti-IL-24 (cat. no.
bs-10576R; BIOSS, Beijing, China), anti-β-actin (cat. no. AP0731;
Bioworld Technology, Inc., St. Louis Park, MN, USA), and
anti-adenovirus early region 1A (E1A; cat. no. sc-374663; Santa
Cruz Biotechnology, Inc., Dallas, TX, USA). The membranes were then
washed with TBS-Tween 20 and incubated with goat anti-rabbit
alkaline phosphatase-conjugated secondary antibodies (1:4,000
dilution, cat. no. ZB-2301; ZSGB-BIO, Zhongshan, Beijing) in
TBS-Tween 20 at room temperature for 2 h and developed using a
nitro-blue tetrazolium chloride/5-bromo-4-chloro-3′-indolyl
phosphate p-toluidine salt color substrate (Promega Corporation,
Madison, WI, USA). The changes in the expression levels of these
proteins were determined using ImageQuant™ TL software (version
8.1; Molecular Dynamics; GE Healthcare Life Sciences, Chalfont, UK)
and are expressed as a ratio relative to the protein level in
PBS-treated cells, which was set at 100%.
Cytopathic effect (CPE) assay
The cells were plated at a density of
1×105 cells in 96- or 48-well plates and treated with
TMZ alone, ZD55-IL-24 alone or varying combinations of TMZ (0, 25,
50, 100, 200 and 400 µM) and ZD55-IL-24 [0, 1, 5 and 10,
multiplicity of infection, (MOI)]. Following a 72- or 120-h culture
at room temperature, the cells were washed with water,
paraformaldehyde-fixed and stained with crystal violet (Amresco,
LLC, Solon, OH, USA).
Statistical analysis
The data are expressed as the mean ± standard
deviation and were analyzed using an independent sample t-test and
analysis of variance as appropriate. The analyses were performed
using SPSS Base version 13.0 for Windows (SPSS, Inc., Chicago, IL,
USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
ZD55-IL-24 efficiently delivers IL-24
into melanoma cells
A key component of the hypothesis of the present
study was the inclusion of IL-24 in the combined antitumor therapy
delivered by ZD55. Therefore, the present study first assessed
whether ZD55-IL-24 was an efficient method of delivering IL-24 to
melanoma tumor cells using the A375 and M14 cell lines. The cells
were treated with ZD55-IL-24+TMZ, ZD55-IL-24 alone or TMZ alone.
The negative control was cells treated with PBS. The present study
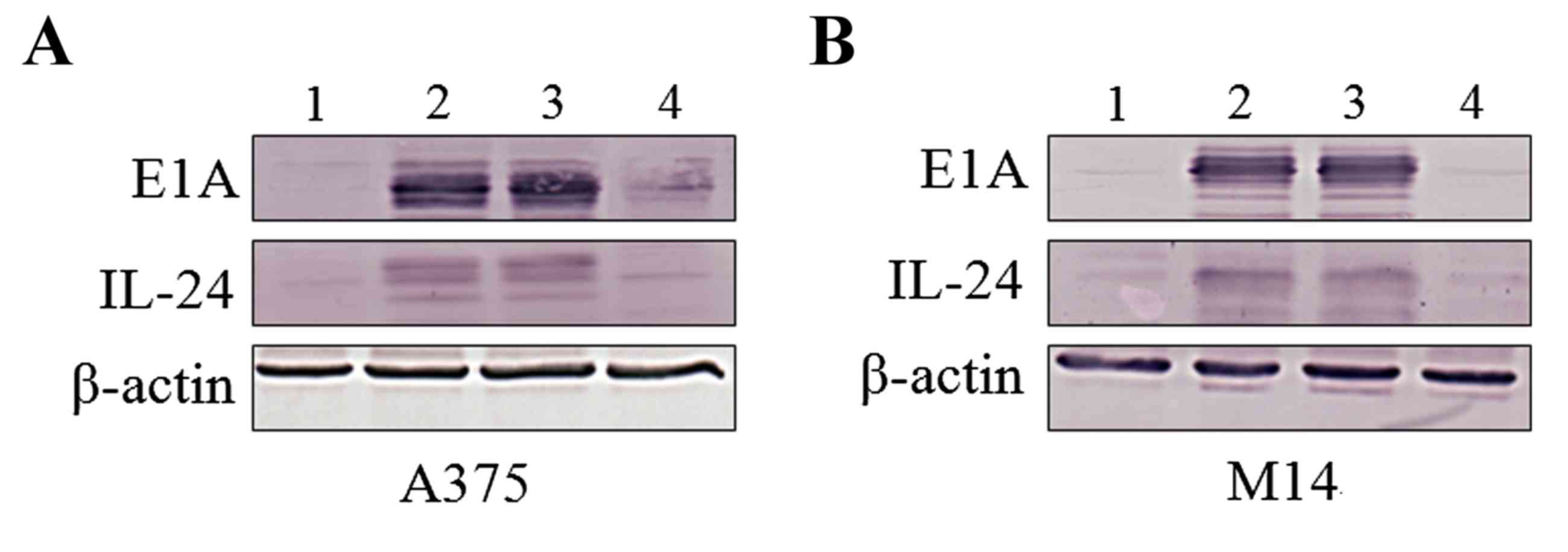
assessed the level of IL-24 protein by immunoblotting. IL-24
protein was clearly detectable XLVIII h subsequent to infection
with ZD55-IL-24 in the A375 (Fig. 2A)
and M14 (Fig. 2B) cells. No
difference was observed in the level of IL-24 expression between
the cells treated with ZD55-IL-24 alone or ZD55-IL-24 + TMZ
(Fig. 2, lanes 2 and 3). These
results demonstrated that ZD55 mediates the stable expression of
high levels of IL-24 in the presence or absence of TMZ. The
adenovirus protein E1A was used to assess whether there were
differences in the level of infection in the presence of TMZ.
Similar levels of E1A were detected in the presence or absence of
TMZ, indicating that TMZ did not reduce the replication capability
of the ZD55 adenovirus (Fig. 2).
Combining ZD55-IL-24 with TMZ
increases the expression of proteins associated with apoptosis
induction
To elucidate the molecular mechanism through which
ZD55-IL-24 and TMZ induced apoptosis, the present study examined
the protein expression levels of NF-κВ, Bcl-2, Mcl-1, Bax and
caspase-3 subsequent to infection with ZD55-IL-24, with or without
TMZ. The marker γ-H2AX was also included as a marker of DNA damage,
a potential apoptotic trigger. The A375 and M14 cells were infected
with ZD55-IL-24 in the presence or absence of TMZ, and the protein
levels of Mcl-1, Bcl-2, Bax, NF-κВ, γ-H2AX and caspase-3 were
determined by immunoblotting. PBS-treated cells were used as the
negative control. Representative western blot images from A375
(Fig. 3A) and M14 (Fig. 3B) cells are shown. The present study
also quantified the protein expression using densitometry, and
expressed the change in protein expression as a ratio of the
protein expression level in PBS-treated cells (which was considered
to be 100%). ZD55-IL-24 alone reduced the protein expression levels
of Mcl-1, Bcl-2 and NF-κB. The addition of TMZ additionally reduced
the protein expression levels of the above proteins. In contrast,
ZD55-IL-24 + TMZ significantly increased the protein expression
levels of Bax and γ-H2AX. TMZ alone did not significantly increase
Bax expression (ZD55-IL-24 + TMZ; Fig.
3).
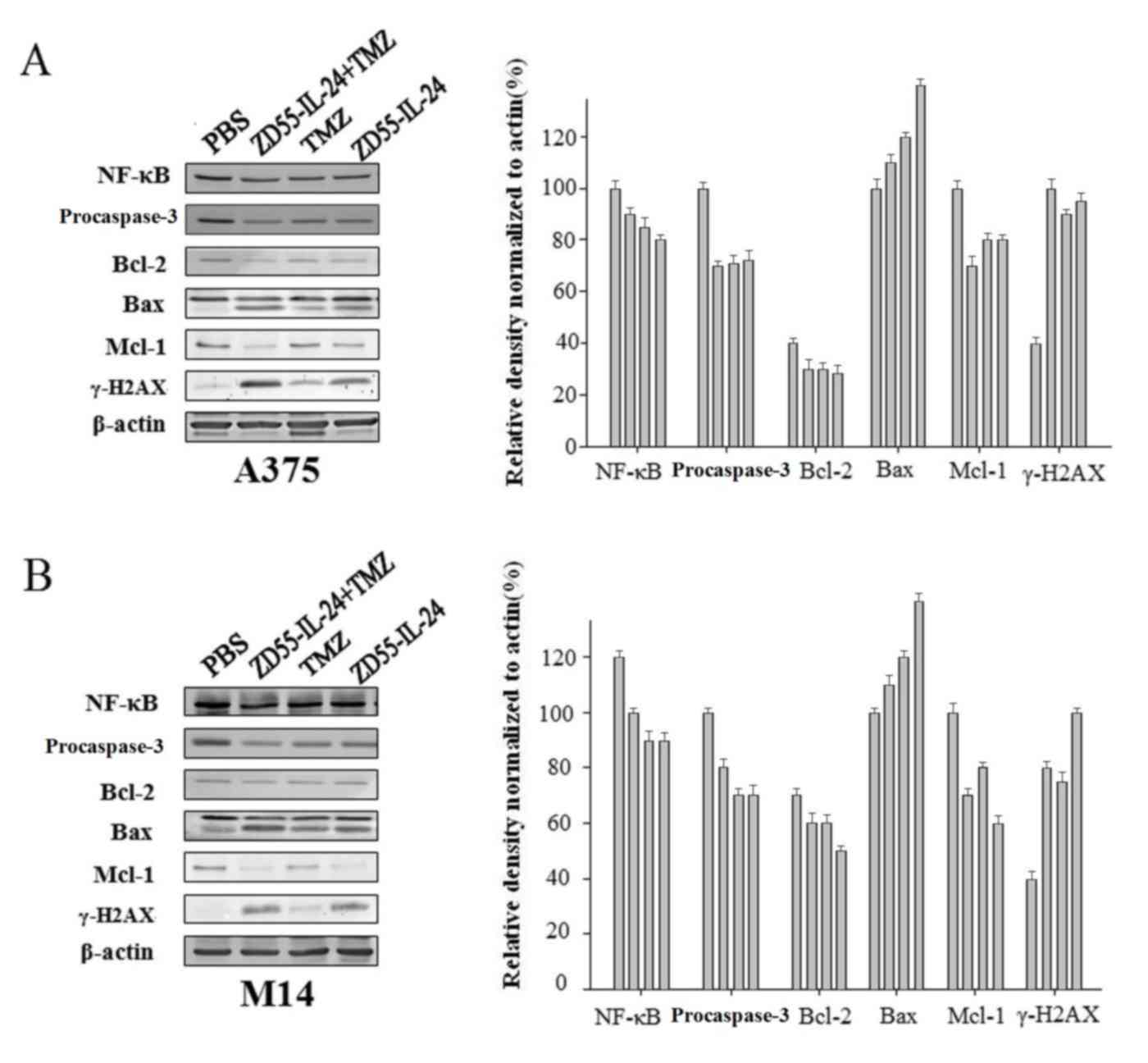 | Figure 3.Combining ZD55-IL-24 with TMZ
increased the expression of proteins associated with apoptosis
induction. Melanoma cells (A) A375 and (B) M14 were treated with
ZD55-IL-24 + TMZ, ZD55-IL-24 only or TMZ only. Cells treated with
PBS were used as the negative control. The cells were lysed 72 h
subsequent to treatment, and the protein expression levels of
proapoptotic (Bax and γ-H2AX) and antiapoptotic (Bcl-2, Mcl-1 and
NF-κB) proteins were assessed. β-actin was used as the loading
control. The change in protein expression level relative to the PBS
control was quantified by densitometry. TMZ, temozolomide; IL,
interleukin; ZD55-IL-24, E1B-55 gene-deleted conditionally
replicating adenovirus expressing the IL-24 gene; Bcl-2, B-cell
lymphoma-2; Bax, Bcl-2-like protein 4; NF-κB, nuclear factor κB;
γ-H2AX, γ-H2A histone family member X; Mcl-1, myeloid cell
leukemia-1. |
ZD55-IL-24 and TMZ upregulate p53
expression
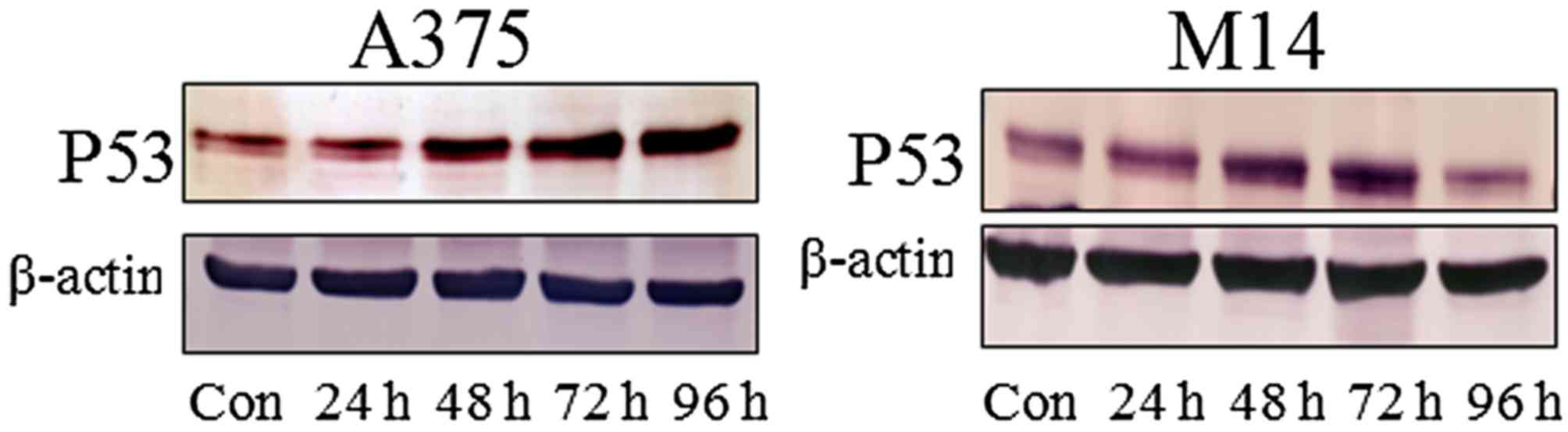
The present study then investigated whether
ZD55-IL-24 regulates the protein expression levels of p53. A375 and
M14 cells were infected with ZD55-IL-24 in the presence or absence
of TMZ, and the expresion of p53 protein was assessed at 24–96 h
post-infection. ZD55-IL-24 treatment increased the protein level of
p53 (Fig. 4). By contrast, TMZ
induced weak p53 expression. The combination of ZD55-IL-24+TMZ
increased the level of p53 in a time-dependent manner.
Combination of ZD55-IL-24 and TMZ
exhibits potent CPEs
Having revealed that proteins associated with the
induction of apoptosis were upregulated by ZD55-IL-24 and TMZ, the
present study then assessed the CPE of the combined treatment. The
CPE of the combined treatment was evaluated across a concentration
gradient of TMZ (µM) and a range of infecting doses of ZD55-IL-24
(MOI) in a systematic matrix (Fig.
5). The cells were stained with crystal violet 7 days
subsequent to infection. As shown in Fig.
5, the CPEs of ZD-55-IL-24 + TMZ were dose dependent. Each
concentration of TMZ tested, including ZD55-IL-24, produced greater
CPE compared with that of TMZ alone in a dose-dependent manner.
Similarly, each ZD55-IL-24 MOI tested including TMZ increased the
CPE in a dose-dependent manner. These results clearly supported the
hypothesis that the combination of TMZ and ZD55-IL-24 exhibits an
increased antitumor activity compared with that caused by either
treatment alone.
Discussion
As conditionally replicating adenoviruses may act as
antitumor agents and delivery vectors for therapeutic genes, the
present study evaluated whether a conditionally replicating
adenovirus lacking the E1B-55 gene and expressing IL-24 may act
synergistically with TMZ to destroy melanoma cells via
apoptosis.
The induction of apoptosis in tumor cells is an
important mechanism of cytotoxicity for the majority of anticancer
therapies (20). The present study
revealed that ZD55-IL-24 + TMZ decreased the levels of NF-κВ, Bcl-2
and Mcl-1, but increased the levels of Bax and γ-H2AX, and induced
a marked activation of caspase-3. The overall change in protein
expression levels suggested that ZD55-IL-24 combined with TMZ
changed the ratio of proapoptotic to antiapoptotic proteins in
melanoma cells, which may have contributed to the induction of
apoptosis, potentially through damaging the tumor cell DNA.
NF-κB activation exhibits an antiapoptotic role, and
promotes cell survival and growth (21). Chemotherapeutic agents and therapeutic
cytokines prevent the prolonged activation of the NF-κB protein
(22). The combination of ZD55-IL-24
+ TMZ inhibited NF-κB protein expression compared with the negative
control and either treatment alone in the melanoma A375 and M14
cells.
In conclusion, the combination of ZD-55-IL-24 and
TMZ was more effective at destroying melanoma cells in vitro
than either treatment alone. Thus, the strategy of combining
conditionally replicating adenoviruses, therapeutic cytokines and
chemotherapy appears to be a promising candidate for the treatment
of patients with malignant melanoma.
References
|
1
|
La Porta CA: Mechanism of drug sensitivity
and resistance in melanoma. Curr Cancer Drug Targets. 9:391–397.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nath K, Nelson DS, Ho AM, Lee SC, Darpolor
MM, Pickup S, Zhou R, Heitjan DF, Leeper DB and Glickson JD: (31) P
and (1) H MRS of DB-1 melanoma xenografts: Lonidamine selectively
decreases tumor intracellular pH and energy status and sensitizes
tumors to melphalan. NMR Biomed. 26:98–105. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jiang G, Li RH, Sun C, Jia HY, Lei TC and
Liu YQ: Efficacy and safety between temozolomide alone and
temozolomide-based double therapy for malignant melanoma: A
meta-analysis. Tumour Biol. 35:315–322. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Plummer ER, Middleton MR, Jones C, Olsen
A, Hickson I, McHugh P, Margison GP, McGown G, Thorncroft M, Watson
AJ, et al: Temozolomide pharmacodynamics in patients with
metastatic melanoma: DNA damage and activity of repair enzymes
O6-alkylguanine alkyltransferase and poly(ADP-ribose) polymerase-1.
Clin Cancer Res. 11:3402–3409. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jiang G, Li LT, Xin Y, Zhang L, Liu YQ and
Zheng JN: Strategy for reversing resistance to temozolomide in
malignant melanoma. Curr Med Chem. 19:3886–3892. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jiang G, Liu YQ, Wei ZP, Pei DS, Mao LJ
and Zheng JN: Enhanced anti-tumor activity by the combination of a
conditionally replicating adenovirus mediated interleukin-24 and
dacarbazine against melanoma cells via induction of apoptosis.
Cancer Lett. 294:220–228. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tian H, Zhang DF, Zhang BF, Li HZ, Zhang
Q, Li LT, Pei DS and Zheng JN: Melanoma differentiation associated
gene-7/interleukin-24 induces caspase-3 denitrosylation to
facilitate the activation of cancer cell apoptosis. J Interferon
Cytokine Res. 35:157–167. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dent P, Yacoub A, Hamed HA, Park MA, Dash
R, Bhutia SK, Sarkar D, Wang XY, Gupta P, Emdad L, et al: The
development of MDA-7/IL-24 as a cancer therapeutic. Pharmacol Ther.
128:375–384. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Emdad L, Lebedeva IV, Su ZZ, Gupta P,
Sauane M, Dash R, Grant S, Dent P, Curiel DT, Sarkar D and Fisher
PB: Historical perspective and recent insights into our
understanding of the molecular and biochemical basis of the
antitumor properties of mda-7/IL-24. Cancer Biol Ther. 8:391–400.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zheng M, Bocangel D, Ramesh R, Ekmekcioglu
S, Poindexter N, Grimm EA and Chada S: Interleukin-24 overcomes
temozolomide resistance and enhances cell death by down-regulation
of O6-methylguanine-DNA methyltransferase in human melanoma cells.
Mol Cancer Ther. 7:3842–3851. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu XY: Targeting gene-virotherapy of
cancer and its prosperity. Cell Res. 16:879–886. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu XY, Li HG, Zhang KJ and Gu JF:
Strategy of Cancer Targeting Gene-Viro-Therapy (CTGVT) a trend in
both cancer gene therapy and cancer virotherapy. Curr Pharm
Biotechnol. 13:1761–1767. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhao L, Gu J, Dong A, Zhang Y, Zhong L, He
L, Wang Y, Zhang J, Zhang Z, Huiwang J, et al: Potent antitumor
activity of oncolytic adenovirus expressing mda-7/IL-24 for
colorectal cancer. Hum Gene Ther. 16:845–858. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang ZY, Wang LQ, Fu CF, Li X, Cui ZL,
Zhang JY, Xue SH, Sun N and Xu F: Combination of targeting
gene-viro therapy with recombinant Fowl-pox viruses with HN and VP3
genes on mouse osteosarcoma. Eur Rev Med Pharmacol Sci. 17:767–776.
2013.PubMed/NCBI
|
|
15
|
Jiang G, Jiang AJ, Cheng Q, Tian H, Li LT
and Zheng JN: A dual-regulated oncolytic adenovirus expressing
interleukin-24 sensitizes melanoma cells to temozolomide via the
induction of apoptosis. Tumour Biol. 34:1263–1271. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhou X, Xie G, Wang S, Wang Y, Zhang K,
Zheng S, Chu L, Xiao L, Yu Y, Zhang Y and Liu X: Potent and
specific antitumor effect for colorectal cancer by CEA and Rb
double regulated oncolytic adenovirus harboring ST13 gene. PLos
One. 7:e475662012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jiang G, Zhang L, Xin Y, Pei DS, Wei ZP,
Liu YQ and Zheng JN: Conditionally replicating adenoviruses
carrying mda-7/IL-24 for cancer therapy. Acta Oncol. 51:285–292.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhao L, Dong A, Gu J, Liu Z, Zhang Y,
Zhang W, Wang Y, He L, Qian C, Qian Q and Liu X: The antitumor
activity of TRAIL and IL-24 with replicating oncolytic adenovirus
in colorectal cancer. Cancer Gene Ther. 13:1011–1022. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Qiu S, Ruan H, Pei Z, Hu B, Lan P, Wang J,
Zhang Z, Gu J, Sun L, Qian C, et al: Combination of targeting
gene-virotherapy with 5-FU enhances antitumor efficacy in malignant
colorectal carcinoma. J Interferon Cytokine Res. 24:219–230. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Berger A, Quast SA, Plötz M, Hein M, Kunz
M, Langer P and Eberle J: Sensitization of melanoma cells for death
ligand-induced apoptosis by an indirubin derivative-Enhancement of
both extrinsic and intrinsic apoptosis pathways. Biochem Pharmacol.
81:71–81. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kong XJ, Duan LJ, Qian XQ, Xu D, Liu HL,
Zhu YJ and Qi J: Tumor-suppressive microRNA-497 targets IKKβ to
regulate NF-κB signaling pathway in human prostate cancer cells. Am
J Cancer Res. 5:1795–1804. 2015.PubMed/NCBI
|
|
22
|
Wilczynski J, Duechler M and Czyz M:
Targeting NF-κB and HIF-1 pathways for the treatment of cancer:
Part I. Arch Immunol Ther Exp (Warsz). 59:289–299. 2011. View Article : Google Scholar : PubMed/NCBI
|