Introduction
Lung cancer is a major cause of morbidity and
mortality worldwide (1,2). Based on its cellular characteristics,
lung cancer is divided into two major types as follows: Non-small
cell lung cancer (NSCLC) and small cell lung cancer. NSCLC accounts
for >80% of lung cancer diagnoses (3). Although great advancements have been
made in non-invasive surgery, chemotherapy, radiotherapy and
immunotherapy to treat human cancer, the 5-year survival rate for
patients with advanced NSCLC is only 15% (3). Tumor metastasis is one of the primary
factors that determines the prognosis, quality of life and survival
rate of patients. Therefore, identifying the molecules and
signaling pathways associated with cancer metastasis is of clinical
significance and may aid in improving the prognosis of patients
with NSCLC.
Previous studies have demonstrated that malignancies
express a number of chemokines (CKs) and CK receptors (CKRs),
suggesting a role for CK/CKR signaling networks in tumor
development and progression (4–8). Among
these CKs and CKRs, the C-X-C motif CK16 (CXCL16)-CXCC receptor
type 6 (CXCR6) signaling axis has been highlighted due to its
distinctive features. CXCL16 can exist in a transmembrane (t) and
soluble (s) form, and CXCR6 is its sole receptor (9–11). Aside
from the roles that CXCL16 and CXCR6 serve in normal biological
processes, CXCL16 and CXCR6 are also aberrantly expressed in
numerous types of human cancer, including prostate, breast,
pancreatic, colorectal and bladder cancer, and in renal cell
carcinoma and nasopharyngeal carcinoma (12–21). The
interaction between CXCL16 and CXCR6 is associated with the growth,
survival, migration, invasion, angiogenesis and the activation of
multiple intracellular signaling pathways in malignant cells
(15–21), suggesting that the CXCL16-CXCR6
interaction may serve an important role in tumorigenesis and
metastasis.
A previous study confirmed the expression of CXCL16
and CXCR6 in human primary lung cancer tissues, and demonstrated
that the activation of the CXCL16-CXCR6 signaling axis promotes the
invasion of A549, 95D and H292 lung cancer cells in vitro
(22), implicating the CXCL16-CXCR6
signaling axis in the development of lung cancer. However, whether
there is variability in CXCL16 and CXCR6 expression between
patients with lung cancer with different clinicopathological
features has not yet been investigated, to the best of our
knowledge. In the present clinical retrospective study, the
association of t-CXCL16, t-CXCR6 and s-CXCL16 levels with
clinicopathological features was investigated in patients with
NSCLC. The data from the present study provide new insights into
potential biomarkers for the early detection of lung cancer, and
into targeted therapy and postoperative follow-up for patients with
NSCLC.
Materials and methods
Tissue sample collection
All procedures involving participants in the present
study were approved by the Human Research Ethics Committee of
Zhongnan Hospital of Wuhan University (Wuhan, China), and written
informed consent was provided by all participants. Tissue
collection was performed as previously described (22). Briefly, human lung cancer tissue (58
cases) and the adjacent normal lung tissue (20 cases) was obtained
from patients who underwent pulmonary lobe resection or
pneumonectomy at Zhongnan Hospital of Wuhan University between
August 2013 and September 2014. Two experienced pathologists
performed the identification of the pathological type and
differentiation degree of NSCLC. Tumor (T) stage was determined
according to the seventh edition of the tumor-node-metastasis (TNM)
staging system of the International Association for the Study of
Lung Cancer (IASLC) in 2009 (23).
The recruitment criteria for patients included a pathological
diagnosis of primary NSCLC, without any other primary tumor
history, intact medical records and follow-up data. The exclusion
criteria were preoperative chemotherapy, radiotherapy, biological
therapy or immunotherapy. The clinicopathological characteristics
of the patients included in the present study are provided in
Table I.
 | Table I.Clinicopathological characteristics of
the patients with non-small cell lung cancer included in the
present study. |
Table I.
Clinicopathological characteristics of
the patients with non-small cell lung cancer included in the
present study.
| Clinicopathological
characteristic | No. of patients | Proportion of
patients (%) |
|---|
| Age (years) |
|
<60 | 32 | 55.2 |
| ≥60 | 26 | 44.8 |
| Gender |
| Male | 42 | 72.4 |
|
Female | 16 | 27.6 |
| Pathological
type |
| AC | 32 | 55.2 |
| SC | 22 | 37.9 |
| ASC | 4 | 6.9 |
| Differentiation
degree |
| Low | 15 | 25.9 |
|
Moderate | 30 | 51.7 |
| High | 13 | 22.4 |
| TMN stage |
| I–II | 30 | 51.7 |
|
III–IV | 28 | 48.3 |
| Smoking |
| + | 37 | 63.8 |
| − | 21 | 36.2 |
| Pleural invasion |
| + | 4 | 6.9 |
| − | 54 | 93.1 |
| Lymph node
metastasis |
| + | 34 | 58.6 |
| − | 24 | 41.4 |
Immunohistochemistry (IHC)
IHC was performed as previously described (17,22).
rabbit polyclonal CXCL16 (cat. no. ab101404; dilution, 1:100) and
rabbit polyclonal CXCR6 (cat. no. ab8023; dilution, 1:100)
antibodies from Abcam (Cambridge, MA, USA) were used as the primary
antibodies in the study. They were validated by the manufacturer
for immunohistochemistry on paraffin-embedded material. The tissues
were fixed with formalin and embedded in paraffin. The 4-µm tissue
sections were deparaffinized with xylene and rehydrated with
ethanol. Antigen retrieval was performed by placing the sections in
0.01 mol/l citrate buffer, pH 6.0, before microwave heating for 15
min at 400 W. Following antigen retrieval, 0.3%
H2O2 for 15 min in PBS was used to block
endogenous peroxidase activity in the 4-µm tissue sections.
Following treatment with citrate buffer (MaiXin Biotechnology, Co.,
Ltd., Fuzhou, China) to clear non-specific binding, the sections
were incubated overnight at 4°C with 25 µg/ml CXCR6 or 20 µg/ml
CXCL16 primary antibodies. The CXCR6 and t-CXCL16 molecules were
visualized by adding horseradish peroxidase (HRP)-labeled mouse
anti-rabbit IgG (cat. no. KIT-9901, dilution, 1:100, MaiXin
Biotechnology, Co., Ltd., Fuzhou, China), which were included in a
detection reagent kit (Elivision™ plus Polyer HRP (Mouse/Rabbit)
IHC Kit, cat. no. KIT-9901, MaiXin Biotechnology, Co., Ltd.,
Fuzhou, China) at 37°C for 15 min. Then 3,3-diaminobenzidine
tetrahydrochloride was used for signal detection and Harris
hematoxylin was used as a counterstain. The reagents for
immunohistochemical analysis, including the citrate buffer,
H2O2, detection kit, DAB and hematoxylin were
purchased from MaiXin Biotechnology, Co. Ltd. A total of 10 µg/ml
rabbit isotype immunoglobulin G (cat. no. AG-0021; dilution, 1:50;
Dingguo Bio Co., Ltd., Shanghai, China) was used as a negative
control.
Scoring of immunohistochemistry
(IHC)
The IHC scoring was performed blindly using a
telepathology system without knowledge of the associated clinical
information, including tumor grade, tumor size and clinical outcome
(17,22). The tissue sections were assigned
scores respectively based on the intensity of immunostaining and
the percentage of positively stained cells. The immunostaining
intensity was observed and scored as follows: No staining (score,
0), light yellow staining (score, 1), light brown staining (score,
2) or brown staining (score, 3). The percentage of positively
stained cells was scored as follows: 5% (score, 0), 5–25% (score,
1), 26–50% (score, 2), 51–75% (score, 3) or >75% (score, 4). The
sum of the immunostaining intensity score and the score for the
percentage of positive cells was the overall score of every tissue
slice, which was defined as follows: <2, negative expression(−);
≥2 positive expression; 2–3, weak expression(+); 4–5, moderate
expression (++); and 6–7 as strong expression (+++) (17,22).
ELISA
A total of 2 ml venous blood was collected using 10
ml syringe in morning, then spaced into vacuum packing tubes
without anticoagulant. The samples were allowed to stand for 30
min. Subsequent to low-speed centrifugal 1,200 × g for 10 min, the
supernatant was collected into EP tubes, and then centrifuged at
19,200 × g for 10 min, Now the supernatant is the serum samples.
The blood sera from 58 patients with NSCLC and 32 normal volunteers
(17 were men and 13 were female, age range 46–72 years) were
collected from between August 2013 and September 2014 and stored at
−80°C until the ELISA analysis was performed. The amount of
s-CXCL16 in each sample was measured using a human CXCL16 ELISA kit
(cat. no. F00514; Shanghai Westang Bio-Tech Co., Ltd., Shanghai,
China), according to the manufacturer's protocol. The CXCL16 assay
kit demonstrated a sensitivity of 40 pg/ml and an intra-assay
coefficient of variation of <12% (22).
Statistical analysis
The data were analyzed using SPSS software (version
17.0; SPSS, Inc., Chicago, IL, USA). The results of the ELISA are
presented as the mean ± standard error and were assessed using a
Student's t-test. The association between CXCL16-CXCR6 expression
and clinicopathological features was analyzed with a χ2
test, Fisher's exact test and Spearman rank correlation coefficient
analysis. A univariate analysis was performed using the
Kaplan-Meier estimator method and a log-rank test. The median
survival time was calculated using SPSS v17.0 (SPSS, Inc., Chicago,
IL, USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
Clinicopathological characteristics of
patients with NSCLC
The age of patients with NSCLC ranged from 43–80
years and the average age was 58.95±9.84 years (Table I). A total of 72.4% of patients with
NSCLC were male and 27.6% were female. The pathological types were
as follows: Adenocarcinoma (AC; 32 cases), squamous carcinoma (SC;
22 cases) and adenosquamous carcinoma (ASC; 4 cases).
Differentiation degrees included low (15 cases), medium (30 cases)
and high (13 cases). A total of 30 patients were identified to have
stage I–II NSCLC and 28 patients were identified to have stage
III–IV NSCLC. Among the 58 patients there were 37 smokers, 4
patients with pleural invasion and 34 patients with lymph node
metastasis.
Association between t-CXCL16 and
t-CXCR6 expression in patients with NSCLC
IHC was performed to detect the expression of
t-CXCL16 and t-CXCR6 protein in human lung tissues derived from
primary NSCLCs (Table II). t-CXCR6-
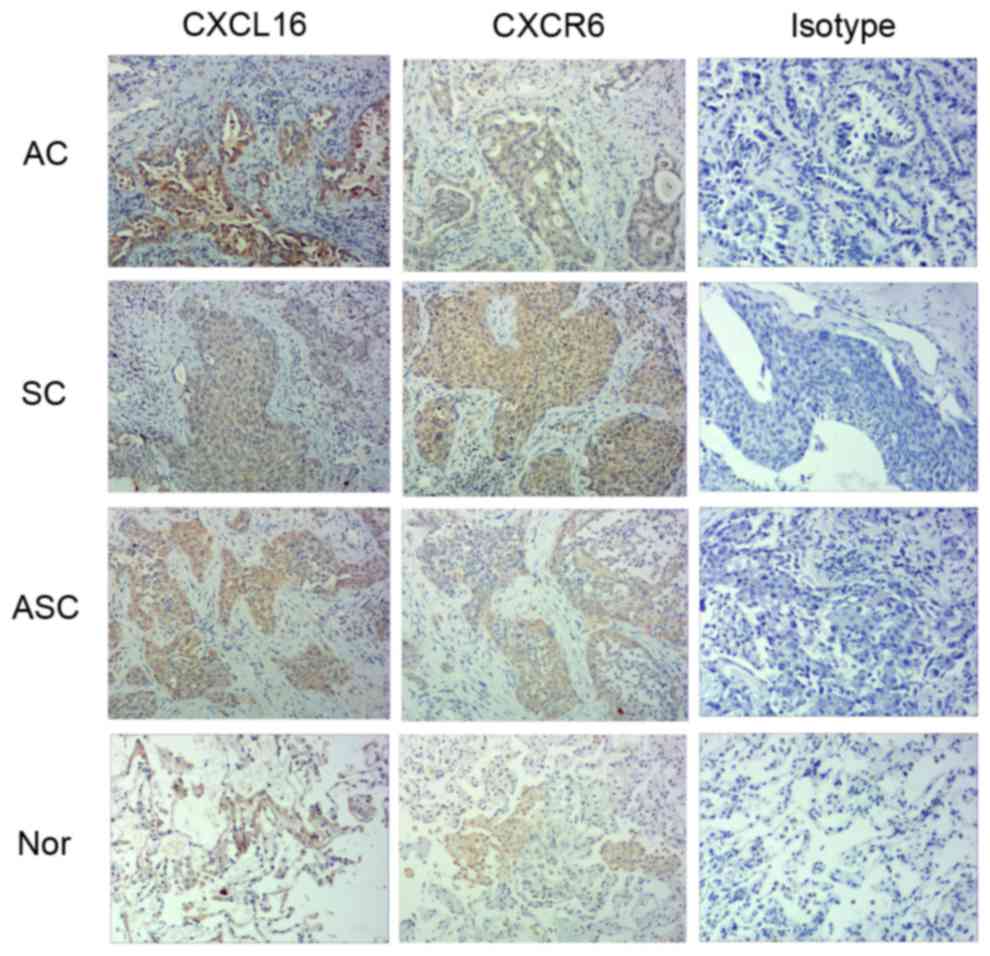
and t-CXCL16-specific staining was clearly observed in the
cytoplasm and membrane of the primary lung cancer cells (Fig. 1). In normal lung tissue, t-CXCL16 and
t-CXCR6 were primarily restricted to the alveolar epithelial cells
and inflammatory cells. According to the scoring of IHC, the
positive expression rate was defined as that the ratio between the
positive expression case (overall score of the tissue slice ≥2) and
all cases in the same group. No significant difference was
identified between the positive expression rate of t-CXCL16 (72.41%
of cases) and that of t-CXCR6 protein (65.52% of cases) (P=0.442;
Table II). Among the 58 patients
with NSCLC, there were 38 cases (65.52%) that co-expressed t-CXCL16
and t-CXCR6 (data not shown).
 | Table II.Positive expression rate of CXCL16 and
CXCR6 in non-small cell human lung cancer tissues. |
Table II.
Positive expression rate of CXCL16 and
CXCR6 in non-small cell human lung cancer tissues.
|
|
| Expression |
|
|
|---|
|
|
|
|
|
|
|---|
| Protein | No. of
patients | Positive | Negative | Positive expression
rate (%) | P-value |
|---|
| CXCL16 | 58 | 42 | 16 | 72.41 | 0.422 |
| CXCR6 | 58 | 38 | 20 | 65.52 |
|
Association between t-CXCL16/t-CXCR6
expression and the clinicopathological characteristics of patients
with NSCLC (Table III)
No significant difference was observed between the
positive expression rate of t-CXCL16 in the <60 years subgroup
compared with that of the ≥60 years subgroup (78.13 vs. 65.38%;
P=0.280). The same was true for t-CXCR6 in the <60 compared with
the ≥60 years old groups (71.88 vs. 57.69%; P=0.258). No
significant differences were identified between the positive
expression rates of t-CXCL16 (71.43 vs. 75%; P=0.28) or t-CXCR6
(64.29 vs. 68.75%; P=0.749) between the male subgroup and the
female subgroups, respectively.
Additionally, no significant differences were
identified between the positive expression rates of t-CXCL16 or
t-CXCR6 in different pathological types of NSCLC (Table III). For t-CXCL16, the positive
expression rates were 75, 68.18 and 75% in the AC, SC and ASC
subgroups (P=0.895), respectively. For t-CXCR6, the positive
expression rates were 65.63, 63.64 and 75% in the AC, SC and ASC
subgroups (P=1.000), respectively. Similar results were observed
when comparing subgroups of patients with NSCLC that was
differentiated to different degrees. For t-CXCL16, the positive
expression rates were 80, 70 and 69.23% in the low, medium and high
subgroups (P=0.799), respectively. For t-CXCR6, the positive
expression rates were 66.67, 63.33 and 69.23% in the low, medium
and high subgroups (P=0.927), respectively. No significant
differences were identified between the expression rates of
t-CXCL16 (100 vs. 70.37%, P=0.480) or t-CXCR6 (75 vs. 64.81%,
P=1.000) in the pleural invasion and non-pleural invasion
subgroups.
When patients were grouped according to TNM stage,
the positive expression rate of t-CXCL16 (60 vs. 85.71%, P=0.029)
and t-CXCR6 (53.33 vs. 78.57%, P=0.043) in stage I–II was
significantly lower compared with that of stage III–IV (Table III). Furthermore, the expression
rate of t-CXCL16 (91.18 vs. 50%, P=0.001) and t-CXCR6 (79.41 vs.
45.83%, P=0.008) in the lymph node metastasis subgroup was
significantly higher compared with that of the corresponding
non-lymph node metastasis subgroup. Although no significant
difference in t-CXCR6 expression was identified between the smoking
and non-smoking subgroups (64.86 vs. 66.67%, P=0.890), the positive
expression rate of t-CXCL16 in the smoking subgroup was
significantly higher compared with that of the non-smoking subgroup
(100 vs. 23.81%, P<0.001).
Association between s-CXCL16
concentration and the clinicopathological characteristics of
patients with NSCLC
An ELISA was performed to compare the concentration
of s-CXCL16 in patients with NSCLC and normal volunteers. As
illustrated in Table IV, the
s-CXCL16 concentration in patients with NSCLC was significantly
higher compared with that of the normal volunteers (572.82±116.05
vs. 329.47±135.38 pg/ml; P<0.001). According to the average
s-CXCL16 concentration, patients with NSCLC were further divided
into two subgroups as follows: High (≥572.82 pg/ml, 24 cases) and
low (<572.82 pg/ml, 34 patients; Tables V and VI). Among the s-CXCL16 high concentration
group containing 24 cases in total, there were 5 patients that did
not express t-CXCL16, 12 patients with weak expression, 5 with
moderate expression and strong expression in 2 patients (Table V). Compared with t-CXCR6, the high
group included 8 patients with negative expression, 11 patients
with weak expression, only one patient with moderate expression and
4 with strong expression (Table VI).
Prominent significant differences were identified between the
t-CXCL16 (72.41 vs. 41.38%; Chi-square=11.389; P=0.001) or t-CXCR6
(65.52 vs. 41.38%; Chi-square=6.791; P=0.009) IHC positive
expression rates and the s-CXCL16 high-level subgroup (Not shown in
Tables V and VI).
 | Table IV.Concentration of s-CXCL16 in patients
with NSCLC and normal volunteers. |
Table IV.
Concentration of s-CXCL16 in patients
with NSCLC and normal volunteers.
| Group | No. of
patients | s-CXCL16
(pg/ml) | P-value |
|---|
| NSCLC | 58 | 572.82±116.05 | <0.001 |
| Normal
volunteers | 32 | 329.47±135.38 |
|
 | Table V.Expression pattern of s-CXCL16 and
t-CXCL16 in patients with non-small cell lung cancer. |
Table V.
Expression pattern of s-CXCL16 and
t-CXCL16 in patients with non-small cell lung cancer.
|
|
| t-CXCL16
concentration |
|
|---|
|
|
|
|
|
|---|
|
|
| − | + | ++ | +++ | Total |
|---|
| s-CXCL16
concentration | Low | 11 | 10 | 10 | 3 | 34 |
|
| High | 5 | 12 | 5 | 2 | 24 |
| Total |
| 16 | 22 | 15 | 5 |
|
 | Table VI.Expression pattern of s-CXCL16 and
t-CXCR6 in patients with non-small cell lung cancer. |
Table VI.
Expression pattern of s-CXCL16 and
t-CXCR6 in patients with non-small cell lung cancer.
|
|
| t-CXCR6
concentration |
|
|---|
|
|
|
|
|
|---|
|
|
| − | + | ++ | +++ | Total |
|---|
| s-CXCL16
concentration | Low | 12 | 11 | 3 | 8 | 34 |
|
| High | 8 | 11 | 1 | 4 | 24 |
| Total |
| 20 | 22 | 4 | 12 |
|
The associations between s-CXCL16 levels (high or
low) and clinicopathological characteristics of the patients are
illustrated in Table VII. There was
no correlation between age and the percentage of cases with a high
level of s-CXCL16 [37.5% (<60 years) vs. 46.15% (≥60 years);
P=0.506]. No significant difference was identified between the
percentage of male patients with a high s-CXCL16 level and the
percentage of female patients with a high s-CXCL16 level (40.48 vs.
43.75%; P=0.821).
 | Table VII.Association between s-CXCL16
concentration and the clinicopathological characteristics of
patients with non-small cell lung cancer. |
Table VII.
Association between s-CXCL16
concentration and the clinicopathological characteristics of
patients with non-small cell lung cancer.
|
|
|
| s-CXCL16 |
|---|
|
|
|
|
|
|---|
| Group | Subgroup | Total no. of
cases | High | Low | PR ((%) | P-value |
|---|
| Age (years) | <60 | 32 | 12 | 20 | 37.50 | 0.506 |
|
| ≥60 | 26 | 12 | 14 | 46.15 |
|
| Gender | Male | 42 | 17 | 25 | 40.48 | 0.821 |
|
| Female | 16 | 7 | 9 | 43.75 |
|
| Pathological
type | AC | 32 | 11 | 21 | 34.38 | 0.487 |
|
| SC | 22 | 11 | 11 | 50.00 |
|
|
| ASC | 4 | 2 | 2 | 50.00 |
|
| Differentiation
degree | Low | 15 | 9 | 6 | 60.00 | 0.224 |
|
| Moderate | 30 | 10 | 20 | 33.33 |
|
|
| High | 13 | 5 | 8 | 38.46 |
|
| TMN stage | I–II | 30 | 8 | 22 | 26.67 | 0.019a |
|
| III–IV | 28 | 16 | 12 | 57.14 |
|
| Smoking | + | 37 | 14 | 23 | 37.84 | 0.467 |
|
| − | 21 | 10 | 11 | 47.62 |
|
| Pleural
invasion | + | 4 | 2 | 2 | 50.00 | 1.000 |
|
| − | 54 | 22 | 32 | 40.74 |
|
| Lymph node
metastasis | + | 34 | 15 | 19 | 44.12 | 0.614 |
|
| − | 24 | 9 | 15 | 37.50 |
|
When grouped according to pathological type, the
percentage of patients with a high s-CXCL16 level was 34.38, 50 and
50% in the AC, SC and ASC subgroups, respectively (P=0.487;
Table VII). No significant
difference was identified between the percentages of patients with
a high s-CXCL16 level in different differentiation degree subgroups
[60% (low) vs. 33.33% (medium) vs. 38.46% (high); P=0.224].
Additionally, the presence or absence of smoking (37.84 vs. 47.62%;
P=0.467), pleural invasion (50 vs. 40.74%; P=1.000) or lymph node
metastasis (44.12 vs. 37.5%; P=0.641) had no effect on the
expression level of s-CXCL16 in patients with NSCLC. However, when
grouped according to TNM stage, the percentage of patients with a
high s-CXCL16 level in the stage I–II subgroup (26.67%) was
significantly lower compared with that of the stage III–IV (57.14%)
subgroup (P=0.019).
Effects of t-CXCL16, t-CXCR6 and
s-CXCL16 expression on patients' prognosis
Professional personnel performed a follow-up every 4
months between January 2014 and January 2015. The follow-up rate
and mortality rate were 100% (58/58) and 13.79% (8/58),
respectively. Within the time period of the present study, the
survival time were 4–18 months and the mean survival time was 16.6
months (data not shown). No significant difference was identified
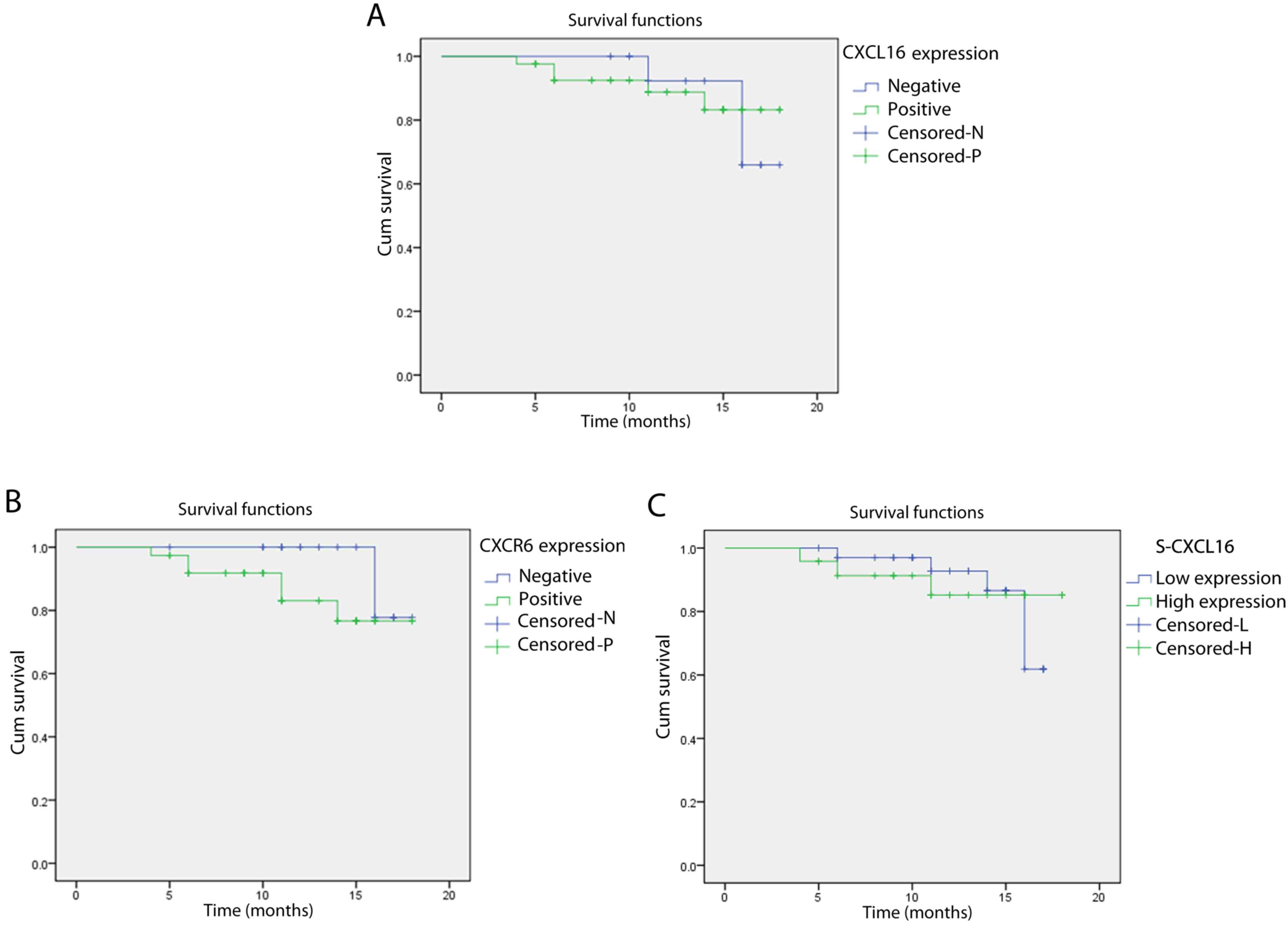
between the survival rate of the t-CXCL16-positive group (88.1%)
compared with the t-CXCL16-negative group (81.25%) (log-rank,
0.008; P=0.931; Fig. 2A).
Additionally, no significant difference was identified between the
t-CXCR6-positive (84.21%) and the t-CXCR6-negative (90%) groups
(log-rank, 1.559; P=0.212; Fig. 2B).
The survival rate of patients with NSCLC [87.5% (high subgroup) vs.
85.29% (low subgroup)] was not associated with the s-CXCL16 level
(log-rank, 0.068; P=0.795; Fig. 2C).
The median survival time was not obtained as the follow-up time was
relatively short and all the survival rates were >50%.
Therefore, it was difficult to obtain the median survival time with
the method used in the present study.
Discussion
The aim of the present study was to investigate the
role of the CXCL16-CXCR6 signaling axis in the progression and
metastasis of human lung cancer. The expression of t-CXCL16,
t-CXCR6 and s-CXCL16 was measured in 58 patients with NSCLC, and
the association between these expression levels and different
clinicopathological features was explored. In accordance with a
previous study (22), t-CXCR6- and
t-CXCL16-specific staining was clearly observed in the cytoplasm
and membrane of the primary lung cancer cells (Fig. 1). The data revealed that t-CXCL16 and
t-CXCR6 were co-expressed in human primary NSCLC tissue, and no
significant difference was identified between the positive
expression rates of t-CXCL16 and t-CXCR6 (P=0.442). This expression
pattern of t-CXCL16 and t-CXCR6 is similar to that observed in a
previous study (22). A total of 91
samples were investigated across the present study and this
previous study. Thus, the co-expression of CXCL16 and CXCR6 may
serve an important role in the development of human lung
cancer.
Age and gender, and the pathological type and
differentiation degree of NSCLC, had no significant effect on
t-CXCL16 or t-CXCR6 expression in NSCLC tissue. However, there was
a significant difference between the positive expression rates of
t-CXCL16 (P=0.029) and t-CXCR6 (P=0.043) of the stage III–IV and
I–II TNM subgroups. The same result was observed when the patients
were grouped according to the occurrence of lymphatic metastasis.
The positive expression rates of t-CXCL16 (P=0.001) and t-CXCR6
(P=0.008) of the lymph node metastasis subgroup were significantly
higher compared with that of the corresponding non-lymph node
metastasis subgroup. Previous studies have demonstrated that the
CXCL16-CXCR6 signaling axis promotes the viability and invasiveness
of lung cancer cell lines in vitro (22). Recent in vivo experiments from
our group have demonstrated that blocking the CXCL16-CXCR6
signaling axis effectively inhibits tumor formation in nude mice
(Hu et al, unpublished data). This previous data, and the
data from the present study, suggest that the CXCL16-CXCR6
signaling axis is associated with human lung tumor metastasis.
The expression pattern of t-CXCL16 was different in
the smoking subgroup when compared with the non-smoking subgroup,
and also when comparing the TNM stage and lymphatic metastasis
status. There was no significant difference in t-CXCR6 expression
between the smoking subgroup and the non-smoking subgroup (P=0.89).
However, all patients with NSCLC from the smoking subgroup
expressed t-CXCL16 at a significantly higher level compared with
those in the non-smoking subgroup (P<0.001). Under normal
conditions, CXCL16 is constitutively expressed by human bronchial
epithelial cells, which is important for the homeostatic regulation
of T cells and resistance to external pathogens (24). During an inflammatory response,
including the response to regular smoking, CXCL16 can be
upregulated (16,25). The role of inflammation in the tumor
microenvironment during tumorigenesis has been investigated
(26,27). It has been demonstrated in prostate
cancer that inflammatory cytokines derived from adjacent
infiltrating CXCR6-positive T cells can stimulate the production of
CXCL16 by cancer cells, and that CXCL16 then further enhances the
growth and proliferation of CXCR6-expressing cancer cells and
primary T cells (16). Thus, the
smoking-associated inflammatory microenvironment, together with an
abnormal increase of CXCL16, may contribute to the high risk of
lung cancer for smokers.
CXCL16 can exist in a t and s form. Thus, in the
present study, the concentration of s-CXCL16 was examined in
patients with NSCLC and in normal volunteers. The s-CXCL16 level in
patients with NSCLC was significantly increased compared with that
in the normal volunteers (P<0.001). No significant differences
were identified between the expression levels of s-CXCL16 among the
age, gender, pathological type, differentiation degree, smoking,
pleural invasion or lymph node metastasis subgroups. This may be
due to the small sample size of the present study. However, the
level of s-CXCL16 in the stage III–IV TNM subgroup was
significantly higher compared with that of the corresponding stage
I–II TNM subgroup (P=0.019). The results from the present study
suggest that s-CXCL16 levels are a novel biomarker for the early
detection and grading of NSCLC. However, further studies
investigating the role that s-CXCL16 serves in tumor metastasis are
required, as the level of s-CXCL16 may not be representative of the
level of t-CXCL16 in the lung.
One limitation of the present study is the small
cohort size, which did not allow for a thorough survival analysis.
Besides, the follow-up period was short and most patients were
still alive at the end of this study, so we cannot get the median
survival time of the patients, and further study should be
conducted for the survival effect. Although a previous study has
investigated the association between CXCL16 expression and the
survival of patients with lung cancer (28), further studies investigating the
prognostic impact of CXCL16 and CXCR6 expression in larger
multicenter cohorts of patients with NSCLC are required.
Additionally, pleural invasion typically occurs in the advanced
stages of NSCLC when surgery is not appropriate. Thus, in the
present study's patient cohort, there were only 4 patients with
pleural metastasis. The underlying molecular mechanisms of pleural
metastasis in NSCLC require further investigation.
In conclusion, the present study demonstrated that
t-CXCL16 and t-CXCR6 are co-expressed in human NSCLC. The TNM stage
and lymph node metastasis status were positively correlated with
the expression levels of t-CXCL16 and t-CXCR6, suggesting that the
CXCL16-CXCR6 signaling axis serves a role in the development and
metastasis of lung cancer. Additionally, there was a significant
increase in s-CXCL16 levels in patients with NSCLC, suggesting that
s-CXCL16 could be used as a biomarker for the early detection of
lung cancer. In addition, the expression of t-CXCL16 was
significantly increased in patients with NSCLC that smoked compared
with patients that did not smoke, which provides insight into a
potential underlying molecular mechanism for the high risk of lung
cancer in smokers.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81270753 and
81471511), Key project of Hubei provincial health and Family
Planning Commission (grant no. WJ2017Z006), the Health and Family
Planning Commission of Hubei Province (grant no. WJ2015MB111) and
the Personnel Training Plan of The Health Care System of Beijing
(grant no. 2013-3-021).
References
|
1
|
Bray F, Ren JS, Masuyer E and Ferlay J:
Global estimates of cancer prevalence for 27 sites in the adult
population in 2008. Int J Cancer. 132:1133–1145. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wood SL, Pernemalm M, Crosbie PA and
Whetton AD: The role of the tumor-microenvironment in lung
cancer-metastasis and its relationship to potential therapeutic
targets. Cancer Treat Rev. 40:558–566. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gerber PA, Hippe A, Buhren BA, Müller A
and Homey B: Chemokines in tumour associated angiogenesis. Biol
Chem. 390:1213–1223. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Raman D, Baugher PJ, Thu YM and Richmond
A: Role of chemokines in tumor growth. Cancer Lett. 256:137–165.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Vandercappellen J, Van Damme J and Struyf
S: The role of CXC chemokines and their receptors in cancer. Cancer
Lett. 267:226–244. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sarvaiya PJ, Guo D, Ulasov I, Gabikian P
and Lesniak MS: Chemokines in tumor progression and metastasis.
Oncotarget. 4:2171–2185. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Keeley EC, Mehrad B and Strieter RM: CXC
chemokines in cancer angiogenesis and metastases. Adv Cancer Res.
106:91–111. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Matloubian M, David A, Engel S, Ryan JE
and Cyster JG: A transmembrane CXC chemokine is a ligand for
HIV-coreceptor Bonzo. Nat Immunol. 1:298–304. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shimaoka T, Kume N, Minami M, Hayashida K,
Kataoka H, Kita T and Yonehara S: Molecular cloning of a novel
scavenger receptor for oxidized low density lipoprotein, SR-PSOX,
on macrophages. J Biol Chem. 275:40663–40666. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wilbanks A, Zondlo SC, Murphy K, Mak S,
Soler D, Langdon P, Andrew DP, Wu L and Briskin M: Expression
cloning of the STRL33/BONZO/TYMSTR ligand reveals elements of CC,
CXC and CX3C chemokines. J Immunol. 166:5145–5154. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hattermann K, Ludwig A, Gieselmann V,
Held-Feindt J and Mentlein R: The chemokine CXCL16 induces
migration and invasion of glial precursor cells via its receptor
CXCR6. Mol Cell Neurosci. 39:133–141. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hara T, Katakai T, Lee JH, Nambu Y,
Nakajima-Nagata N, Gonda H, Sugai M and Shimizu A: A transmembrane
chemokine, CXC chemokine ligand 16, expressed by lymph node
fibroblastic reticular cells has the potential to regulate T cell
migration and adhesion. Int Immunol. 18:301–311. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Huang Y, Zhu XY, Du MR, Wu X, Wang MY and
Li DJ: Chemokine CXCL16, a scavenger receptor, induces
proliferation and invasion of firsttrimester human trophoblast
cells in an autocrine manner. Hum Reprod. 21:1083–1091. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Deng L, Chen N, Li Y, Zheng H and Lei Q:
CXCR6/CXCL16 functions as a regulator in metastasis and progression
of cancer. Biochim Biophys Acta. 1806:42–49. 2010.PubMed/NCBI
|
|
16
|
Darash-Yahana M, Gillespie JW, Hewitt SM,
Chen YY, Maeda S, Stein I, Singh SP, Bedolla RB, Peled A, Troyer
DA, et al: The chemokine CXCL16 and its receptor, CXCR6, as markers
and promoters of inflammation-associated cancers. PLoS One.
4:e66952009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hu W, Zhen X, Xiong B, Wang B, Zhang W and
Zhou W: CXCR6 is expressed in human prostate cancer in vivo and is
involved in the in vitro invasion of PC3 and LNCap cells. Cancer
Sci. 99:1362–1369. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Meijer J, Ogink J, Kreike B, Nuyten D, de
Visser KE and Roos E: The chemokine receptor CXCR6 and its ligand
CXCL16 are expressed in carcinomas and inhbit proliferation. Cancer
Res. 68:4701–4708. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gutwein P, Schramme A, Sinke N,
Abdel-Bakky MS, Voss B, Obermüller N, Doberstein K, Koziolek M,
Fritzsche F, Johannsen M, et al: Tumoural CXCL16 expression is a
novel prognostic marker of longer survival times in renal cell
cancer patients. Eur J Cancer. 45:478–489. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ou DL, Chen CL, Lin SB, Hsu CH and Lin LI:
Chemokine receptor expression profiles in nasopharyngeal carcinoma
and their association with metastasis and radiotherapy. J Pathol.
210:363–373. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lee JT, Lee SD, Lee JZ, Chung MK and Ha
HK: Expression analysis and clinical significance of CXCL16/CXCR6
in patients with bladder cancer. Oncol Lett. 5:229–235.
2012.PubMed/NCBI
|
|
22
|
Hu W, Liu Y, Zhou W, Si L and Ren L:
CXCL16 and CXCR6 are coexpressed in human lung cancer in vivo and
mediate the invasion of lung cancer cell lines in vitro. PLoS One.
9:e990562014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Groome PA, Bolejack V, Crowley JJ, Kennedy
C, Krasnik M, Sobin LH and Goldstraw P; IASLC International Staging
Committee, : Cancer Research and Biostatistics; Observers to the
Committee; Participating Institutions: The IASLC lung cancer
staging project: Validation of the proposals for revision of the T
N and M descriptors and consequent stage groupings in the
forthcoming (seventh) edition of the TNM classification of
malignant tumours. J Thorac Oncol. 2:694–705. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Day C, Patel R, Guillen C and Wardlaw AJ:
The chemokine CXCL16 is highly and constitutively expressed by
human bronchial epithelial cells. Exp Lung Res. 35:272–283. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sallusto F, Mackay CR and Lanzavecchia A:
The role of chemokine receptors in primary, effector, and memory
immune responses. Annu Rev Immunol. 18:593–620. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kamp DW, Shacter E and Weitzman SA:
Chronic inflammation and cancer: The role of the mitochondria.
Oncology (Williston Park). 25:400–410, 413. 2011.PubMed/NCBI
|
|
27
|
Pikarsky E, Porat RM, Stein I, Abramovitch
R, Amit S, Kasem S, Gutkovich-Pyest E, Urieli-Shoval S, Galun E and
Ben-Neriah Y: NF-kappaB functions as a tumour promoter in
inflammation-associated cancer. Nature. 431:461–466. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hald SM, Kiselev Y, Al-Saad S, Richardsen
E, Johannessen C, Eilertsen M, Kilvaer TK, Al-Shibli K, Andersen S,
Busund LT, et al: Prognostic impact of CXCL16 and CXCR6 in
non-small cell lung cancer: Combined high CXCL16 expression in
tumor stroma and cancer cells yields improved survival. BMC Cancer.
15:4412015. View Article : Google Scholar : PubMed/NCBI
|