Introduction
Cholangiocarcinoma (CCA) is a relatively rare
hepatobiliary malignancy, with an incidence of <1 case per
100,000 individuals, which accounts for 3% of all gastrointestinal
malignancies worldwide (1,2). Known risk factors for the development of
CCA include primary sclerosing cholangitis, choledochal cysts and
hepatolithiasis, as well as liver flukes in Asian populations
(2).
Patients with extra-hepatic cholangiocarcinoma often
present with symptoms of biliary obstruction, including painless
jaundice, pale stools, dark urine and pruritis. Intra-hepatic
disease may present differently, often with less specific symptoms,
such as malaise, abdominal pain and weight loss (3).
Commonly used diagnostic imaging modalities for CCA
include computed tomography (CT) and magnetic resonance
cholangio-pancreatography. Endoscopic ultrasound and endoscopic
retrograde cholangio-pancreatography are useful for imaging distal
extra-hepatic lesions, as these techniques allow stenting to
relieve biliary obstruction and cytological brushings to be
obtained (3,4). Diagnosis may also be supported by
elevated levels of the tumor marker, carcinoembryonic antigen 19–9
(4).
Surgical resection remains the standard treatment
for cholangiocarcinoma, however 5-year postoperative survival rates
remain poor at 27–37% for patients with extra-hepatic disease and
23–42% for patients with intra-hepatic disease and clear surgical
margins (3,4).
In the current study, two cases of CCA, in patients
who had previously undergone external beam radiotherapy (EBRT) for
the treatment of non-Hodgkin's lymphoma (NHL), are presented. The
clinical data of the patients are presented and the medical records
of the patients are retrospectively reviewed. The study details the
clinical history, investigations and outcome of these two cases of
this rarely encountered phenomenon, which may ultimately be
considered an unusual but important variant of CCA. We hypothesize
that the biliary epithelium is particularly sensitive to EBRT and
review the current literature to investigate the proposed etiology
of secondary malignancies following EBRT.
Case report
Case 1
In August 1981, a 10-year-old female was diagnosed
with metastatic abdominal NHL [Ann Arbor stage IV (5)], after presenting to Westmead Children's
Hospital (Sydney, Australia) with massive hepatosplenomegaly. The
patient was administered combined chemotherapy and radiotherapy
with the LSA2L2 (6) protocol for 3
years, and received prophylactic cranial (24 Gy) and abdominal (7.2
Gy) irradiation for residual lymphoma. Abdominal irradiation was
terminated early due to marrow suppression. The patient was well
when discharged and was followed up in the hospital's Late-Effects
Clinic for 22 years.
In July 2011, the patient presented with symptomatic
cholelithiasis, and underwent an uncomplicated laparoscopic
cholecystectomy (normal cholangiogram). However, at 2 months
post-surgery, the patient presented with painless, obstructive
jaundice. Endoscopic retrograde cholangiopancreatography (ERCP)
proved technically difficult and following two failed attempts, a
biliary stricture was identified. The stricture was subsequently
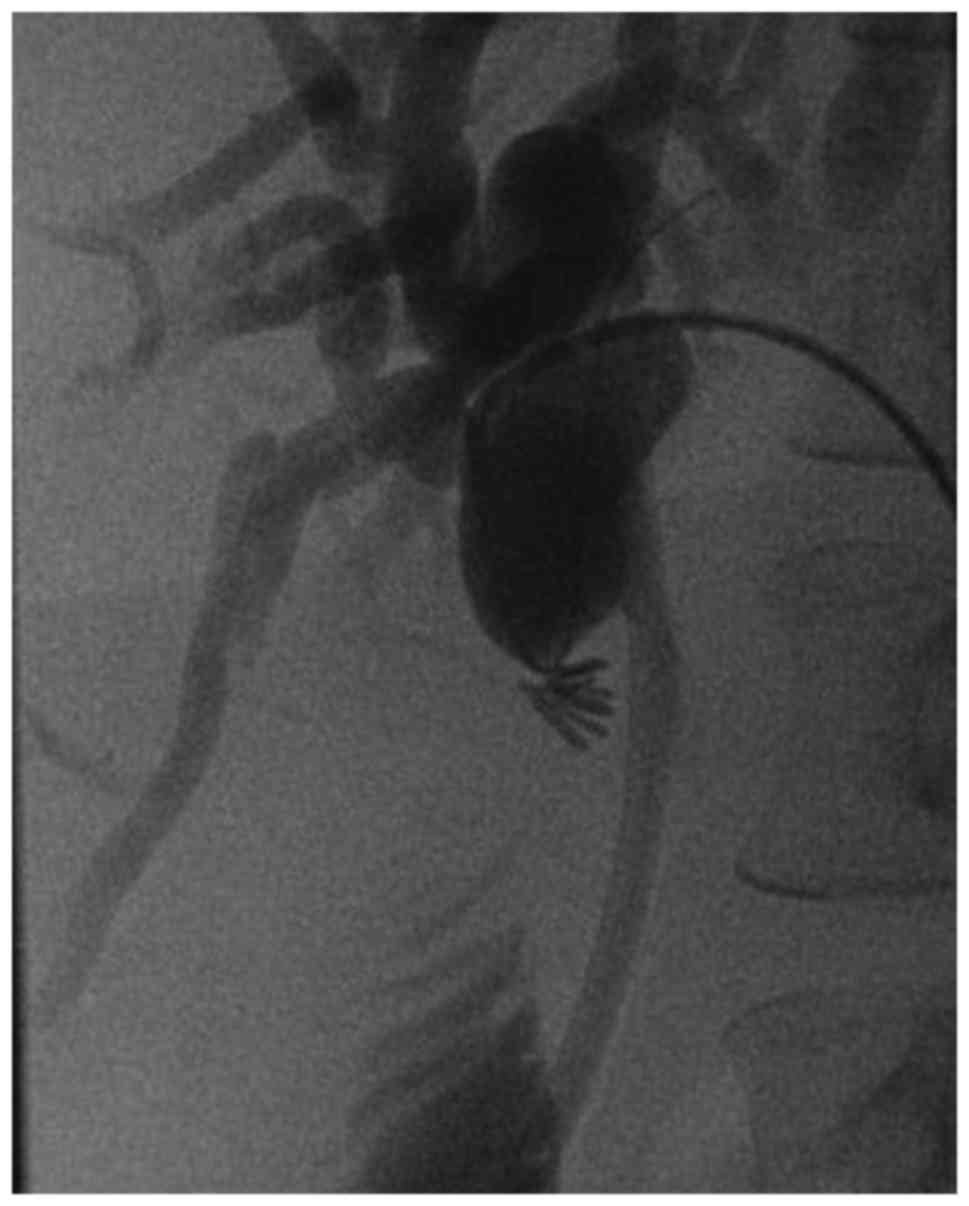
dilated and stented using an antegrade, percutaneous transhepatic
(PTC) approach (Fig. 1). The
stricture was located 1 cm proximal to the common hepatic duct and
1 cm into the common bile duct. During follow-up ERCP, progression
of the stricture was identified and a new plastic stent was
inserted. No evidence of malignancy was identified on radiological
or biochemical examination and thus, a benign bile duct stricture
was presumptively diagnosed and the patient was referred for
hepatico-jejunostomy.
During the hepatico-jejunostomy, widespread omental
and abdominal metastatic disease was identified and thus, surgery
was abandoned. Histopathological examination of the peritoneal
biopsy demonstrated adenocarcinoma of pancreaticobiliary origin
with a typical morphology and immunohistochemical profile
[cytokeratin (CK)7+, CK20−]. Subsequent
positron emission tomography (PET) scans showed uptake surrounding
the biliary stent, and CCA was diagnosed.
The patient was administered 3 cycles (21
days/cycle) of combined chemotherapy with cisplatin and
gemcitabine, which was changed to paclitaxel when disease
progression was observed. A further PTC and ERCP with a metal stent
were required to relieve the common bile duct obstruction. The
patient was discharged to a palliative care unit and succumbed to
the disease at 41 years of age, 31 years after the diagnosis of NHL
and the delivery of EBRT, and 12 months after the diagnosis of
CCA.
Case 2
In May 1997, a 42-year-old male was diagnosed with
stage IVB NHL (5) following an
inguinal node biopsy for a suspicious groin lymphadenopathy at
Royal North Shore Hospital (Sydney, Australia). The patient
subsequently underwent six cycles (21 days/cycle) of
cyclophosphamide, hydroxydaunorubicin, vincristine and prednisone
(CHOP) chemotherapy, achieving complete remission for 8 years. At
this time in July 2005, the patient relapsed with widespread
lymphadenopathy and was administered six further cycles of CHOP
chemotherapy, achieving remission for another 3 years. The patient
relapsed again 3 years later in May 2008, presenting with
abdominal, back and flank pain. A PET scan revealed uptake in the
left upper abdomen and para-aortic nodes. Four cycles (21
days/cycle) of rituximab, ifosfamide, carboplatin and etoposide
salvage chemotherapy were subsequently administered and an
autologous stem cell transplant was performed the following year.
The transplant was followed by abdominal EBRT to the left upper
mesenteric mass; a total of 38 Gy of radiation was delivered.
The patient was presented to the Upper
Gastrointestinal Service 4 years later, in June 2012 with painless
obstructive jaundice. Blood tests revealed a bilirubin level of 300
µmol/l (normal range, 3–20 µmol/l) and a carcinoembryonic antigen
19–9 level of 15,120 U/ml (normal range, <37 U/ml). A CT scan
revealed mid-common bile duct obstruction. An ERCP with stenting
was performed and cytology confirmed the diagnosis of CCA due to
the presence of adenocarinoma on histological staining samples from
the common bile duct. A staging laparoscopy revealed widespread
metastatic disease. The patient was subsequently transferred to a
palliative care facility in Sydney, Australia and succumbed to the
disease at 58 years of age, 16 years after the initial diagnosis of
NHL, 4 years after the administration of EBRT and 2 months after
the diagnosis of CCA.
Written informed consent was obtained from the
families of each patient for publication of the study data.
Discussion
Modern cancer therapies have led to increases in the
quality of life and prognosis of cancer patients, allowing for the
observation of long-term sequelae of cancer therapies. The risk of
secondary malignancies following delivery of EBRT has been
identified previously in a number of studies (7–10). Known
risk factors for this include a younger age at diagnosis, high
radiation dose and being of the female gender (11).
In 1948, Cahan and Woodard (12) proposed certain classic criteria that
were required for the diagnosis of secondary malignancies due to
prior radiotherapy treatment. These criteria remain widely accepted
as a conservative guide and stipulate that affected patients must
possess the following: i) A prior history of radiation treatment;
ii) an asymptomatic latent period of several years; iii) occurrence
of a second malignancy within the previously irradiated field; and
iv) histopathology of a secondary malignancy distinct from that of
the primary malignancy (12).
Primary cancers that have been consistently
associated with a high risk of EBRT-associated secondary malignant
neoplasm (SMN), include breast cancer, prostate cancer and lymphoma
(including Hodgkin's and non-Hodgkin's subtypes) (11). The most commonly described SMNs vary
with regard to primary cancer site and radiation field. In patients
treated for NHL, the most common SMNs include leukaemia and lung
cancer (7–9,13) as well
as melanoma in Australian populations (14).
At present, the association between ionizing
radiation exposure and hepatobiliary malignancy remains unclear.
Recently, Dores et al (15)
reported an increased risk of pancreatic malignancy in patients
treated with moderate to high doses of EBRT for Hodgkin's lymphoma.
Furthermore, the study demonstrated that this risk was higher in
patients treated with both EBRT and chemotherapy, which indicates a
possible interaction between the two treatment modalities in
contributing to SMN. Similarly, Morton et al (16) reported an increased risk of stomach
cancer with EBRT, and showed that this risk was associated with the
sub-diaphragmatic radiation dose.
Radiation exposure associated with hepatobiliary
malignancy has previously been reported within the context of
Thorotrast (17,18) and in survivors of atomic bomb fallout
exposure in Nagasaki and Hiroshima (19,20).
In total, 5 cases of EBRT-associated CCA have been
previously reported in the literature; 3 of these cases occurred
18–23 years after EBRT for urogenital carcinoma (21), and two cases occurred following
abdominal EBRT for Hodgkin's lymphoma and teratocarcinoma,
respectively (22). Radiation-induced
liver disease is a known complication of EBRT (23), and there have also been reports of
patients developing hepatocellular carcinoma (24) and benign biliary strictures (25,26)
following EBRT.
The molecular mechanism of EBRT-induced biliary
carcinogenesis remains unclear. However, it has been postulated
that chronic inflammation secondary to biliary epithelial injury,
and the accompanying disruption to bile flow, are important factors
in the development of carcinogenesis (27–29).
Persistent inflammation is hypothesized to damage DNA mismatch
repair genes and tumor suppressor genes, thus promoting
carcinogenesis (27). Cheng et
al (23) previously suggested
that hepatocytes may be more susceptible to radiation injury than
the biliary epithelium, however, the susceptibility of these
cell-types to radiation has not yet been reported.
In the present study, in the two cases reported, the
histopathology was consistent with primary CCA. Considering the
reported occurrence of CCA and other hepatobiliary complications
(21–26,30,31), we
hypothesize that chronic inflammatory reactions and resultant
changes to the biliary epithelium following abdominal EBRT were
fundamental to the development of CCA. However, it is unclear
whether this represents a radiation-associated variant of the
disease.
CCA continues to exhibit a poor prognosis. Thus,
risk factors must be closely assessed to improve patient prognosis.
Abdominal EBRT must be recognized as a risk factor of SMNs and
thus, a heightened index of suspicion and more intensive
surveillance of patients may facilitate an earlier diagnosis.
References
|
1
|
Gatto M, Bragazzi MC, Semeraro R, Napoli
C, Gentile R, Torrice A, Gaudio E and Alvaro D: Cholangiocarcinoma:
Update and future perspectives. Dig Liver Dis. 42:253–260. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tyson GL and El-Serag HB: Risk factors for
cholangiocarcinoma. Hepatology. 54:173–184. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Van Beers BE: Diagnosis of
cholangiocarcinoma. HPB (Oxford). 10:87–93. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ghouri YA, Mian I and Blechacz B: Cancer
review: Cholangiocarcinoma. J Carcinog. 14:12015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Carbone PP, Kaplan HS, Musshoff K,
Smithers DW and Tubiana M: Report of the Committee on Hodgkin's
Disease Staging Classification. Cancer Res. 31:1860–1861.
1971.PubMed/NCBI
|
|
6
|
Wollner N, Burchenal JH, Lieberman PH,
Exelby P, D'Angio G and Murphy ML: Non-Hodgkin's lymphoma in
children. A comparative study of two modalities of therapy. Cancer.
37:123–134. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sacchi S, Marcheselli L, Bari A,
Marcheselli R, Pozzi S, Luminari S, Lombardo M, Buda G, Lazzaro A,
Gobbi PG, et al: Secondary malignancies after treatment for
indolent non-Hodgkin's lymphoma: A 16-year follow-up study.
Haematologica. 93:398–404. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Travis LB, Curtis RE, Boice JD Jr, Hankey
BF and Fraumeni JF Jr: Second cancers following non-Hodgkin's
lymphoma. Cancer. 67:2002–2009. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Travis LB, Curtis RE, Glimelius B,
Holowaty E, van Leeuwen FE, Lynch CF, Adami J, Gospodarowicz M,
Wacholder S and Inskip P: Second cancers among long-term survivors
of non-Hodgkin's lymphoma. J Natl Cancer Inst. 85:1932–1937. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xu XG, Bednarz B and Paganetti H: A review
of dosimetry studies on external-beam radiation treatment with
respect to second cancer induction. Phys Med Biol. 53:R193–R241.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tubiana M: Can we reduce the incidence of
second primary malignancies occurring after radiotherapy? A
critical review. Radiother Oncol. 91:4–15. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cahan WG and Woodard HQ: Sarcoma arising
in irradiated bone; report of eleven cases. Cancer. 1:3–29. 1948.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mudie NY, Swerdlow AJ, Higgins CD, Smith
P, Qiao Z, Hancock BW, Hoskin PJ and Linch DC: Risk of second
malignancy after non-Hodgkin's lymphoma: A British cohort study. J
Clin Oncol. 24:1568–1574. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Brennan P, Coates M, Armstrong B, Colin D
and Boffetta P: Second primary neoplasms following non-Hodgkin's
lymphoma in New South Wales, Australia. Br J Cancer. 82:1344–1347.
2000.PubMed/NCBI
|
|
15
|
Dores GM, Curtis RE, van Leeuwen FE,
Stovall M, Hall P, Lynch CF, Smith SA, Weathers RE, Storm HH,
Hodgson DC, et al: Pancreatic cancer risk after treatment of
Hodgkin lymphoma. Ann Oncol. 25:2073–2079. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Morton LM, Dores GM, Curtis RE, Lynch CF,
Stovall M, Hall P, Gilbert ES, Hodgson DC, Storm HH, Johannesen TB,
et al: Stomach cancer risk after treatment for hodgkin lymphoma. J
Clin Oncol. 31:3369–3377. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Andersson M and Storm HH: Cancer incidence
among Danish Thorotrast-exposed patients. J Natl Cancer Inst.
84:1318–1325. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
van Kaick G, Dalheimer A, Hornik S, Kaul
A, Liebermann D, Lührs H, Spiethoff A, Wegener K and Wesch H: The
german thorotrast study: Recent results and assessment of risks.
Radiat Res. 152:S64–S71. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cologne JB, Tokuoka S, Beebe GW, Fukuhara
T and Mabuchi K: Effects of radiation on incidence of primary liver
cancer among atomic bomb survivors. Radiat Res. 152:364–373. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Preston DL, Ron E, Tokuoka S, Funamoto S,
Nishi N, Soda M, Mabuchi K and Kodama K: Solid cancer incidence in
atomic bomb survivors: 1958–1998. Radiat Res. 168:1–64. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Biermann CW, Fröschle G, Kuhlencordt R,
Schwarz R and Gonnermann D: Bile duct carcinoma after abdominal
irradiation for urologic malignancies. Helv Chir Acta.
60:1131–1136. 1994.(In German). PubMed/NCBI
|
|
22
|
Burmeister BH and Turner SL: External beam
radiation therapy as an agent in the aetiology of carcinoma of the
bile duct: A report on two patients. Clin Oncol (R Coll Radiol).
7:48–49. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cheng JC, Wu JK, Huang CM, Huang DY, Cheng
SH, Lin YM, Jian JJ, Yang PS, Chuang VP and Huang AT:
Radiation-induced liver disease after radiotherapy for
hepatocellular carcinoma: Clinical manifestation and dosimetric
description. Radiother Oncol. 63:41–45. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Greten TF, Manns MP, Reinisch I and
Kaatsch P: Hepatocellular carcinoma occurring after successful
treatment of childhood cancer with high dose chemotherapy and
radiation. Gut. 54:7322005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cherqui D, Palazzo L, Piedbois P,
Charlotte F, Duvoux C, Duron JJ, Fagniez PL and Valla D: Common
bile duct stricture as a late complication of upper abdominal
radiotherapy. J Hepatol. 20:693–697. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nakakubo Y, Kondo S, Katoh H and Shimizu
M: Biliary stricture as a possible late complication of radiation
therapy. Hepatogastroenterology. 47:1531–1532. 2000.PubMed/NCBI
|
|
27
|
Jaiswal M, LaRusso NF, Burgart LJ and
Gores GJ: Inflammatory cytokines induce DNA damage and inhibit DNA
repair in cholangiocarcinoma cells by a nitric oxide-dependent
mechanism. Cancer Res. 60:184–190. 2000.PubMed/NCBI
|
|
28
|
Schottenfeld D and Beebe-Dimmer J: Chronic
inflammation: A common and important factor in the pathogenesis of
neoplasia. CA Cancer J Clin. 56:69–83. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wise C, Pilanthananond M, Perry BF, Alpini
G, McNeal M and Glaser SS: Mechanisms of biliary carcinogenesis and
growth. World J Gastroenterol. 14:2986–2989. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gorea G, Demy M, van Nhieu J Tran, Tigori
J, Aubé C, Cherqui D, Oberti F, Caroli-Bosc FX and Calès P:
Radiation-induced cholangitis with hepatocellular Carcinoma.
Gastroenterol Clin Biol. 34:35–39. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lawrence TS, Robertson JM, Anscher MS,
Jirtle RL, Ensminger WD and Fajardo LF: Hepatic toxicity resulting
from cancer treatment. Int J Radiat Oncol Biol Phys. 31:1237–1248.
1995. View Article : Google Scholar : PubMed/NCBI
|