Introduction
Ovarian cancer is the fourth leading cause of
cancer-associated mortality in women, with a 5-year survival rate
of 25–35% in Western countries (1).
Ovarian cancer often remains undetected until a late stage, and is
commonly referred to as a silent killer (2–8).
Currently, the standard therapy for advanced ovarian cancer
includes surgery and systemic chemotherapy (5,9–11). Since 1990, combined platinum-based
agents and paclitaxel (Taxol; Bristol-Myers Squibb, New York, NY,
USA) have become the first-line chemotherapy for ovarian cancer
(12,13). Despite a high initial sensitivity to
chemotherapy and frequent complete clinical response, the majority
of patients with advanced-stage tumors may relapse and gradually
develop resistance to the majority of chemotherapeutic drugs
(14–16). It has not yet been possible to
overcome multidrug resistance (MDR) in the clinical settings.
Much effort has been focused on identifying natural
compounds that may reverse the MDR phenotype of cancer cells and/or
sensitize MDR cancer cells to chemotherapy without undesirable side
effects (17–19). Traditional Chinese medicines are
excellent starting materials, since they are not only a rich source
for diverse chemicals, but have been used in humans for thousands
of years (20–22). Recently, certain natural compounds
have been shown to be capable of reversing the drug resistance of
ovarian cancer (23). Abouzeid et
al (24) and Sarisozen et
al (25) reported that
transferring-targeted combination micelles of curcumin and
paclitaxel have a clear synergistic effect against
paclitaxel-resistant SK-OV-3TR cells. Yang et al (26) demonstrated that tectorigenin may
sensitize paclitaxel-resistant human ovarian cancer cells through
downregulating the Akt and nuclear factor-κB signaling pathways. Li
et al (27) revealed that
emodin can sensitize paclitaxel-resistant human ovarian cancer
cells to paclitaxel-induced apoptosis in vitro. Zhou et
al (28) reported that silibinin
is able to restore paclitaxel sensitivity in paclitaxel-resistant
human ovarian carcinoma cells.
Cucurbitacin B (CuB) is a particularly potent member
of the triterpenoid family isolated from plants, and has shown
anti-proliferative effects on various cancer cells in vitro
and in vivo (29). CuB may
induce cell cycle arrest and apoptosis in cancer cells (30–42).
In the present study, the inhibitory effect of CuB
on the paclitaxel-resistant human ovarian cancer A2780/Taxol cell
line and its parental A2780 cell line was assessed. To the best of
our knowledge, the present study demonstrated for the first time
that CuB inhibits the growth of these two types of cells through
induction of cell cycle arrest and apoptosis, by a number of
molecular mechanisms.
Materials and methods
Reagents
CuB (with a purity of 98%) was obtained from
Professor Yihui Deng's laboratory (Department of Pharmacy, Shenyang
Pharmaceutical University, Shenyang, China). Primary antibodies
directed against B-cell lymphoma-2 (Bcl-2; cat. no. sc-783),
caspase-3 (cat. no. sc-271028), p53 (cat. no. sc-126), p21 (cat.
no. sc-6246), P-glycoprotein (P-gp; cat. no. sc-8313) and β-actin
(cat. no. sc-130300) were purchased from Santa Cruz Biotechnology,
Inc. (Dallas, TX, USA). Secondary antibodies directed against
rabbit (Peroxidase Conjugated AffiniPure Goat Anti-rabbit IgG (cat.
no. ZB-2301), or mouse (Peroxidase Conjugated AffiniPure Goat
anti-mouse IgG (cat. no. ZB-2305) were from ZSGB-BIO (Beijing,
China). The ECL Western Blotting Detection reagent was purchased
from GE Healthcare Life Sciences (Chalfont, UK). Dulbecco's
modified Eagle's medium (DMEM), RPMI-1640 medium and fetal bovine
serum (FBS) were obtained from Thermo Fisher Scientific, Inc.
(Waltham, MA, USA). The Annexin V-fluorescein isothiocyanate (FITC)
apoptosis detection kit, propidium iodide (PI), Hoechst 33258,
dimethyl sulfoxide (DMSO) and other reagents were purchased from
Sigma-Aldrich (Merck KGaA, Darmstadt, Germany).
Cell cultures
Ovarian carcinoma A2780 and paclitaxel-resistant
A2780 (A2780/Taxol) ovarian carcinoma cell lines were purchased
from Nanjing KeyGen Biotech Co., Ltd. (Nanjing, China). Cells were
maintained in DMEM (A2780) or RPMI-1640 with 800 ng/ml paclitaxel
(A2780/Taxol) supplemented with 10% FBS, 100 U/ml penicillin and
100 µg/ml streptomycin. The cells were cultured in a humidified
atmosphere containing 5% CO2 at 37°C.
Cell viability assay
The viability of A2780 and A2780/Taxol cells was
evaluated by counting the numbers of viable cells at 24, 48 and 72
h following the addition of 10 µl CuB to achieve final
concentrations of 0.0625, 0.125, 0.25, 0.5 and 1 µM. The cells were
stained with Trypan Blue and counted using a hemocytometer. The
final concentration of 0.5% DMSO produced no inhibition of cell
growth. At least three replicate experiments were performed with
three wells per concentration.
Cell cycle analysis
A total of 8×105 A2780/Taxol cells and
their parental A2780 cells were incubated with 0.0625, 0.125, 0.25,
0.5 or 1 µM CuB or DMSO for 24 h. The cells were harvested, washed
with iced-cold PBS and fixed in 70% ethanol at −20°C for 24 h.
Subsequently, cells were washed with PBS and resuspended in PBS
containing 100 µg/ml PI (Sigma-Aldrich; Merck KGaA), 50 µg/ml
ribonuclease A and 0.1% Triton X-100 in the dark for 30 min at
37°C. Flow cytometry analysis was performed on a FACSCalibur
instrument (BD Biosciences, Franklin Lakes, NJ, USA) using the
ModFit LT program (version 3.0; BD Biosciences).
Annexin V/PI staining
Apoptosis of the cells was detected with an Annexin
V-FITC Apoptosis Detection kit (Sigma-Aldrich; Merck KGaA). Annexin
V-FITC is a sensitive probe for identifying cells undergoing
apoptosis, as phosphatidylserine exposure occurs early in the
apoptotic process. PI is a non-specific DNA dye that is excluded
from live cells with intact plasma membranes, but can be
incorporated into non-viable cells. Upon detection, single-positive
populations may present as early apoptotic (Annexin
V+/PI−) or necrotic cells (Annexin
V−/PI+), whereas double-positive populations
(Annexin V+/PI+) are indicative of late stage
apoptosis (43).
Hoechst 33258 staining
A2780/Taxol and parental A2780 cells
(3×105 cells) were grown on coverslips placed on 6-well
plates at 37°C overnight. Following 0.25, 0.5 and 1 µM CuB
treatment, the cells were fixed with methanol and acetic acid (3/1
v/v) at 4°C for 15 min, and stained with Hoechst 33258 for 30 min
in the dark at 37°C. Subsequent to washing in PBS 3 times, the
cells were mounted with a medium containing 80% glycerol (cat. no.
G5516; Sigma-Aldrich; Merck KGaA) in PBS. The processed cells were
then observed under a fluorescence microscope with 3 fields of view
(magnification, ×40; Olympus Corporation, Tokyo, Japan).
Western blot analysis
Cells (1×106 cells/well) seeded onto
6-well plates were treated with 0.0625, 0.125, 0.25, 0.5 and 1 µM
CuB or DMSO. The medium was aspirated, and the cells were washed
with cold PBS. They were then scraped, washed twice with cold PBS
followed by centrifugation at 1,000 × g for 5 min at 4°C. The
pellet was resuspended in lysis buffer supplemented with proteases
and phosphatase inhibitors (cat. no. P8340; Sigma-Aldrich; Merck
KGaA) and incubated for 1 h at 4°C. The lysate was collected by
centrifugation at 14,000 × g for 40 min at 4°C, and the supernatant
was stored at −20°C. For western blot analysis, 50 µg proteins were
resolved in 8–10% PAGE and transferred to polyvinylidene difluoride
membranes. The blot was blocked with a blocking buffer (5% nonfat
dry milk/0.1% Tween-20 in TBS) for 1 h at room temperature, and
next incubated with appropriate primary antibodies directed against
Bcl-2 (1:200), caspase-3 (1:200), p53 (1:1,000), p21 (1:1,000),
P-gp (1:200) or β-actin (1:1,000) overnight at 4°C. The blots were
then incubated with horseradish peroxidase-conjugated secondary
antibodies (1:1,000–2,000) for 2 h at room temperature and detected
with enhanced chemiluminescence, and protein levels were quantified
using a Tanon 5200 Chemiluminescent Imaging system (version 1.02;
Tanon Science and Technology Co., Ltd., Shanghai, China). β-actin
was detected on the same membrane and used as loading control.
Evaluation of multidrug resistance in
ovarian cancer cells
The multidrug resistance of ovarian cancer cells was
evaluated by the cell count and P-gp expression. A2780/Taxol and
A2780 cells at a density of 8×105 cells/well were
cultured with docetaxel (0.001, 0.01, 0.1, 1 and 10 µM),
doxorubicin (0.01, 0.1, 1, 10 and 100 µM), cisplatin (1, 3, 10, 30
and 100 µM) and gemcitabine (0.01, 0.1, 1, 10 and 100 µM) for 24 h.
The viability of the cells was determined using a cell counting
assay.
Statistical analysis
All values are expressed as the mean ± standard
deviation. Comparison between groups was analyzed by one-way
analysis of variance followed by Fisher's least significant
difference test using SPSS software (version 16.0; SPSS, Inc.,
Chicago, IL, USA). Two-sided P<0.05 was considered to indicate a
statistically significant difference.
Results
Evaluation of MDR in A2780/Taxol
cells
The MDR of A2780/Taxol cells was evaluated by
treating the A2780 and A2780/Taxol cells with docetaxel (0.001,
0.01, 0.1, 1 and 10 µM), doxorubicin (0.01, 0.1, 1, 10 and 100 mM),
cisplatin (1, 3, 10, 30 and 100 µM) and gemcitabin (0.01, 0.1, 1,
10 and 100 µM). As shown in Fig. 1A,
the A2780/Taxol cells were resistant not only to docetaxel
[A2780/Taxol, half-maximal inhibitory concentration
(IC50) >10 µM vs. A2780, IC50=0.11±0.22
µM], but also to the common chemotherapeutic drug doxorubicin
(A2780/Taxol, IC50 >100 µM vs. A2780,
IC50=8.30±2.50 µM). However, such cells exhibited no
resistance to cisplatin (A2780/Taxol, IC50=41.53±7.48 µM
vs. A2780, IC50=42.52±14.47 µM) or gemcitabine
(A2780/Taxol, IC50=20.40±3.16 µM vs. A2780,
IC50=18.19±2.47 µM) at high concentrations. Compared
with A2780 cells, A2780/Taxol cells displayed an increase of 90.91-
and 12.05-fold resistance to docetaxel and doxorubicin,
respectively. By western blot analysis, the paclitaxel-resistant
A2780/Taxol cells also exhibited elevated P-gp protein expression
compared with A2780 cells (Fig.
1B).
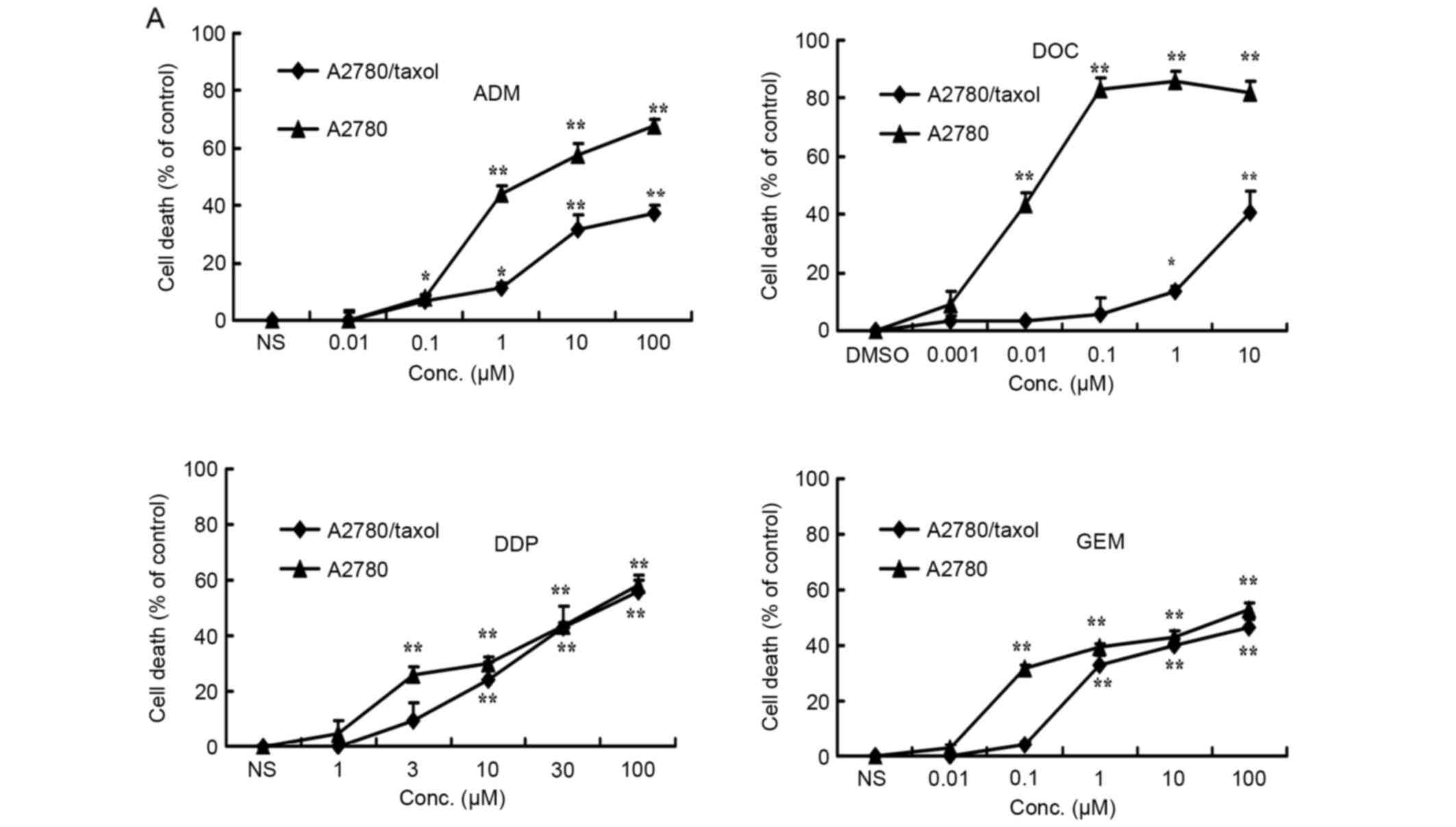 | Figure 1.Evaluation of multidrug resistance in
ovarian cancer cells. A2780/Taxol and A2780 cells at a density of
8×105 cells/well were cultured with docetaxel (0.001,
0.01, 0.1, 1 and 10 µM), doxorubicin (0.01, 0.1, 1, 10 and 100 µM),
cisplatin (1, 3, 10, 30 and 100 µM) and gemcitabine (0.01, 0.1, 1,
10 and 100 µM) for 24 h. (A) The viability of the cells was
determined by a cell counting assay. Each point represents the mean
± standard deviation of three independent experiments. *P<0.05,
**P<0.01. (B) P-gp expression in A2780/Taxol and A2780 cells.
Each point represents the mean of three independent experiments.
**P<0.01 compared with the control. DOC, docetaxel; GEM,
gemcitabine; ADM, doxorubicin; DDP, cisplatin; P-gp,
P-glycoprotein; Conc, concentration. |
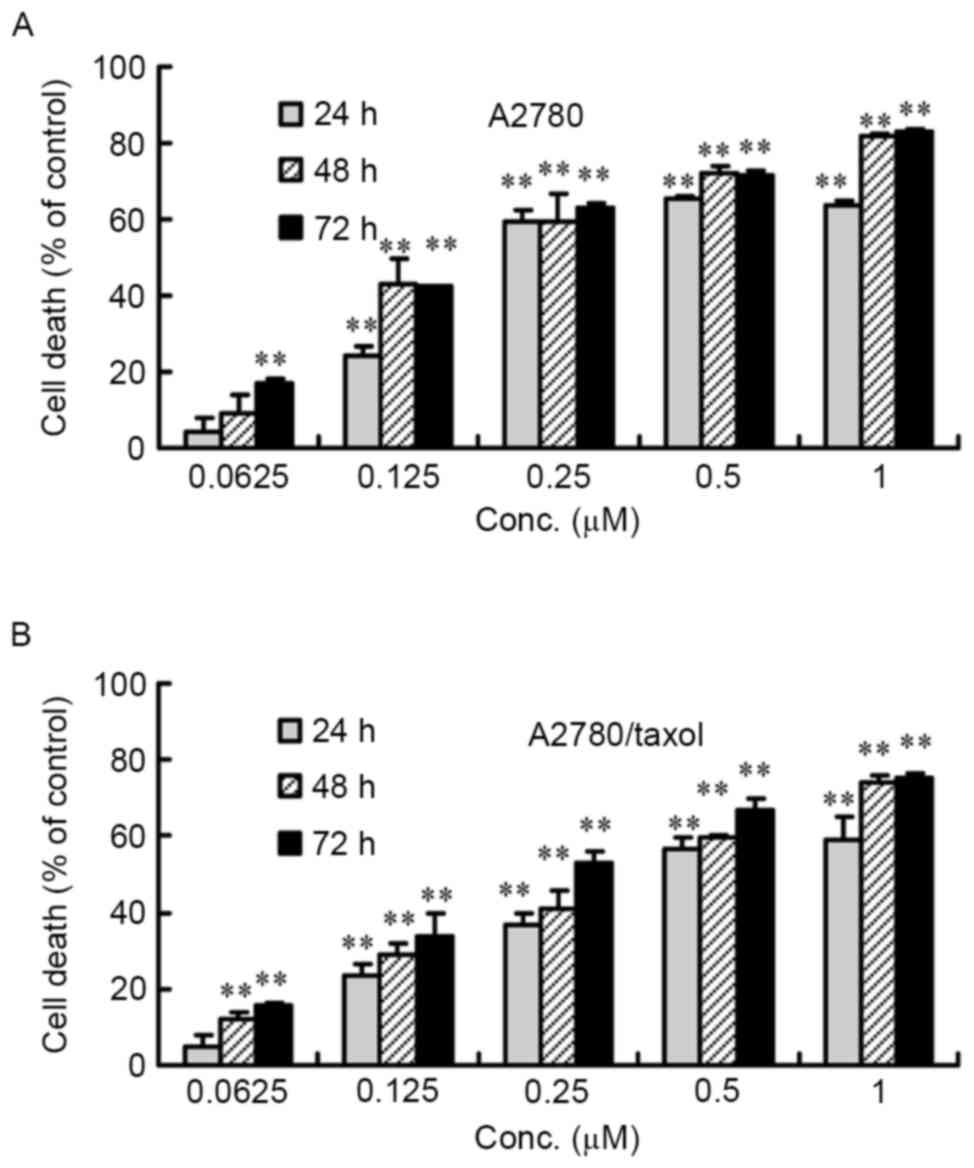
Inhibition of the viability of
A2780/Taxol and A2780 cells by CuB
A2780/Taxol and A2780 cells were treated with
0.0625–1 µM of CuB for 24–72 h, and cell proliferation was measured
by counting the number of viable cells. As shown in Fig. 2, cell proliferation was inhibited by
CuB in a dose- and time-dependent manner. Similar IC50
values for CuB in the two cell lines were derived
(IC50=0.27 µM after 72 h or 0.35 µM after 48 h for
A2780/Taxol cells, and IC50=0.21 µM after 72 h or 0.25
µM after 48 h for A2780 cells). These results indicated that CuB
has a potent anti-proliferative effect on ovarian cancer cells,
regardless of whether they had resistance to paclitaxel (Fig. 2).
Effect of CuB on the cell cycle
A2780/Taxol and A2780 cells were treated with
various concentration of CuB for 24 h. Following PI staining, the
population in various phases of the cell cycle was determined by
fluorescence-activated cell sorting (FACS) analysis. As shown in
Fig. 3A, the percentage of
G2/M phase cells had increased from 8.49±0.95% in the
control cells to 55.22±2.10% (1 µM CuB) in A2780 cells, and from
12.34±1.66% in DMSO-treated to 43.47±2.61% (1 µM CuB) in
A2780/Taxol cells.
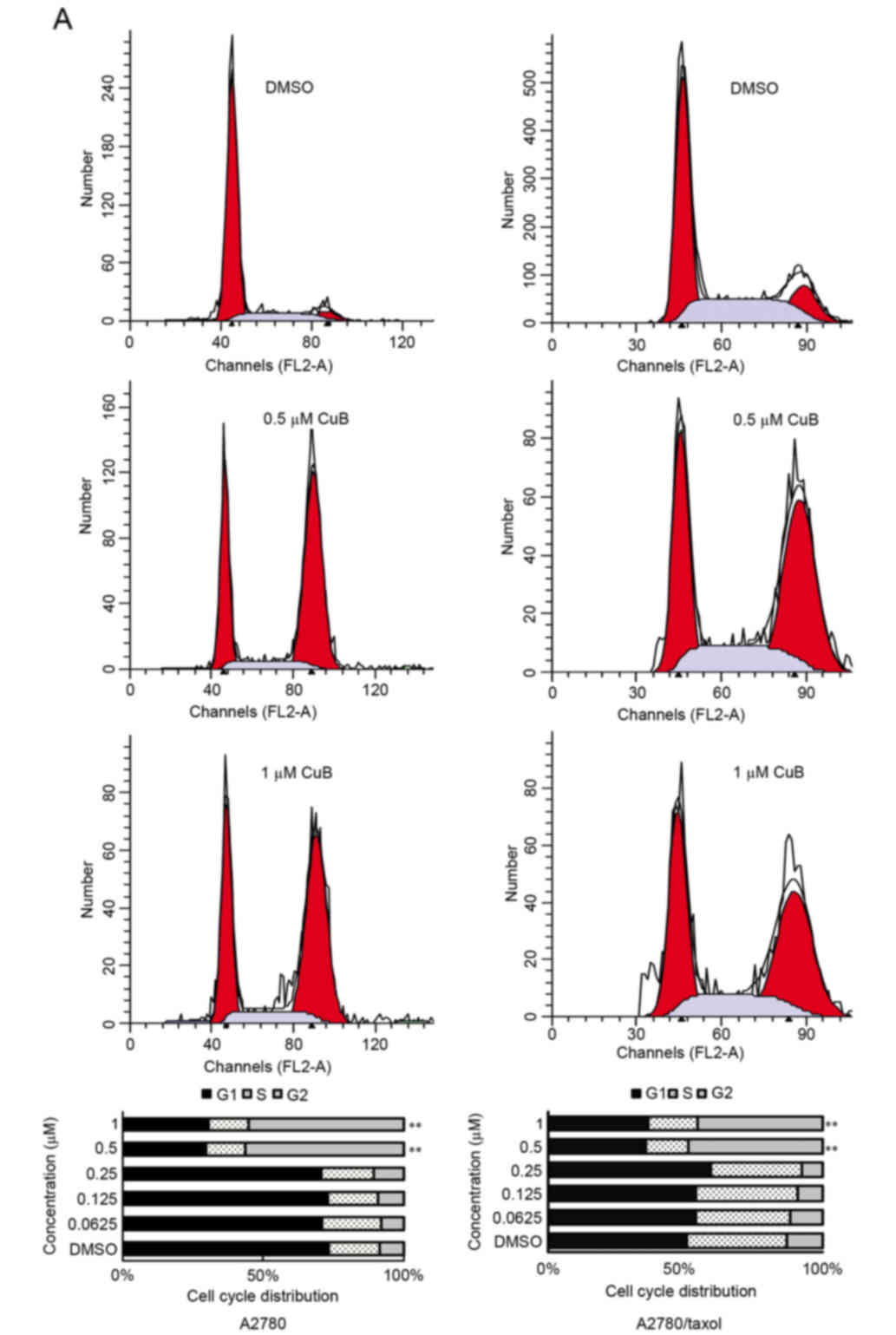 | Figure 3.Effect of CuB on cell cycle
distribution and apoptosis induction. A2780/Taxol and A2780 cells
(1×106) were treated with various concentrations of CuB
for 24 h. (A) Cell cycle analysis by flow cytometry. The percentage
of G2/M phase cells increased from 8.49±0.95% in the control DMSO
group to 55.22±2.10% in A2780 cells treated with 1 µM CuB, and from
12.34±1.66% in the control DMSO group to 43.47±2.61% in A2780/Taxol
cells treated with 1 µM CuB.**P<0.01 compared with the control.
(B) Apoptosis analysis by flow cytometry. As shown by FACS, there
appeared to be a dose-dependent increase in apoptotic cells in
CuB-treated samples compared with the controls. The proportion of
apoptotic cells increased from 2.03±0.23 to 11.57±2.03%, and from
1.37±0.44 to 8.77±1.24% in A2780 and A2780/Taxol cells,
respectively. **P<0.01 compared with the control. (C) Cells with
Hoechst 33258 staining (magnification, ×40). (D) Cell morphology
under light microscopy (magnification, ×40). CuB, cucurbitacin;
DMSO, dimethyl sulfoxide; FITC, fluorescein isothiocyanate; PI,
propidium iodide; Conc, concentration. |
Effect of CuB on cell apoptosis
As shown by FACS, there appeared to be a
dose-dependent increase in apoptotic cells in CuB-treated samples
compared with the controls. The proportion of apoptotic cells
increased from 2.03±0.23 to 11.57±2.03%, and from 1.37±0.44 to
8.77±1.24% in A2780 and A2780/Taxol cells, respectively (P<0.01;
Fig. 3B). In addition, marked
morphological changes suggestive of apoptosis, including chromatin
condensation, nuclear fragmentation and apoptotic bodies, were
observed by Hoechst 33258 staining (Fig.
3C).
Effect of CuB on the expression of p53
and p21
The present study investigated whether CuB modulates
the expression of p53 and p21 in ovarian cancer cells. As shown in
Fig. 4A, CuB treatment could
upregulate the expression of p53 and p21 in a dose-dependent
manner. Treatment of A2780/Taxol and A2780 cells with 0.25 µM CuB
for 24 h significantly upregulated the expression of the p21
(P<0.05), while CuB significantly upregulated the expression of
the p53 at 0.5 µM (P<0.05; Fig.
4A).
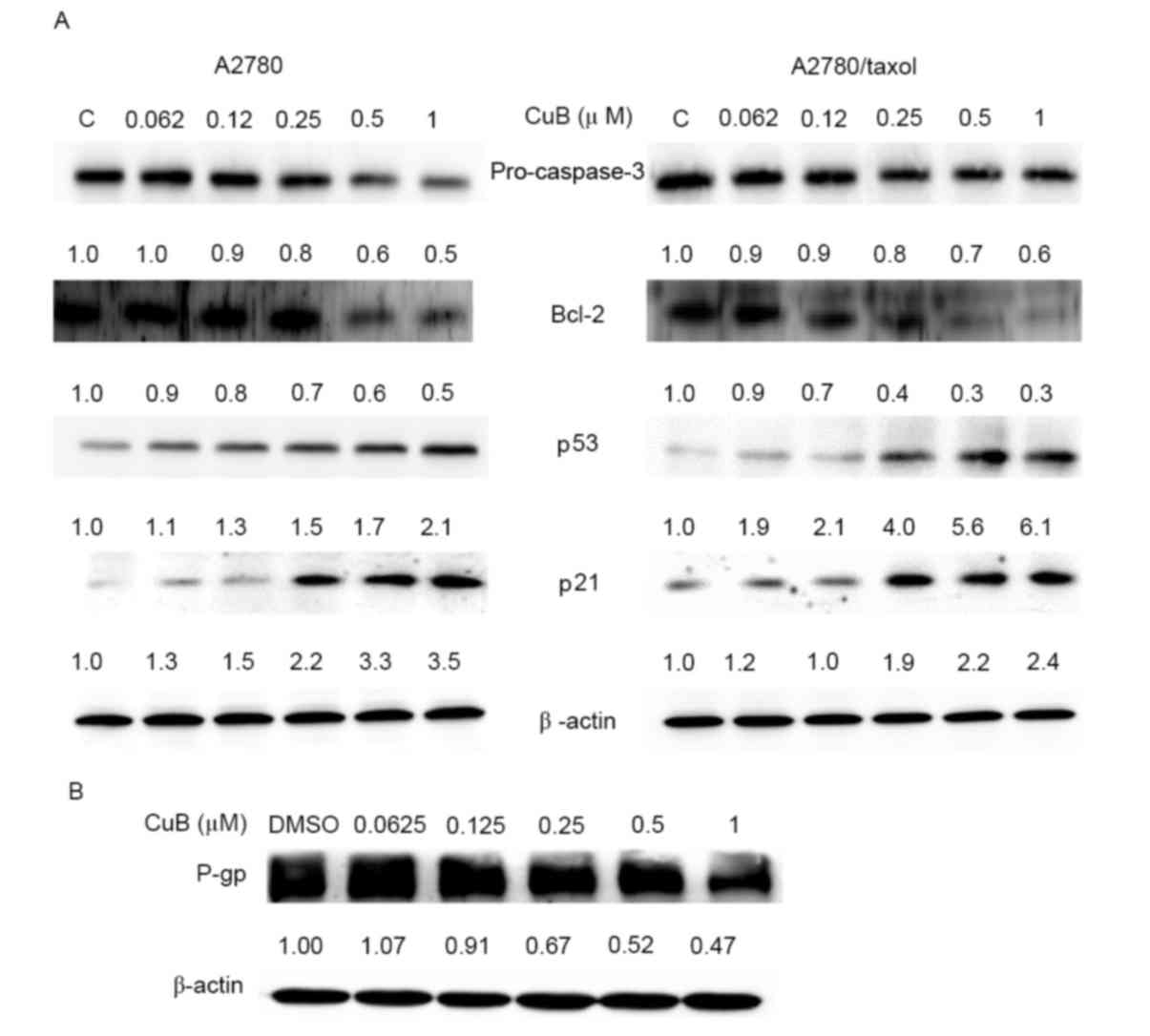 | Figure 4.Western blot analysis of Bcl-2, p53,
p21, caspase-3 and P-gp protein expression. (A) A2780 and
A2780/Taxol cells were treated with various concentrations of CuB
for 24 h, and the protein expression of Bcl-2, p53, p21 and
caspase-3 was analyzed. (B) A2780/Taxol cells were treated with
various concentrations of CuB for 24 h, and the protein expression
of P-gp was analyzed. Upon CuB treatment, the cells were harvested
and processed for western blotting. β-actin was used as an internal
control. Immunoblots are representative of three independent
experiments. Bcl-2, B-cell lymphoma 2; CuB, cucurbitacin; C, DMSO;
P-gp, P-glycoprotein. |
Effect of CuB on the expression of
Bcl-2 and caspase-3
Bcl-2 is an important member of the Bcl-2 family of
proteins, which regulate cell apoptosis (44). Bcl-2 is considered an important
anti-apoptotic protein and is classified as an oncogene (45). As shown in Fig. 4A, Bcl-2 is expressed in A2780/Taxol
and A2780 cells, and CuB could suppress Bcl-2 expression in a
dose-dependent manner. In addition, CuB treatment significantly
decreased the intensity of pro-caspase-3 expression (Fig. 4A).
Effect of CuB on P-gp expression
P-gp is an important protein of the cell membrane,
which pumps various foreign substances out of the cell (19). P-gp is responsible for decreased drug
accumulation in MDR cells, and can mediate the development of
resistance to anticancer drugs (46).
The present study determined whether CuB downregulates the
expression of P-gp in A2780/Taxol cells. As confirmed by western
blot analysis, CuB treatment suppressed the expression of P-gp in
the A2780/Taxol cell line in a dose-dependent manner (Fig. 4B).
Discussion
The high mortality of ovarian cancer is usually due
to the failure of early detection and lack of effective therapies
for late-stage cancers (3,5). The standard therapy for advanced ovarian
cancer consists of extensive surgical resection followed by
combination chemotherapy with paclitaxel/carboplatin (47). However, such drugs frequently induce
resistance (48). Recently, natural
compounds with a high potential and relatively low toxicity have
been explored for use as single agents or in combination with a
conventional chemotherapeutic agent (24). For instance, curcumin isolated from
curry spice has been demonstrated to potentiate the antitumor
activity of various chemotherapeutic agents in a wide range of
cancer cells, including drug-resistant ovarian cancer (24,25,49,50).
CuBs are isolated from various plants, which have
been used as folk medicines in countries such as China and India
(32). In recent years, their
anti-proliferative effect has been demonstrated on a variety of
cancer cells by in vivo and in vitro experiments
(32,35,42,51). The
present study confirmed that CuB also has an inhibitory effect on
the growth of paclitaxel-resistant A2780/Taxol cells and their
parental A2780 cells in a dose- and time-dependent manner. Through
a series of experiments, it was demonstrated that CuB treatment
resulted in the accumulation of cells at the G2/M phase
of the cell cycle. CuB could also induce cell apoptosis in a
dose-dependent manner. These results indicated that the inhibitory
effect of CuB on paclitaxel-resistant A2780/Taxol cells was due to
the induction of cell cycle arrest as well as apoptosis.
MDR is the major cause of failure in anticancer
treatment (19). The mechanisms
associated with MDR may be classified into two major categories:
Pump (P-gp) resistance and non-pump (apoptosis) resistance
(52). The former is mainly caused by
overexpression of the P-gp protein, which is capable of reducing
the intracellular accumulation of drugs by expelling the drugs
outside of the cell (53). This may
lead to resistance to a wide range of anticancer drugs, including
doxorubicin, vinblastine, dactinomycin and paclitaxel (54–56).
Approximately 50% of human cancers overexpress P-gp at levels
sufficient to confer MDR (56–59).
Through the present study, it was demonstrated that the MDR of
A2780/Taxol cells may be in part attributed to overexpression of
the P-gp protein, while CuB may significantly inhibit P-gp
expression.
The non-pump resistance mechanism is the result of
an anti-cell death effect, which mainly improves survival and
suppresses apoptosis (60). Usually,
resistance to anticancer drugs is associated with a low propensity
to apoptosis (61). Increased
expression of anti-apoptotic proteins, including Bcl-2, has been
noted in MDR cells (42,62,63).
Therefore, inhibition of Bcl-2 may be a strategy to overcome MDR.
The present results demonstrated that CuB induced the apoptosis of
A2780/Taxol cells in a dose-dependent manner, which was accompanied
by decreased expression of pro-caspase-3 and Bcl-2.
p53 is commonly considered as a tumor-suppressor
gene, which can regulate genes involved in apoptosis and may
modulate mitochondrial proteins, including Bcl-2, Bcl-2-associated
X protein, Bcl-2 homologous antagonist/killer and Bcl-extra large,
to promote the release of cytochrome c and apoptosis
(64). It may also induce cell cycle
arrest and/or apoptosis in response to cellular stresses, including
ionizing radiation, ultraviolet light, growth factor deprivation,
reactive oxygen species and DNA damage induced by various cytotoxic
agents (65). There has been
accumulating evidence that the status of p53 has a significant
impact on drug sensitivity (66–68). As
confirmed by the present study, CuB treatment increases the protein
levels of p53 and p21 in a dose-dependent manner.
In summary, to the best of our knowledge, the
present study demonstrated for the first time that CuB can inhibit
the growth of paclitaxel-resistant A2780/Taxol cells and induce
cell apoptosis through upregulation of p53 and p21, downregulation
of Bcl-2, activation of caspase-3 and suppression of P-gp. This
compound may therefore provide an adjuvant therapy for the
treatment of paclitaxel-resistant ovarian cancer.
Acknowledgements
The present study was jointly sponsored by the
Natural Science Foundation of China (grant no. 81473446), the
Natural Science Foundation of Liaoning Province (grant no.
2013021081) and the Natural Science Foundation of Chongqing City
(grant no. cstc2013jcyjA1587).
References
|
1
|
Bai X, Ma Y and Zhang G: Butein suppresses
cervical cancer growth through the PI3K/AKT/mTOR pathway. Oncol
Rep. 33:3085–3092. 2015.PubMed/NCBI
|
|
2
|
Arend RC, Londoño-Joshi AI, Straughn JM Jr
and Buchsbaum DJ: The Wnt/β-catenin pathway in ovarian cancer: A
review. Gynecol Oncol. 131:772–779. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Arikan SK, Kasap B, Yetimalar H, Yildiz A,
Sakarya DK and Tatar S: Impact of prognostic factors on survival
rates in patients with ovarian carcinoma. Asian Pac J Cancer Prev.
15:6087–6094. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li H, Zeng J and Shen K: PI3K/AKT/mTOR
signaling pathway as a therapeutic target for ovarian cancer. Arch
Gynecol Obstet. 290:1067–1078. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Muccioli M and Benencia F: Toll-like
receptors in ovarian cancer as targets for immunotherapies. Front
Immunol. 5:3412014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Teo MC: Update on the management and the
role of intraperitoneal chemotherapy for ovarian cancer. Curr Opin
Obstet Gynecol. 26:3–8. 2013. View Article : Google Scholar
|
|
7
|
Wei W, Dizon D, Vathipadiekal V and Birrer
MJ: Ovarian cancer: Genomic analysis. Ann Oncol. 24 Suppl
10:x7–x15. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhao Y, Gou WF, Chen S, Takano Y, Xiu YL
and Zheng HC: BTG1 expression correlates with the pathogenesis and
progression of ovarian carcinomas. Int J Mol Sci. 14:19670–19680.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bookman MA: First-line chemotherapy in
epithelial ovarian cancer. Clin Obstet Gynecol. 55:96–113. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Raja FA, Counsell N, Colombo N, Pfisterer
J, du Bois A, Parmar MK, Vergote IB, Gonzalez-Martin A, Alberts DS,
Plante M, et al: Platinum versus platinum-combination chemotherapy
in platinum-sensitive recurrent ovarian cancer: A meta-analysis
using individual patient data. Ann Oncol. 24:3028–3034. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Schwab CL, English DP, Roque DM and Santin
AD: Taxanes: Their impact on gynecologic malignancy. Anticancer
Drugs. 25:522–535. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pellicciotta I, Yang CP, Venditti CA,
Goldberg GL and Shahabi S: Response to microtubule-interacting
agents in primary epithelial ovarian cancer cells. Cancer Cell Int.
13:332013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sorbe B, Graflund M, Nygren L and Horvath
G: A study of docetaxel weekly or every three weeks in combination
with carboplatin as first line chemotherapy in epithelial ovarian
cancer: Hematological and non-hematological toxicity profiles.
Oncol Lett. 5:1140–1148. 2013.PubMed/NCBI
|
|
14
|
Colombo PE, Fabbro M, Theillet C, Bibeau
F, Rouanet P and Ray-Coquard I: Sensitivity and resistance to
treatment in the primary management of epithelial ovarian cancer.
Crit Rev Oncol Hematol. 89:207–216. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Leamon CP, Lovejoy CD and Nguyen B:
Patient selection and targeted treatment in the management of
platinum-resistant ovarian cancer. Pharmgenomics Pers Med.
6:113–125. 2013.PubMed/NCBI
|
|
16
|
Lopez J, Banerjee S and Kaye SB: New
developments in the treatment of ovarian cancer-future
perspectives. Ann Oncol. 24 Suppl 10:x69–x76. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cort A and Ozben T: Natural product
modulators to overcome multidrug resistance in cancer. Nutr Cancer.
67:411–423. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Si M, Zhao J, Li X, Tian JG, Li YG and Li
JM: Reversion effects of curcumin on multidrug resistance of
MNNG/HOS human osteosarcoma cells in vitro and in vivo through
regulation of P-glycoprotein. Chin Med J (Engl). 126:4116–4123.
2013.PubMed/NCBI
|
|
19
|
Abdallah HM, Al-Abd AM, El-Dine RS and
El-Halawany AM: P-glycoprotein inhibitors of natural origin as
potential tumor chemo-sensitizers: A review. J Adv Res. 6:45–62.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bansal T, Jaggi M, Khar RK and Talegaonkar
S: Emerging significance of flavonoids as P-glycoprotein inhibitors
in cancer chemotherapy. J Pharm Pharm Sci. 12:46–78. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lee CH: Reversing agents for ATP-binding
cassette drug transporters. Methods Mol Biol. 596:325–340. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wu CP, Calcagno AM and Ambudkar SV:
Reversal of ABC drug transporter-mediated multidrug resistance in
cancer cells: Evaluation of current strategies. Curr Mol Pharmacol.
1:93–105. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhao BX, Sun YB, Wang SQ, Duan L, Huo QL,
Ren F and Li GF: Grape seed procyanidin reversal of p-glycoprotein
associated multi-drug resistance via down-regulation of NF-κB and
MAPK/ERK mediated YB-1 activity in A2780/T cells. PLoS One.
8:e710712013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Abouzeid AH, Patel NR, Sarisozen C and
Torchilin VP: Transferrin-targeted polymeric micelles co-loaded
with curcumin and paclitaxel: Efficient killing of
paclitaxel-resistant cancer cells. Pharm Res. 31:1938–1945. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sarisozen C, Abouzeid AH and Torchilin VP:
The effect of co-delivery of paclitaxel and curcumin by
transferrin-targeted PEG-PE-based mixed micelles on resistant
ovarian cancer in 3-D spheroids and in vivo tumors. Eur J Pharm
Biopharm. 88:539–550. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yang YI, Lee KT, Park HJ, Kim TJ, Choi YS,
Shih IeM and Choi JH: Tectorigenin sensitizes paclitaxel-resistant
human ovarian cancer cells through downregulation of the Akt and
NFκB pathway. Carcinogenesis. 33:2488–2498. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li J, Liu P, Mao H, Wanga A and Zhang X:
Emodin sensitizes paclitaxel-resistant human ovarian cancer cells
to paclitaxel-induced apoptosis in vitro. Oncol Rep. 21:1605–1610.
2009.PubMed/NCBI
|
|
28
|
Zhou L, Liu P, Chen B, Wang Y, Wang X,
Internati M Chiriva, Wachtel MS and Frezza EE: Silibinin restores
paclitaxel sensitivity to paclitaxel-resistant human ovarian
carcinoma cells. Anticancer Res. 28:1119–1127. 2008.PubMed/NCBI
|
|
29
|
Iwanski GB, Lee DH, En-Gal S, Doan NB,
Castor B, Vogt M, Toh M, Bokemeyer C, Said JW, Thoennissen NH and
Koeffler HP: Cucurbitacin B, a novel in vivo potentiator of
gemcitabine with low toxicity in the treatment of pancreatic
cancer. Br J Pharmacol. 160:998–1007. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Aribi A, Gery S, Lee DH, Thoennissen NH,
Thoennissen GB, Alvarez R, Ho Q, Lee K, Doan NB, Chan KT, et al:
The triterpenoid cucurbitacin B augments the antiproliferative
activity of chemotherapy in human breast cancer. Int J Cancer.
132:2730–2737. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Dakeng S, Duangmano S, Jiratchariyakul W,
U-Pratya Y, Bögler O and Patmasiriwat P: Inhibition of Wnt
signaling by cucurbitacin B in breast cancer cells: Reduction of
Wnt-associated proteins and reduced translocation of
galectin-3-mediated β-catenin to the nucleus. J Cell Biochem.
113:49–60. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gao Y, Islam MS, Tian J, Lui VW and Xiao
D: Inactivation of ATP citrate lyase by Cucurbitacin B: A bioactive
compound from cucumber, inhibits prostate cancer growth. Cancer
Lett. 349:15–25. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Guo J, Wu G, Bao J, Hao W, Lu J and Chen
X: Cucurbitacin B induced ATM-mediated DNA damage causes G2/M cell
cycle arrest in a ROS-dependent manner. PLoS One. 9:e881402014.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Guo J, Zhao W, Hao W, Ren G, Lu J and Chen
X: Cucurbitacin B induces DNA damage, G2/M phase arrest, and
apoptosis mediated by reactive oxygen species (ROS) in leukemia
K562 cells. Anticancer Agents Med Chem. 14:1146–1153. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gupta P and Srivastava SK: Inhibition of
integrin-HER2 signaling by Cucurbitacin B leads to in vitro and in
vivo breast tumor growth suppression. Oncotarget. 5:1812–1828.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ma J, Zi Jiang Y, Shi H, Mi C, Li J, Nan J
Xing, Wu X, Lee J Joon and Jin X: Cucurbitacin B inhibits the
translational expression of hypoxia-inducible factor-1α. Eur J
Pharmacol. 723:46–54. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Shang Y, Guo XX, Li WW, Rao W, Chen ML, Mu
LN and Li SJ: Cucurbitacin-B inhibits neuroblastoma cell
proliferation through up-regulation of PTEN. Eur Rev Med Pharmacol
Sci. 18:3297–3303. 2014.PubMed/NCBI
|
|
38
|
Yin D, Wakimoto N, Xing H, Lu D, Huynh T,
Wang X, Black KL and Koeffler HP: Cucurbitacin B markedly inhibits
growth and rapidly affects the cytoskeleton in glioblastoma
multiforme. Int J Cancer. 123:1364–1375. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhang M, Zhang H, Sun C, Shan X, Yang X,
Li-Ling J and Deng Y: Targeted constitutive activation of signal
transducer and activator of transcription 3 in human hepatocellular
carcinoma cells by cucurbitacin B. Cancer Chemother Pharmacol.
63:635–642. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhang M, Sun C, Shan X, Yang X, Li-Ling J
and Deng Y: Inhibition of pancreatic cancer cell growth by
cucurbitacin B through modulation of signal transducer and
activator of transcription 3 signaling. Pancreas. 39:923–929. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhang M, Bian ZG, Zhang Y, Wang JH, Kan L,
Wang X, Niu HY and He P: Cucurbitacin B inhibits proliferation and
induces apoptosis via STAT3 pathway inhibition in A549 lung cancer
cells. Mol Med Rep. 10:2905–2911. 2014.PubMed/NCBI
|
|
42
|
Zheng Q, Liu Y, Liu W, Ma F, Zhou Y, Chen
M, Chang J, Wang Y, Yang G and He G: Cucurbitacin B inhibits growth
and induces apoptosis through the JAK2/STAT3 and MAPK pathways in
SH-SY5Y human neuroblastoma cells. Mol Med Rep. 10:89–94.
2014.PubMed/NCBI
|
|
43
|
Shi W, Li X, Hou X, Peng H, Jiang Q, Shi
M, Ji Y, Liu X and Liu J: Differential apoptosis gene expressions
of rhabdomyosarcoma cells in response to enterovirus 71 infection.
BMC Infect Dis. 12:3272012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Correia C, Lee SH, Meng XW, Vincelette ND,
Knorr KL, Ding H, Nowakowski GS, Dai H and Kaufmann SH: Emerging
understanding of Bcl-2 biology: Implications for neoplastic
progression and treatment. Biochim Biophys Acta. 1853:1658–1671.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Schacter JL, Henson ES and Gibson SB:
Estrogen regulation of anti-apoptotic Bcl-2 family member Mcl-1
expression in breast cancer cells. PLoS One. 9:e1003642014.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Li C, Sun BQ and Gai XD: Compounds from
Chinese herbal medicines as reversal agents for
P-glycoprotein-mediated multidrug resistance in tumours. Clin
Transl Oncol. 16:593–598. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Seward SM and Winer I: Primary debulking
surgery and neoadjuvant chemotherapy in the treatment of advanced
epithelial ovarian carcinoma. Cancer Metastasis Rev. 34:5–10. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Januchowski R, Zawierucha P, Ruciński M
and Zabel M: Microarray-based detection and expression analysis of
extracellular matrix proteins in drug-resistant ovarian cancer cell
lines. Oncol Rep. 32:1981–1990. 2014.PubMed/NCBI
|
|
49
|
Huq F, Yu JQ, Beale P, Chan C, Arzuman L,
Nessa MU and Mazumder ME: Combinations of platinums and selected
phytochemicals as a means of overcoming resistance in ovarian
cancer. Anticancer Res. 34:541–545. 2014.PubMed/NCBI
|
|
50
|
Saxena V and Hussain MD: Polymeric mixed
micelles for delivery of curcumin to multidrug resistant ovarian
cancer. J Biomed Nanotechnol. 9:1146–1154. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Ren Y, Yu K, Sun S, Li Z, Yuan J, Han XD,
Shi J and Zhen L: JSI124 inhibits breast cancer cell growth by
suppressing the function of B cells via the downregulation of
signal transducer and activator of transcription 3. Oncol Lett.
8:928–932. 2014.PubMed/NCBI
|
|
52
|
Li JM, Zhang W, Su H, Wang YY, Tan CP, Ji
LN and Mao ZW: Reversal of multidrug resistance in MCF-7/Adr cells
by codelivery of doxorubicin and BCL2 siRNA using a folic
acid-conjugated polyethylenimine hydroxypropyl-β-cyclodextrin
nanocarrier. Int J Nanomedicine. 10:3147–3162. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Jansson PJ, Yamagishi T, Arvind A,
Seebacher N, Gutierrez E, Stacy A, Maleki S, Sharp D, Sahni S and
Richardson DR: Di-2-pyridylketone 4,4-dimethyl-3-thiosemicarbazone
(Dp44mT) overcomes multidrug resistance by a novel mechanism
involving the hijacking of lysosomal P-glycoprotein (Pgp). J Biol
Chem. 290:9588–9603. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Drinberg V, Bitcover R, Rajchenbach W and
Peer D: Modulating cancer multidrug resistance by sertraline in
combination with a nanomedicine. Cancer Lett. 354:290–298. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Hu T, To KK, Wang L, Zhang L, Lu L, Shen
J, Chan RL, Li M, Yeung JH and Cho CH: Reversal of P-glycoprotein
(P-gp) mediated multidrug resistance in colon cancer cells by
cryptotanshinone and dihydrotanshinone of Salvia miltiorrhiza.
Phytomedicine. 21:1264–1272. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Januchowski R, Wojtowicz K,
Sujka-Kordowska P, Andrzejewska M and Zabel M: MDR gene expression
analysis of six drug-resistant ovarian cancer cell lines. Biomed
Res Int. 2013:2417632013. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Abraham J, Salama NN and Azab AK: The role
of P-glycoprotein in drug resistance in multiple myeloma. Leuk
Lymphoma. 56:26–33. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Tomiyasu H, Watanabe M, Sugita K,
Goto-Koshino Y, Fujino Y, Ohno K, Sugano S and Tsujimoto H:
Regulations of ABCB1 and ABCG2 expression through MAPK pathways in
acute lymphoblastic leukemia cell lines. Anticancer Res.
33:5317–5323. 2013.PubMed/NCBI
|
|
59
|
Vasconcelos FC, Silva KL, Souza PS, Silva
LF, Moellmann-Coelho A, Klumb CE and Maia RC: Variation of MDR
proteins expression and activity levels according to clinical
status and evolution of CML patients. Cytometry B Clin Cytom.
80:158–166. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Vtorushin SV, Khristenko KY, Zavyalova MV,
Perelmuter VM, Litviakov NV, Denisov EV, Dulesova AY and
Cherdyntseva NV: The phenomenon of multi-drug resistance in the
treatment of malignant tumors. Exp Oncol. 36:144–156.
2014.PubMed/NCBI
|
|
61
|
Zhao H, Peng C, Ruan G, Zhou J, Li Y and
Hai Y: Adenovirus-delivered PDCD5 counteracts adriamycin resistance
of osteosarcoma cells through enhancing apoptosis and inhibiting
Pgp. Int J Clin Exp Med. 7:5429–5436. 2014.PubMed/NCBI
|
|
62
|
Ji T, Gong D, Han Z, Wei X, Yan Y, Ye F,
Ding W, Wang J, Xia X, Li F, et al: Abrogation of constitutive
Stat3 activity circumvents cisplatin resistant ovarian cancer.
Cancer Lett. 341:231–239. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Su J, Zhou L, Xia MH, Xu Y, Xiang XY and
Sun LK: Bcl-2 family proteins are involved in the signal crosstalk
between endoplasmic reticulum stress and mitochondrial dysfunction
in tumor chemotherapy resistance. Biomed Res Int. 2014:2343702014.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Chakraborty S, Mazumdar M, Mukherjee S,
Bhattacharjee P, Adhikary A, Manna A, Chakraborty S, Khan P, Sen A
and Das T: Restoration of p53/miR-34a regulatory axis decreases
survival advantage and ensures Bax-dependent apoptosis of non-small
cell lung carcinoma cells. FEBS Lett. 588:549–559. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Bykov VJ and Wiman KG: Mutant p53
reactivation by small molecules makes its way to the clinic. FEBS
Lett. 588:2622–2627. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Vibhuti A, Muralidhar K and Dwarakanath
BS: Differential cytotoxicity of the glycolytic inhibitor
2-deoxy-D-glucose in isogenic cell lines varying in their p53
status. J Cancer Res Ther. 9:686–692. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Schwermer M, Lee S, Köster J, van Maerken
T, Stephan H, Eggert A, Morik K, Schulte JH and Schramm A:
Sensitivity to cdk1-inhibition is modulated by p53 status in
preclinical models of embryonal tumors. Oncotarget. 6:15425–15435.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Sabbatino F, Fusciello C, Somma D, Pacelli
R, Poudel R, Pepin D, Leonardi A, Carlomagno C, Scarpati G Della
Vittoria, Ferrone S and Pepe S: Effect of p53 activity on the
sensitivity of human glioblastoma cells to PARP-1 inhibitor in
combination with topoisomerase I inhibitor or radiation. Cytometry
A. 85:953–961. 2014. View Article : Google Scholar : PubMed/NCBI
|