Introduction
Nasopharyngeal carcinoma (NPC) arises from the
epithelium of the nasopharyngeal mucosa. The incidence of NPC
varies between countries, however among the Caucasian population,
the incidence is <1 case per 100,000 individuals (1,2). In
contrast, NPC ranks among the most common of the head and neck
cancers in Asia, particularly in Hong Kong and the Guangdong
Province in Southern China, where the incidence is 25 cases per
100,000 individuals (1,2). The majority of patients with NPC are
initially diagnosed with advanced disease, resulting in a high rate
of mortality (1,2). Recently, healthcare providers have begun
to examine the possible roles of lifestyle and genetic factors in
the development of NPC. The notion that salted fish consumption is
a classical risk factor of NPC in China has been denied by Lau
et al (3). They also reported
that vegetable consumption appears to help protect against NPC
(3). Numerous therapeutic strategies
have been investigated to improve the prognosis for NPC patients,
including surgical techniques, chemotherapy, radiation and targeted
therapies (4,5). However, patients with NPC, and
particularly those with relapsed NPC, continue to have a poor
survival rate. Many of the current treatments for NPC have high
toxicities (6). Therefore, there is
an urgent need to identify novel drugs that are more effective, but
less toxic for the treatment of NPC.
Apogossypolone (ApoG2) is a novel derivative of
gossypol, which is a polyphenolic substance extracted from
cottonseed (7). ApoG2 is an effective
inhibitor of cancer cell proliferation and suppresses tumor growth
by inducing apoptosis (7).
Additionally, ApoG2 is less toxic to normal cells than gossypol. In
laboratory and clinical studies, ApoG2 has shown potent antitumor
activity for several types of malignant tumors including prostate
(7), breast (8), gastric (9)
and pancreatic cancer (10), myeloma
(11) and chronic lymphocytic
leukemia (12). Several studies have
now demonstrated that ApoG2 induces tumor cell apoptosis by
blocking the B-cell lymphoma-2 (Bcl-2) signaling pathway (13,14). Bcl-2
is an anti-apoptotic protein that serves a critical role in
promoting tumor cell survival and tumor growth (15,16). An
agent that selectively inhibits Bcl-2 expression and/or activity
would be hypothesized to induce tumor cell apoptosis and inhibit
tumor growth.
Given the previous studies supporting an antitumor
role for ApoG2, the present study designed an in vitro and
in vivo model to evaluate its effects and mechanisms in NPC,
a tumor with high morbidity and mortality, particularly in Southern
China.
Materials and methods
Cell lines, ApoG2, and experimental
reagents
The human NPC CNE-2 cell line was obtained from the
Cancer Institute of the Southern Medical University (Guangzhou,
China). ApoG2 was provided by the University of Michigan (Michigan,
USA). An ApoG2 stock solution was freshly prepared at a
concentration of 20 mmol/l in 100% dimethyl sulfoxide (DMSO) on the
day of the experiment, and then diluted to the specific
concentrations (0, 5, 10, 20, 40, 60 and 80 µmol/l) required for a
particular study.
Control groups in the experiments were treated with
0.1% DMSO alone. All primary antibodies used in western blot
analysis were rabbit anti-human monoclonal antibodies. The
anti-Bcl-2 antibody was purchased from Santa Cruz Biotechnology,
Inc. (Santa Cruz, CA, USA; sc-492; dilution 1:1,000), the
anti-beclin-1 antibody was purchased from Cell Signaling
Technology, Inc. (Danvers, MA, USA; 3495; dilution, 1:1,000) and
the anti-β-actin antibody was purchased from Abmart Biomedical
(Shanghai, China; P30002; dilution, 1:2,000). The secondary
antibodies were biotin-labeled goat anti-rabbit antibodies
purchased from Boster Biotechnology Inc. (Wuhan, China; BA1003;
dilution, 1:400). A pre-stained protein ladder was purchased from
Thermo Fisher Scientific, Inc., Waltham, MA, USA), and other
reagents and laboratory supplies were purchased from Hyclone; GE
Healthcare Life Sciences (Logan, UT, USA).
Cell culture
The human NPC CNE-2 cell line was maintained in RPMI
1640 culture medium supplemented with 10% fetal bovine serum (FBS),
100 U/ml penicillin, and 100 µg/ml streptomycin (Gibco; Thermo
Fisher Scientific, Inc.) in a humidified incubator at 37°C
containing 5% CO2. Subcultures were initiated when the
cell density reached ~80%. Cells to be harvested were trypsinized
(0.025% trypsin and 0.02% EDTA) and then washed twice with PBS.
Cell viability assay
Human NPC CNE-2 cells were seeded at a density of
5,000 cells/well in flat-bottom 96-well plates (100 µl per well). A
total of 24 h later the cells were treated with ApoG2 at increasing
concentrations (0, 5, 10, 20, 40, 60 and 80 µmol/l), and cell
viability was determined after 24, 48, and 72 h using the Cell
Counting kit-8 assay (CCK-8; Dojindo Molecular Technologies, Inc.,
Kumamoto, Japan) according the manufacturer's protocol. All cell
viability assays were performed in triplicate.
Hoechst 33258 staining
Human NPC CNE-2 cells were seeded in 6-well plates
(50,000 cells/well) and incubated overnight at 37°C. Subsequently,
the cells were treated with 40 µmol/l ApoG2 for 48 h and then fixed
in 4% formaldehyde for 10 min. The cells were then washed twice
with ice-cold PBS and stained with 0.5 ml of blue nucleic acid
counterstain solution Hoechst 33258 (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) for 5 min at room temperature. The cells were
examined under an inverted fluorescence microscope. Cells with
punctate and condensed nuclei were identified as apoptotic
cells.
Flow cytometry analysis of apoptotic
cells
Human NPC cell line CNE-2 cells were seeded in
6-well plates and treated with 40 µmol/l ApoG2. Following a 48-h
incubation at 37°C, cells were harvested, washed twice with
ice-cold PBS, and resuspended in a binding buffer. The cells were
then stained with Annexin V/propidium iodide solution (BD
Biosciences, San Jose, CA, USA) at room temperature, and analyzed
using a Becton Dickinson FACScan flow cytometer (BD
Biosciences).
Analysis of autophagy by
immunofluorescence staining
Human NPC CNE-2 cells were treated with 40 µmol/l
ApoG2 for 48 h then washed, harvested and centrifuged at 1,500 × g
for 10 min at 4°C. The pelleted cells were initially fixed for 1 h
in a 2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer for 1 h,
and then in 1% osmium tetroxide in 0.1 M cacodylate buffer for 1 h.
The cells were then dehydrated with ethanol and infiltrated with
Araldite resin. An ultramicrotome was used to obtain specimens of
70–80 nm thickness. Finally, the cells were stained with uranyl
acetate and lead citrate, and any features of autophagy were
observed using transmission electron microscopy (TEM).
Autophagy detection by flow cytometry
analysis
Human NPC cell line CNE-2 cells were treated with 40
µmol/l ApoG2 for 48 h as aforementioned and incubated with 1 mg/l
acridine orange for 15 min. The cells were then repeatedly washed
with ice-cold PBS to remove residual dye, and images were captured
using an inverted fluorescence microscope equipped with a 100 W
mercury lamp (490 nm band pass blue excitation filters, a 500 nm
dichroic mirror and a 515 nm long pass barrier filter). Autophagy
was quantified based on the mean number of cells showing intense
red staining. In total, 3 microscopic fields containing ≥50 cells
per field were analyzed when determining the results obtained with
each set of experimental conditions. Fluorescence intensity was
analyzed using the FACScan software system (BD Biosciences).
Western blot analysis
Human NPC CNE-2 cells were treated with ApoG2 at
increasing concentrations (0, 5, 10, 20, 40, 60 and 80 µmol/l) for
48 h; after which they were washed twice with ice-cold PBS and
lysed in 0.5 ml lysis buffer for 20 min at 4°C. Protein
concentrations were determined using a BCA Protein assay kit
(Thermo Fisher Scientific, Inc.). Samples with equivalent amounts
of total protein were loaded and separated by 12% SDS-PAGE, and
then transferred to a polyvinylidene difluoride membrane.
Immunoblotting was performed using primary antibodies against
Bcl-2, beclin-1, and β-actin, and a horseradish
peroxidase-conjugated anti-IgG as the secondary antibody. The blots
were developed using ECL chemiluminescent reagent (Cell Signaling
Technology, Inc.).
In vivo tumor model in BALB/c-nu
mice
A total of 30 male nude mice (BALB/c-nu) of 4 weeks
old, weighting 16–19 g, were purchased from the Provincial Animal
Center (Guangdong, China), and maintained under a fixed 12 h
light/dark photoperiod (lights on from 7:00 to 19:00) with food and
water available ad libitum. They were raised individually
housed in a stainless steel cage in a room maintained at 25±1°C
with 70±4% relative humidity. All animal studies were performed in
accordance with guidelines provided in the Guide for the Care and
Use of Laboratory Animals (14).
The study protocols were approved by the Animal
Investigation Committee of Sun Yat-Sen University (Guangzhou,
China). The human NPC CNE-2 cells suspended in serum-free culture
medium were inoculated subcutaneously into the flank region of
BALB/c-nu mice (5×106 cells/mouse). When the tumor size
reached ~4 mm in diameter, the mice were randomly assigned to
subgroups consisting of a control group and an ApoG2-treatment
group. All pharmacologic agents were administered by
intraperitoneal injection at a dose of 120 mg/kg every other day
for 3 weeks. The weight of each mouse and the tumor volume was
monitored every other day. Tumor measurements were obtained using
vernier calipers, and tumor volumes were calculated as AxBxB/2;
where A and B represent tumor length and width, respectively. At 2
weeks post-inoculation, the mice were anesthetized using 10%
chloral hydrate (47335; Sigma-Aldrich; Merck KGaA injected
intraperitoneally (300 mg/kg). Immediately after, they were
euthanized by dislocated cervical vertebra and their tumor
xenografts were removed. The percent inhibition of tumor growth was
calculated as (1-T/C)x100%; where T and C represent the average
tumor weight in the ApoG2 and control group, respectively.
Statistical analysis
All statistical analysis was performed using SPSS
statistics software for Windows, version 13.0 (SPSS Inc., Chicago,
IL, USA). Data was calculated as a percentage obtained from at
least 3 duplicate experiments and expressed as the mean ± standard
deviation. Statistical differences between mean values were
analyzed using the Student's t-test or one-way analysis of
variance. P<0.05 was considered to indicate a statistically
significant difference.
Results
ApoG2 decreased CNE-2 cell
viability
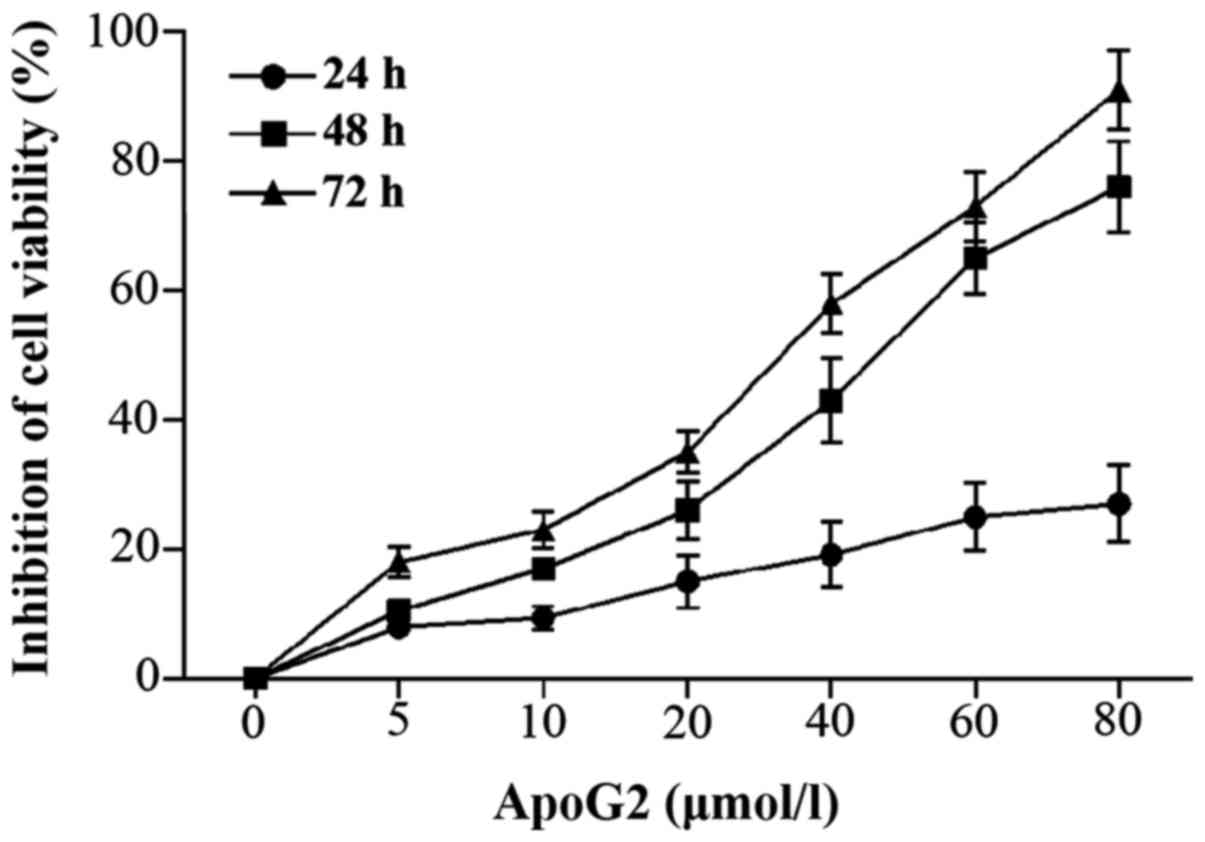
The CCK-8 assay results indicated that ApoG2
significantly decreased the viability of the human NPC cell line
CNE-2 cells in a time and dose-dependent manner
(Ftime=2041.671, Ptime<0.001;
Fconcentration=1819.354,
Pconcentration<0.001; Fig.
1). The results also suggest an interaction between ApoG2
concentration and exposure time (F=202.540, P<0.001). The 50%
inhibitory concentration (IC50) for ApoG2 after a 72 h
exposure was 23.61 µmol/l.
ApoG2 induced apoptosis in CNE-2
cells
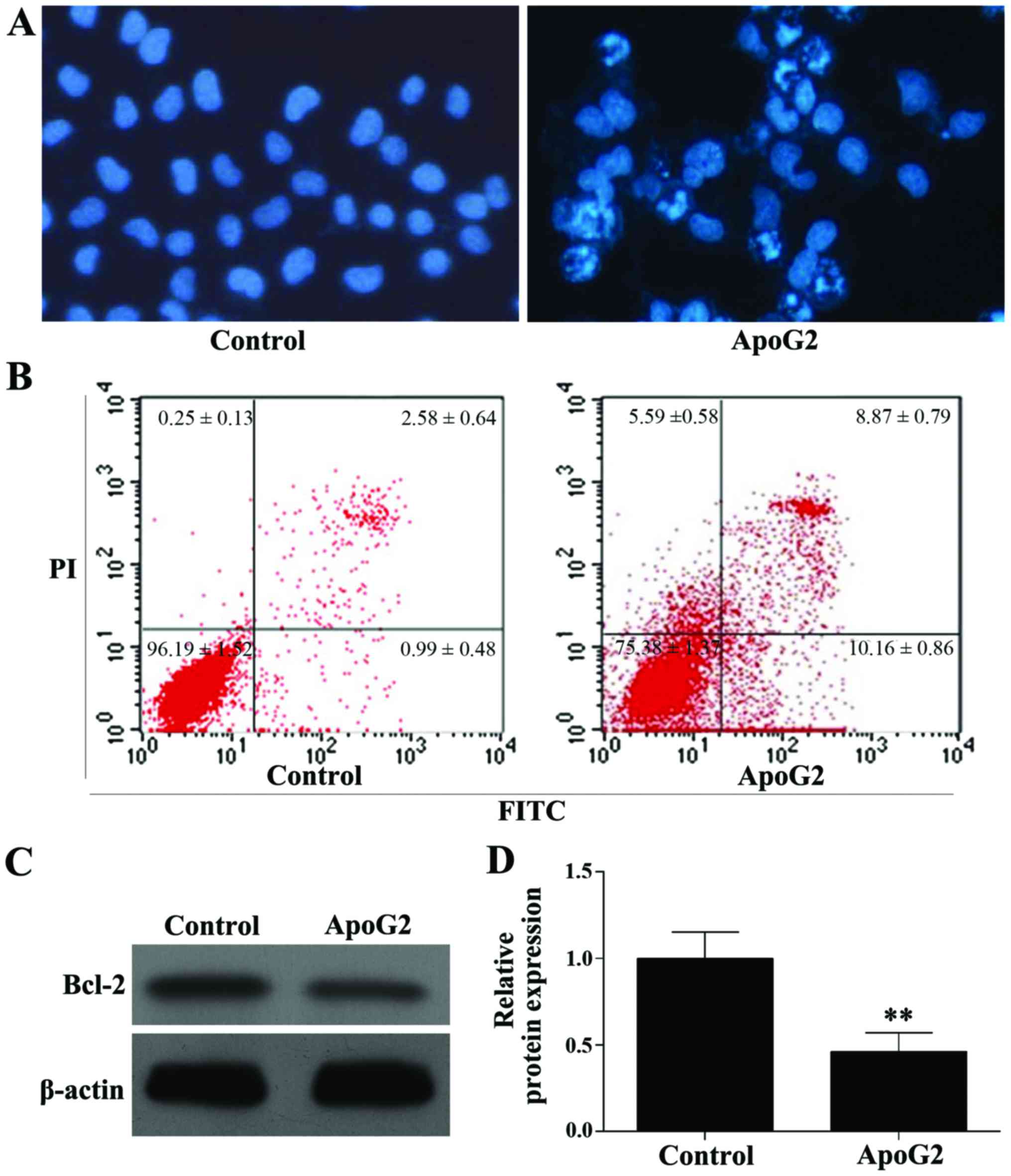
Hoechst-33258 nucleic acid staining (blue)
demonstrated apoptosis in CNE-2 cells following incubation with
ApoG2. Cell nuclear pyknosis, chromosome fragmentation, chromatin
condensation, the formation of apoptotic bodies, and other
apoptotic features were observed in ApoG2-treated cells but not in
control cells (Fig. 2A). Flow
cytometry results indicated apoptosis rates of 3.90±0.34 and
19.52±1.18% in the control and ApoG2 treated cells, respectively,
and this difference was statistically significant (F=485.294,
P<0.001; Fig. 2B). Western blot
analysis showed that ApoG2 significantly decreased expression of
Bcl-2 protein in CNE-2 cells, when compared with expression in
control cells (F=68.909, P=0.001; Fig. 2C
and D).
ApoG2 induced autophagy in CNE-2
cells
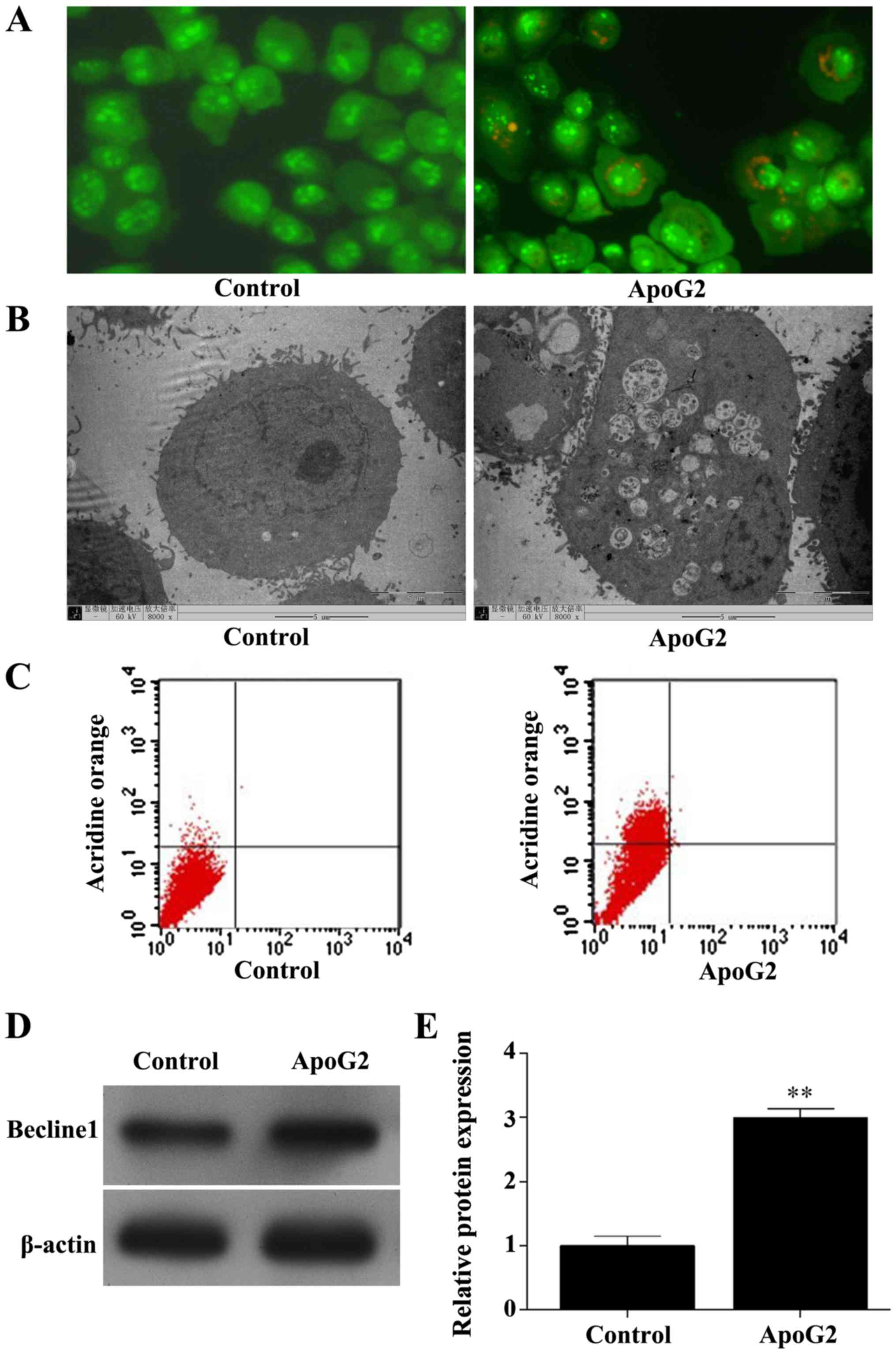
Control cells stained with acridine orange showed a
cell nucleus and cytoplasm that appeared bright green. In contrast,
ApoG2-treated cells showed bright red fragments of cytoplasm and
nucleus contained within acidic autophagosomes (Fig. 3A). Increased numbers of large vacuoles
and double-layered membrane structures were observed by TEM in
ApoG2-treated cells, but not in control cells (Fig. 3B). Flow cytometry results indicated
that 0.92±3.10% of control cells exhibited fluorescence compared
with 28.24±7.35% of ApoG2-treated cells (F=31.035, P=0.003;
Fig. 3C). Additionally, ApoG2
treatment significantly increased beclin-1 protein expression in
CNE-2 cells (F=497.906, P<0.001; Fig.
3D and E).
ApoG2 suppressed tumor growth in the
BALB/c-nu mice
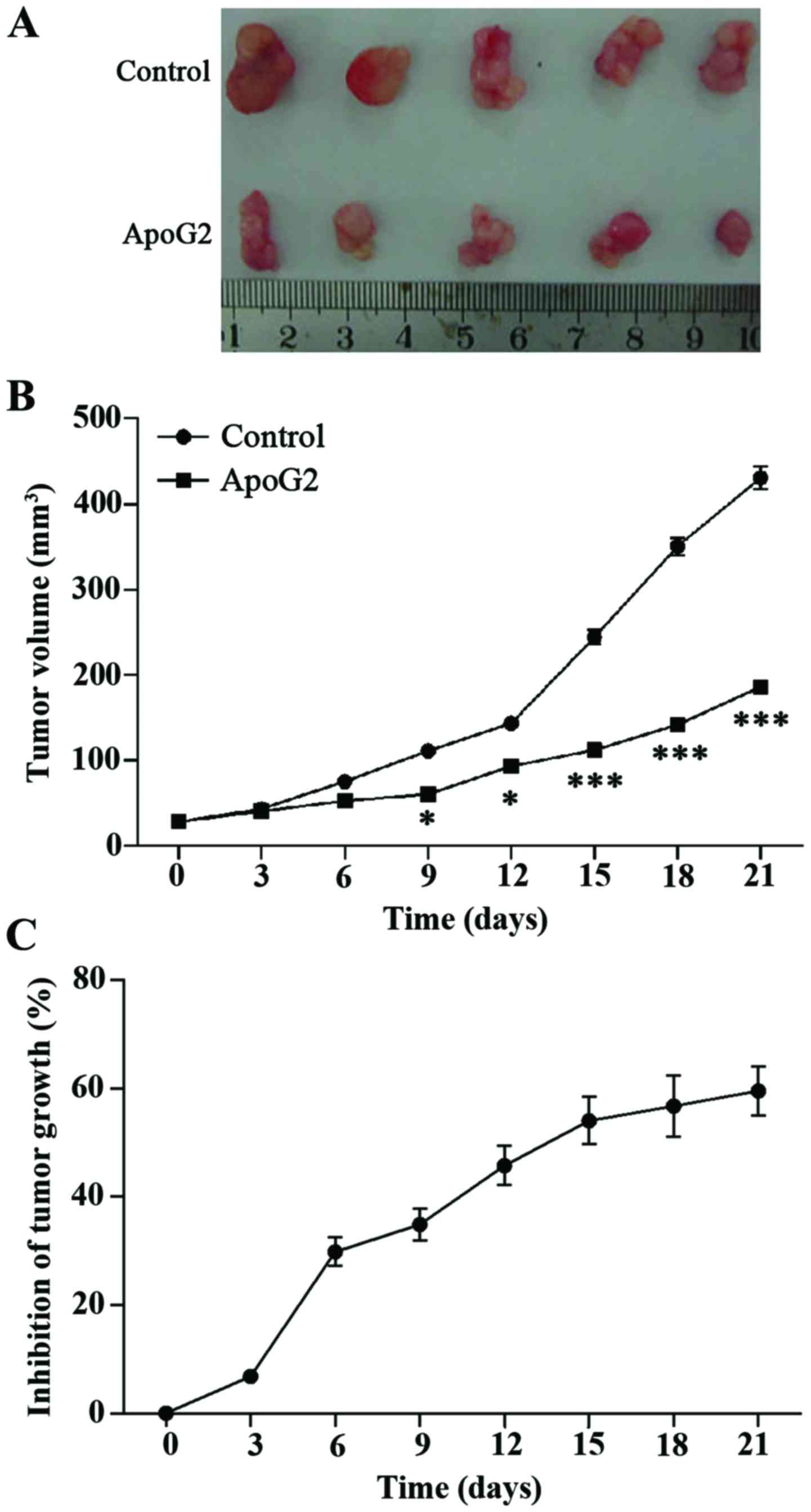
The in vivo study of subcutaneously grafted
CNE-2 human NPC cells in BALB/c-nu mice showed that ApoG2 treatment
inhibited tumor growth by 65.49% (P<0.05). The mice showed no
adverse reactions to treatment throughout the study. Based on these
results, the ApoG2 treatment was demonstrated to suppress tumor
growth in this in vivo mouse model (Fig. 4).
Discussion
NPC is associated with a high mortality rate
worldwide, but is a particularly prevalent primary malignancy in
China (17). Xu et al
(18) have reported that the
incidence of mortality due to NPC in China is 1.99/100,000
individuals, and mortality in males with NPC is greater than in
females at 2.81/100,000 vs. 1.14/100,000, respectively (19). Chemoradiotherapy remains the standard
treatment for locally-advanced and lymph node-positive NPC, and
platinum-based chemotherapy treatment regimens have recently
demonstrated superiority (17).
Previous studies have demonstrated that lifestyle changes may to
help to prevent NPC, and that early detection can improve patient
survival (17,19). Despite the use of conventional
chemotherapy, radiation and biological therapy and preventive
strategies such lifestyle changes, there remains a requirement to
identify safe and effective treatments for NPC.
The present study used in vitro and in
vivo models of NPC to demonstrate that ApoG2 is capable of
decreasing NPC tumor cell viability and suppressing tumor growth.
Apoptosis, or programmed cell death, assists in eliminating
unhealthy cells that are generated in physiological or pathological
conditions, oncogene activation, hypoxia, or during chemotherapy or
radiation (18,20). As an important anti-apoptosis protein,
Bcl-2 serves a critical role in regulating cell survival and
modulates the activity of tumor growth-associated molecules
(21,22). Several studies have demonstrated that
overexpression of Bcl-2 enhances tumor cell growth whilst silencing
of Bcl-2 with small interfering RNA significantly inhibited the
growth of tumor cells (23–25). It is also known that chemotherapy
resistance in patients with NPC correlates with overexpression of
Bcl-2 while inhibition of Bcl-2 enhances sensitivity to
chemotherapy (26,27). As a result, targeted downregulation of
Bcl-2 expression is considered an effective strategy for
sensitizing tumor cells to chemotherapy.
Autophagy has been widely accepted as a type of
programmed cell death. However, it remains unclear whether
autophagy is beneficial or detrimental for tumor growth. Autophagy
serves to eliminate superfluous or damaged organelles and initiates
the formation of multiple double-membrane vacuoles that fuse with
lysosomes to form intracellular bodies termed ‘autophagosomes’
(28,29). Certain external factors such as
nutrient deprivation and specific medications can induce autophagy
and lead to decreased cell viability. On the basis of these
published findings on the importance of apoptosis and autophagy in
tumor biology, the components of the present study were
planned.
The results of the current study indicated that
ApoG2 significantly decreased Bcl-2 expression, leading to human
NPC CNE-2 cell apoptosis. Immunofluorescence assays, flow cytometry
analysis, and TEM imaging suggested that ApoG2 treatment
significantly decreased autophagy in CNE-2 cells, which may be a
mechanism for the observed ApoG2-induced decrease in CNE-2 cell
viability. The present study additionally identified that ApoG2
treatment significantly increased expression of beclin-1 protein,
which has been reported as a marker of autophagy (30,31). The
results of the current study are supported by several other studies
that have investigated ApoG2 in NPC using either in vitro or
xenograft models (32–36).
Data from the present study support a role for ApoG2
in the regulation of Bcl-2 and beclin-1 expression in the NPC CNE-2
cell line, inducing apoptosis and autophagy, which modulate tumor
cell viability and tumor growth. Based on these observations, the
present study suggests that additional larger studies on the role
of ApoG2 should be performed to investigate its potential role as
an antitumor agent for use in the treatment of patients with
NPC.
The limitations of the present study include the
small study size and the use of an NPC cell line. Cell lines may
not reflect the behavior of human tumors arising in the nasopharynx
that can be heterogeneous and exhibit low-grade to high-grade
differentiation and behavior. In the in vivo mouse model,
the ‘tumors’ evaluated were subcutaneously implanted and would not
be expected to behave in the same way as tumors arising in the
nasopharynx.
There are remaining questions to be answered
regarding the mechanism of action of ApoG2 in NPC prior to
conducting clinical safety and efficacy studies in humans. It is
possible, for example, that autophagy and the promotion of
apoptosis may promote tumor cell survival in the clinical
situation. The appropriate clinical dose of ApoG2 additionally
remains to be determined for patients with NPC. There is the
possibility of cross-talk between apoptosis and autophagy, and the
mechanism by which ApoG2 induces tumor cell autophagy remains to be
studied. In addition, due to the fact that anti-Bcl-2 therapy has
been reported to sensitize tumor cells to chemotherapy and
radiation therapy, the present study proposed to test the effects
of combined ApoG2 therapy with chemotherapy and/radiotherapy on the
development of NPC in the study models.
In conclusion, these results support a role for
ApoG2 in inhibiting the growth of human NPC cells by inducing
apoptosis and autophagy. Additional controlled clinical studies
could be planned, to define safety, efficacy and dosing regimens
for ApoG2 as a potential treatment for patients with NPC.
Acknowledgements
The present study was supported by Science and
Technology projects in Guangzhou, China (grant no.
2011y2-00019-3).
Glossary
Abbreviations
Abbreviations:
|
ApoG2
|
apogossypolone
|
|
NPC
|
nasopharyngeal carcinoma
|
References
|
1
|
Wang Y, Zhang Y and Ma S: Racial
differences in nasopharyngeal carcinoma in the United States.
Cancer Epidemiol. 37:793–802. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kimura Y, Suzuki D, Tokunaga T,
Takabayashi T, Yamada T, Wakisaka N, Yoshizaki T, Murata H, Miwa K,
Shoujaku H, et al: Epidemiological analysis of nasopharyngeal
carcinoma in the central region of Japan during the period from
1996 to 2005. Auris Nasus Larynx. 38:244–249. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lau HY, Leung CM, Chan YH, Lee AW, Kwong
DL, Lung ML and Lam TH: Secular trends of salted fish consumption
and nasopharyngeal carcinoma: A multi-jurisdiction ecological study
in 8 regions from 3 continents. BMC Cancer. 13:2982013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xu T, Shen C, Ou X, He X, Ying H and Hu C:
The role of adjuvant chemotherapy in nasopharyngeal carcinoma with
bulky neck lymph nodes in the era of IMRT. Oncotarget.
7:21013–21022. 2016.PubMed/NCBI
|
|
5
|
Xu T, Liu Y, Dou S, Li F, Guan X and Zhu
G: Weekly cetuximab concurrent with IMRT aggravated
radiation-induced oral mucositis in locally advanced nasopharyngeal
carcinoma: Results of a randomized phase II study. 51:875–879.
2015.
|
|
6
|
Li H, Wang DL, Liu XW, Chen MY, Mo YX,
Geng ZJ and Xie CM: MRI signal changes in the skull base bone after
endoscopic nasopharyngectomy for recurrent NPC: A serial study of 9
patients. Eur J Radiol. 82:309–315. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhan YH, Huang XF, Hu XB, An QX, Liu ZX
and Zhang XQ: Growth inhibition and apoptosis induction of human
umbilical vein endothelial cells by apogossypolone. Asian Pac J
Cancer Prev. 14:1791–1795. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Niu X, Li S, Wei F, Huang J, Wu G, Xu L,
Xu D and Wang S: Apogossypolone induces autophagy and apoptosis in
breast cancer MCF-7 cells in vitro and in vivo. Breast Cancer.
21:223–230. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xin J, Zhan YH, Xia LM, Zhu HW, Nie YZ,
Liang JM and Tian J: ApoG2 as the most potent gossypol derivatives
inhibits cell growth and induces apoptosis on gastric cancer cells.
Biomed Pharmacother. 67:88–95. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Banerjee S, Choi M, Aboukameel A, Wang Z,
Mohammad M, Chen J, Yang D, Sarkar FH and Mohammad RM: Preclinical
studies of apogossypolone, a novel pan inhibitor of bcl-2 and
mcl-1, synergistically potentiates cytotoxic effect of gemcitabine
in pancreatic cancer cells. Pancreas. 39:323–331. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lin J, Wu YJ, Yang DJ and Zhao YQ: Effect
of apogossypolone on induction apoptosis in multiple myeloma cells
and its mechanisms. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 17:92–98.
2009.(In Chinese). PubMed/NCBI
|
|
12
|
Balakrishnan K, Aggarwal S, Wierda W and
Gandhi V: Bax and Bak are required for apogossypolone, a
BH3-mimetic, induced apoptosis in chronic lymphocytic leukemia
cells. Leuk Lymphoma. 54:1097–1100. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cheng P, Ni Z, Dai X, Wang B, Ding W, Rae
Smith A, Xu L, Wu D, He F and Lian J: The novel BH-3 mimetic
apogossypolone induces Beclin-1- and ROS-mediated autophagy in
human hepatocellular carcinoma [corrected] cells. Cell Death Dis.
4:e4892013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sun J, Li ZM, Hu ZY, Lin XB, Zhou NN, Xian
LJ, Yang DJ and Jiang WQ: ApoG2 inhibits antiapoptotic Bcl-2 family
proteins and induces mitochondria-dependent apoptosis in human
lymphoma U937 cells. Anticancer Drugs. 19:967–974. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hockenbery DM: Bcl-2 in cancer,
development and apoptosis. J Cell Sci Suppl. 18:51–55. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Raffo AJ, Perlman H, Chen MW, Day ML,
Streitman JS and Buttyan R: Overexpression of bcl-2 protects
prostate cancer cells from apoptosis in vitro and confers
resistance to androgen depletion in vivo. Cancer Res. 55:4438–4445.
1995.PubMed/NCBI
|
|
17
|
National Research Council (US), .
Committee for the Update of the Guide for the Care and Use of
Laboratory Animals. ‘Guide for the Care and Use of Laboratory
Animals. ’ Guide for the Care & Use of Laboratory Animals.
103:1072–1073. 2011.
|
|
18
|
Xu ZJ, Zheng RS, Zhang SW, Zou XN and Chen
WQ: Nasopharyngeal carcinoma incidence and mortality in China in
2009. Chin J Cancer. 32:453–460. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mesia R, Pastor M, Grau JJ and del Barco
E; SEOM: SEOM clinical guidelines for the treatment of
nasopharyngeal carcinoma 2013. Clin Transl Oncol. 15:1025–1029.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Piro LD: Apoptosis, Bcl-2 antisense, and
cancer therapy. Oncology (Williston Park). 18 13 Suppl 10:S5–S10.
2004.
|
|
21
|
Kontos CK, Christodoulou MI and Scorilas
A: Apoptosis-related BCL2-family members: Key players in
chemotherapy. Anticancer Agents Med Chem. 14:353–374. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kaneko T, Zhang Z, Mantellini MG, Karl E,
Zeitlin B, Verhaegen M, Soengas MS, Lingen M, Strieter RM, Nunez G
and Nör JE: Bcl-2 orchestrates a cross-talk between endothelial and
tumor cells that promotes tumor growth. Cancer Res. 67:9685–9693.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tucker CA, Kapanen AI, Chikh G, Hoffman
BG, Kyle AH, Wilson IM, Masin D, Gascoyne RD, Bally M and Klasa RJ:
Silencing Bcl-2 in models of mantle cell lymphoma is associated
with decreases in cyclin D1, nuclear factor-kappaB, p53, bax, and
p27 levels. Mol Cancer Ther. 7:749–758. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tekedereli I, Alpay SN, Akar U, Yuka E,
Ayugo-Rodriguez C, Han HD, Sood AK, Lopez-Berestein G and Ozpolat
B: Therapeutic silencing of Bcl-2 by systemically administered
siRNA nanotherapeutics inhibits tumor growth by autophagy and
apoptosis and enhances the efficacy of chemotherapy in orthotopic
xenograft models of ER (−) and ER (+) breast cancer. Mol Ther
Nucleic Acids. 2:e1212013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Du P, Cao H, Wu HR, Zhu BS, Wang HW, Gu
CW, Xing CG and Chen W: Blocking Bcl-2 leads to autophagy
activation and cell death of the HEPG2 liver cancer cell line.
Asian Pac J Cancer Prev. 14:5849–5854. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Akar U, Chaves-Reyez A, Barria M, Tari A,
Sanguino A, Kondo Y, Kondo S, Arun B, Lopez-Berestein G and Ozpolat
B: Silencing of Bcl-2 expression by small interfering RNA induces
autophagic cell death in MCF-7 breast cancer cells. Autophagy.
4:669–679. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Paoluzzi L, Gonen M, Gardner JR, Mastrella
J, Yang D, Holmlund J, Sorenen M, Leopold L, Manova K, Marcucci G,
et al: Targeting Bcl-2 family members with the BH3 mimetic AT-101
markedly enhances the therapeutic effects of chemotherapeutic
agents in in vitro and in vivo models of B-cell lymphoma. Blood.
111:5350–5358. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lieber J, Kirchner B, Eicher C, Warman SW,
Seitz G, Fuchs J and Armeanu-Ebinger S: Inhibition of Bcl-2 and
Bcl-X enhances chemotherapy sensitivity in hepatoblastoma cells.
Pediatr Blood Cancer. 55:1089–1095. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lamb CA, Yoshimori T and Tooze SA: The
autophagosome: Origins unknown, biogenesis complex. Nat Rev Mol
Cell Biol. 14:759–774. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yang Z and Klionsky DJ: Eaten alive: A
history of macroautophagy. Nat Cell Biol. 12:814–822. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xu HD, Wu D, Gu JH, Ge JB, Wu JC, Han R,
Liang ZQ and Qin ZH: The pro-survival role of autophagy depends on
Bcl-2 under nutrition stress conditions. PLoS One. 8:e632322013.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tian S, Lin J, Jun Zhou J, Wang X, Li Y,
Ren X, Yu W, Zhong W, Xiao J, Sheng F, et al: Beclin 1-independent
autophagy induced by a Bcl-XL/Bcl-2 targeting compound, Z18.
Autophagy. 6:1032–1041. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
He JH, Liao XL, Wang W, Li DD, Chen WD,
Deng R, Yang D, Han ZP, Jiang JW and Zhu XF: Apogossypolone, a
small-molecule inhibitor of Bcl-2, induces radiosensitization of
nasopharyngeal carcinoma cells by stimulating autophagy. Int J
Oncol. 45:1099–1108. 2014.PubMed/NCBI
|
|
34
|
Hu ZY, Wang J, Cheng G, Zhu XF, Huang P,
Yang D and Zeng YX: Apogossypolone targets mitochondria and light
enhances its anticancer activity by stimulating generation of
singlet oxygen and reactive oxygen species. Chin J Cancer.
30:41–53. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hu ZY, Sun J, Zhu XF, Yang D and Zeng YX:
ApoG2 induces cell cycle arrest of nasopharyngeal carcinoma cells
by suppressing the c-Myc signaling pathway. J Transl Med. 7:742009.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hu ZY, Zhu XF, Zhong ZD, Sun J, Wang J,
Yang D and Zeng YX: ApoG2, a novel inhibitor of antiapoptotic Bcl-2
family proteins, induces apoptosis and suppresses tumor growth in
nasopharyngeal carcinoma xenografts. Int J Cancer. 123:2418–2429.
2008. View Article : Google Scholar : PubMed/NCBI
|