Introduction
Nasopharyngeal carcinoma (NPC) is a common malignant
head and neck cancer in Southeast Asia and Southern China, with an
incidence rate of >20 per 100,000 people per year (nearly
100-fold higher than the rate in Western countries) (1). Fractionated radiotherapy (RT) with a
fraction dose of 2 Gy to a total dose of 60–70 Gy is a
common/standard strategy for treating NPC due to its advantages in
the preservation of normal tissues (2,3). However,
surviving tumor cells following fractionated RT may be
radioresistant, particularly in patients with advanced NPC, and
this cell population contributes to the majority of RT failures
(4). Thus, improving radiation
sensitization in the treatment of NPC remains a challenge.
It has been reported that the
phosphatidylinositol-4, 5-bisphosphate 3-kinase (PI3K)/Akt
signaling pathway regulates cellular functions involving mechanisms
of radioresistance in head and neck cancer, including intrinsic
radioresistance, tumor cell proliferation and hypoxia (5). Shimura (6)
previously overviewed the molecular mechanisms of cancer
radioresistance acquirement during fractionated RT. It is assumed
that fractionated RT damages the DNA of cancer cells and induces
DNA double strand breaks (DSBs), which subsequently activate
DNA-dependent protein kinase (PK) and then Akt. Activated Akt
induces the phosphorylation of glycogen synthase kinase (GSK) 3β at
serine 9, which decreases cyclin D1 proteolysis. As a consequence,
cyclin D1 accumulates in the nucleus of cells in S phase, thus
perturbing DNA replication, triggering the DNA damage checkpoint
and mediating more DNA DSBs. DNA DSBs in turn induce the activation
of DNA-PK and eventually mediate cyclin D1 overexpression, which
leads to the proliferation and progression of cancer cells and
eventually to radioresistance (6).
Mammalian target of rapamycin (mTOR), which is
activated by phosphorylated Akt, is a core regulator of protein
translation (7). It has been reported
that the Akt/mTOR signaling pathway is also responsible for cancer
radioresistance (8–10). Additionally, mTOR can decrease the
kinase activity of GSK3β, responsible for the degradation of cyclin
D1 (11). Previous studies have shown
that mTOR inhibition improves the radiosensitivity of prostate,
breast, bladder and brain cancer (12–15).
Rapamycin, an important inhibitor of mTOR and a drug
used for suppressing autoimmune responses in organ transplantation
(16), has been reported to act as a
radiosensitizer in the RT treatment of prostate, breast, bladder
and brain cancer (12–15). However, the anti-tumor effects of the
combination of rapamycin and ionizing radiation (IR) for treating
NPC are still unknown. In the present study, it was hypothesized
that rapamycin could overcome the radioresistance of NPC, and that
the presence of rapamycin could improve the anti-tumor effects of
IR in the treatment of NPC. Thus, in the present study, the effect
of the combination of rapamycin and IR on the DNA damage and cycle
status of NPC cells was investigated using an in vitro model
of NPC.
Materials and methods
Cell culture and viability assays
HNE1 NPC cells were obtained from the Cancer
Research Institute of Central South University (Changsha, China).
Cells were cultured in RPMI medium (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) containing fetal bovine serum (10% v/v, Thermo
Fisher Scientific, Inc.), L-glutamine (2 mM), penicillin (100 U/ml)
and streptomycin (100 µg/ml), and maintained at 37°C and 5% CO2.
Cell viability was assessed using an MTT assay according to the
manufacturer's protocol (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany).
Western blotting
Cells were treated with rapamycin, IR or a
combination of both, while untreated cells served as the control.
Following IR treatment for 24 h, cells were scraped on ice and
re-suspended for 30 min at 4°C in cold radioimmunoprecipitation
assay lysis buffer containing a cocktail of phosphatase and
protease inhibitors (Roche Diagnostics, Indianapolis, IN, USA).
Clear lysates were obtained upon centrifugation at 4°C and 10,000 ×
g for 10 min. The protein concentration of the lysate was
measured using Pierce BCA Protein Assay kit (Thermo Fisher
Scientific, Inc.). Protein samples (50 µg) were loaded onto 10%
polyacrylamide gels, and electrophoresis was subsequently
performed. The proteins in the gels were then transferred to
polyvinylidene fluoride membranes at 100 V for 90 min. The
membranes were blocked with a phosphate buffer saline (PBS)
solution containing 5% skimmed milk or 5% bovine serum albumin at
37°C for 1 h, and then western blotted with the following rabbit
anti-human monoclonal primary antibodies at 4°C overnight:
Anti-phosphorylated Akt (Ser473 phosphorylation, cat. no. #4046),
anti-total Akt (cat. no. #4685), anti-phosphorylated GSK3β (Ser9
phosphorylation, cat. no. #5558), anti-total GSK3β (cat. no.
#12456), anti-phosphorylated S6 (Ser240/244 phosphorylation, cat.
no. #5364), anti-total S6 (cat. no. #2217), anti-cyclin D1 (cat.
no. #2978) and anti-β-actin (cat. no. #4970). All primary
antibodies were purchased from Cell Signaling Technology, Inc.
(Danvers, MA, USA) and diluted to 1:1,000. Upon washing five times
with PBST (PBS containing 0.1% Tween-20), the membranes were
incubated with the appropriate anti-rabbit secondary antibodies
(dilution, 1:2,000; cat. no. #7074; Cell Signaling Technology,
Inc.) at 37°C for 2 h. Following five washes in PBST, an enhanced
chemiluminescence detection system (GE Healthcare Life Sciences,
Chalfont, UK) was used to reveal the protein bands of interest on
X-ray films (Kodak, Rochester, New York, USA). The films were
scanned using an SCX-4321NS imaging system (Samsung Group, Seoul,
Korea), and the protein levels were normalized to β-actin.
Analysis of phosphorylated histone
subunit 2AX (γH2AX) foci
Residual DNA damage in irradiated or non-irradiated
HNE1 cells was assessed by measuring residual γH2AX foci. Cells
were treated with rapamycin (100 nmol/l) for 10 h, followed by
treatment with or without 4 Gy of irradiation, and then cultured
for 24 h. Subsequently, the number of residual γH2AX foci per cell
was counted in five random visual fields under with a DM 2000 LED
microscope (Leica Microsystems GmbH, Wetzlar, Germany) (17).
Cell cycle assay
HNE1 cells were plated onto T25 tissue culture
flasks (Falcon; Corning Incorporated, Corning, NY, USA) and
incubated overnight to allow cells to reach mid-log growth phase.
Tumor cells were treated with rapamycin (100 nmol/l) for 10 h prior
to irradiation and cultured for 24 h post-irradiation. All adherent
cells were suspended using 0.5% trypsin/EDTA (Thermo Fisher
Scientific, Inc.) and harvested upon washing with PBS. Following
another wash with ice-cold 70% ethanol, the cells were incubated
with a mixture of 200 µg/ml RNaseA with 50 µg/ml propidium iodide
in a dark room for 30 min at room temperature. Cell cycle status
was examined using flow cytometry (BD Accuri™; BD Biosciences,
Franklin Lakes, NJ, USA). Data were representative of three
independent experiments.
Statistical analysis
All statistical analyses were performed using the
SPSS 12.0 software package (SPSS, Inc., Chicago, IL, USA).
Differences between groups were compared using analysis of variance
and then a post-hoc test. All tests were two-tailed and P<0.05
was considered to indicate a statistically significant
difference.
Results
Protein expression and phosphorylation
in HNE1 cells following various treatments
Upon exposure to radiation, the phosphorylation of
Akt (Ser473) in HNE1 cells increased in a time-dependent manner and
peaked at 180 min post-IR (Fig. 1A).
The phosphorylation of GSK3β, one of the downstream targets of Akt
(6), was retarded in the presence of
rapamycin, which resulted in the decrease of cyclin D1 levels
(Fig. 1B). In addition, rapamycin
targeted S6, a downstream target of mTOR (7), and decreased the phosphorylation of S6
in irradiated HNE1 cells (Fig.
1B).
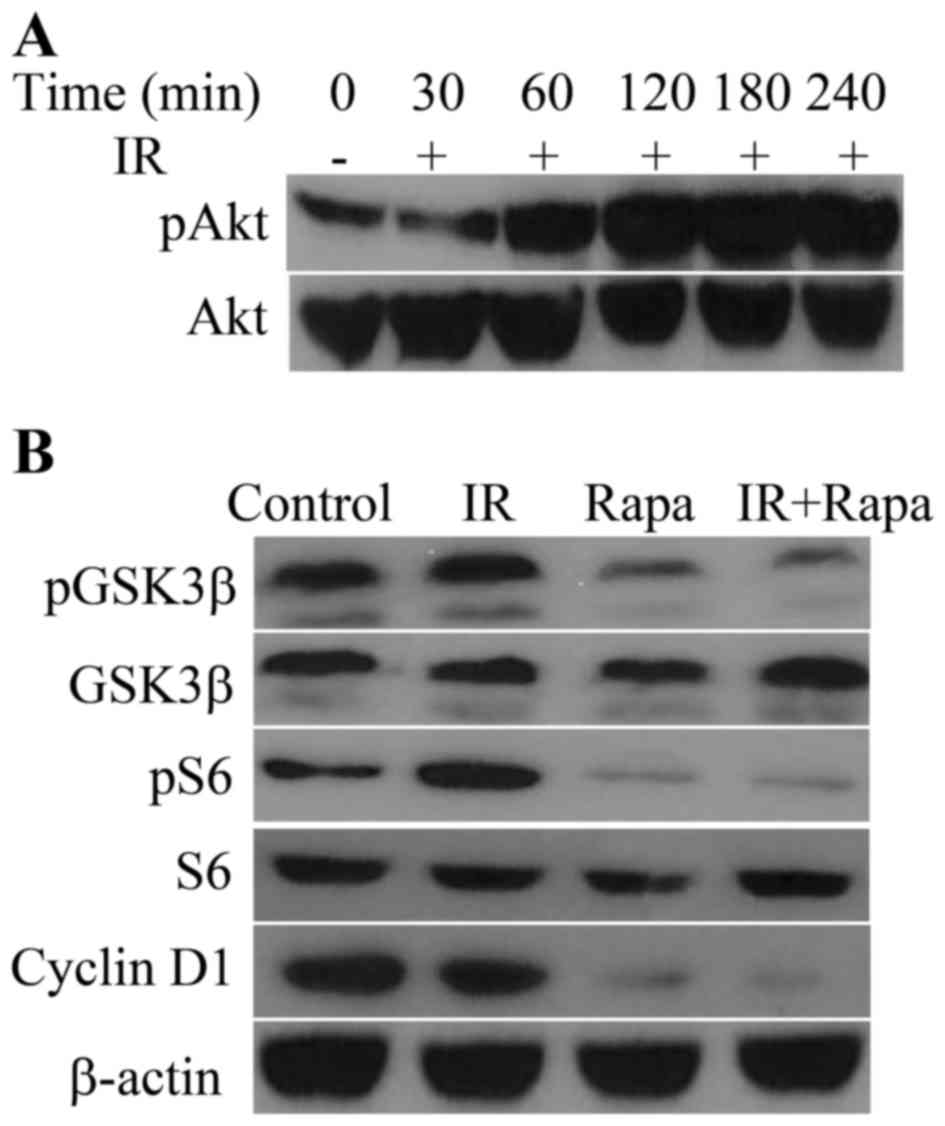 | Figure 1.Effects of IR or/and rapamycin on
Akt-associated pathways. (A) Phosphorylation of Akt in HNE1 cells
following treatment with a radiation dose of 4 Gy for 0, 30, 60,
120, 180 and 240 min. (B) Phosphorylation of GSK3β and ribosomal
protein S6, and expression of GSK3β, S6, cyclin D1 and β-actin, in
HNE1 cells treated with a radiation dose of 4 Gy, 100 nM rapamycin
or both. Untreated HNE1 cells served as the control. IR, ionizing
radiation; Rapa, rapamycin; GSK, glycogen synthase kinase; p,
phosphorylated. |
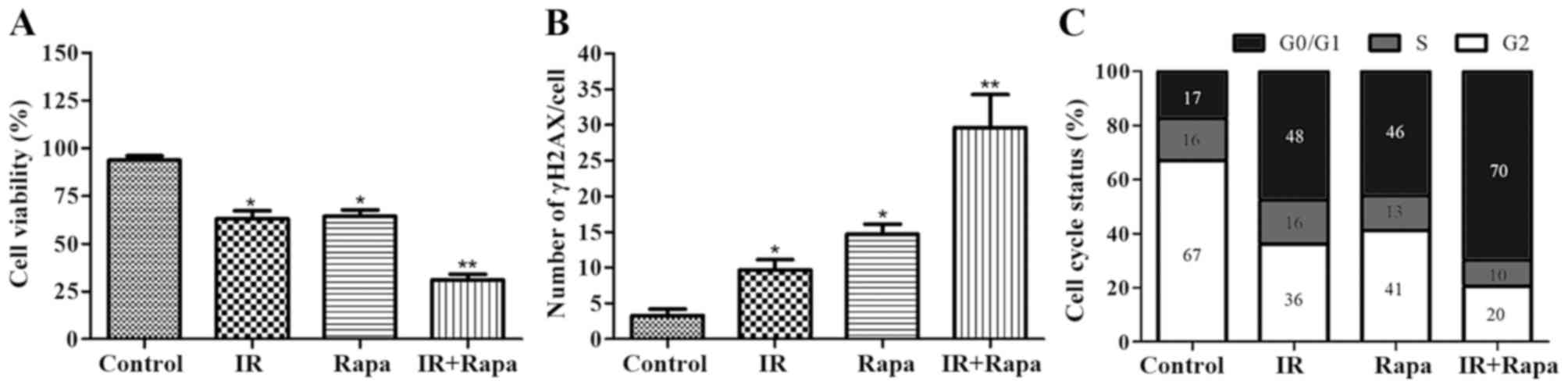
Cell viability, DNA damage and cell
cycle status in HNE1 cells following various treatments
Cell viability was significantly lower, and the
number of residual γH2AX foci was significantly higher, in the
groups subjected to treatment with IR (4 Gy) and/or rapamycin (100
nmol/l) than in the untreated control group (Fig. 2A). Furthermore, as compared with cells
treated with IR alone, lower cell viability and a higher number of
residual γH2AX foci were detected in cells treated with both
rapamycin and IR (Fig. 2B). For cell
cycle status analysis, treatment with both rapamycin and IR led to
an increase in the number of cells in G1 phase and a decrease in
the number of cells in S phase compared with cells treated with
either IR or rapamycin alone, thus indicating an effect of
rapamycin on G1 arrest (Fig. 2C).
Discussion
Although fractionated RT can treat the majority of
NPC patients, the efficacy of RT is limited in certain cases such
as advanced and recurrent NPC (3,18).
Furthermore, fractionated RT may increase the radioresistance of
surviving cancer cells (6,9,19,20). It has been demonstrated that the
activation of the PI3K/Akt signaling pathway serves a vital role in
the radioresistance of head and neck cancer (5). IR induces DNA DSBs, which consequently
activate the PI3K/Akt signaling pathway for DNA DSB repair
(6). This phenomenon was reproduced
in the present study, in which the phosphorylation of Akt at Ser473
in HNE1 cells was increased in a time-dependent manner following
exposure to a dose of 4 Gy of IR. Once the PI3K/Akt signaling
pathway is activated, downstream cascades, including the Akt/mTOR
and Akt/GSK3β/cyclin D1 signaling pathways, are triggered, thus
increasing the growth, proliferation, survival and motility of
cancer cells (5), which eventually
facilitates radioresistance acquirement in head and neck cancer
(21).
Rapamycin is an inhibitor of mTOR, and can prevent
the phosphorylation of GSK3β at serine 9 to consequently increase
cyclin D1 proteolysis (11). Previous
studies have shown that inhibition of these Akt-associated
pro-survival signaling pathways enhances the radiosensitivity to RT
of various cancers (12–15). This improvement may be due to,
firstly, the antifungal characteristics of rapamycin itself, and
secondly, to the excessive autophagy induced by rapamycin (15). In addition, Liu et al (22) demonstrated that leukemia inhibitory
factor (LIF) activated p70 S6 kinase (p70S6K) and its downstream
molecules, GSK3α/β, thus leading to the proliferation of NPC cells.
It is well known that mTOR is the upstream regulator of p70S6K
(7). In the study by Liu et al
(22), mTOR inhibitors, including
rapamycin and everolimus, significantly attenuated the LIF-induced
activation of p70S6K and consequently suppressed the proliferation
of NPC cells. A consistent result was observed in the present
study, since rapamycin alone significantly suppressed the viability
and progression of NPC, and improved the anti-tumor effects of IR
in the treatment of NPC. The present study demonstrated that
rapamycin decreased the phosphorylation of S6 [a downstream target
of mTOR (7)], GSK3β and cyclin D1 [a
downstream target of GSK3β (6)] in
HNE1 cells.
S6, which is activated by phosphorylated p70S6K (a
downstream target of mTOR), is a ribosomal protein and a crucial
component of the translation machinery of messenger RNA (23). Reduction of activated S6 decreases
protein synthesis and cell growth, and prolongs the G1 phase of the
cell cycle (24). Cyclin D1 is an
indispensible factor in the transition from G1 to S phase (25). Additionally, cyclin D1 serves a
critical role in DNA damage repair (26). Cancer cells with radioresistance
highly express cyclin D1, which causes the majority of these cells
to enter S phase, and increases radioresistance and progression
(27). In the present study,
rapamycin decreased the phosphorylation of S6 and the expression of
cyclin D1 in NPC, which resulted in decreased cell viability, G1
phase arrest and increased DNA damage in NPC. Additionally, cells
in the late G1 and G2/M phases are most sensitive to IR, whereas
those in S phase have least radiosensitivity (28). In the present study, rapamycin
arrested NPC cells in G1 phase, and therefore sensitized NPC to RT,
which was supported by the observations that the combination of
rapamycin and IR induced lower growth and higher DNA damage in NPC
cells than either rapamycin or IR alone. These results suggested
that rapamycin can retard NPC growth and improve the
radiosensitivity of NPC through inhibiting both the Akt/mTOR/S6 and
Akt/GSK3β/cyclin D1 pro-survival signaling pathways. A recent phase
I clinical trial documented the safety and tolerance of everolimus,
another mTOR inhibitor, in the treatment of head and neck cancer
when administered in combination with weekly cisplatin and
intensity-modulated RT (29). As a
widely used drug for suppressing autoimmune responses in organ
transplantation, rapamycin is safe and may be used as a
radiosensitizer in the RT of NPC. However, further in vivo
studies using xenografts and immunohistochemical analysis remain to
be conducted to validate the present results.
In conclusion, rapamycin improves the anti-tumor
effect of IR in the treatment of NPC. The findings of the present
study may facilitate further clinical investigations of combination
therapy using rapamycin as a radiosensitizer to improve patient
prognosis in the treatment of NPC.
Acknowledgements
The present study was supported by grants from the
Science and Technology Agency of Hunan Province (grant nos.
2014SK3272 and 2016WK2035), Health Department of Hunan Province
(grant nos. B2014-144 and A2016005) and Changsha Municipal Science
and Technology Bureau (grant no. k1406004-61).
References
|
1
|
Low SY, Tan BS, Choo HL, Tiong KH, Khoo AS
and Leong CO: Suppression of BCL-2 synergizes cisplatin sensitivity
in nasopharyngeal carcinoma cells. Cancer Lett. 314:166–175. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lee AW, Lin JC and Ng WT: Current
management of nasopharyngeal cancer. Semin Radiat Oncol.
22:233–244. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yoshizaki T, Ito M, Murono S, Wakisaka N,
Kondo S and Endo K: Current understanding and management of
nasopharyngeal carcinoma. Auris Nasus Larynx. 39:137–144. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Orlandi E, Tomatis S, Potepan P, Bossi P,
Mongioj V, Carrara M, Palazzi M, Franceschini M, Bergamini C,
Locati L, et al: Critical analysis of locoregional failures
following intensity-modulated radiotherapy for nasopharyngeal
carcinoma. Future Oncol. 9:103–114. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bussink J, van der Kogel AJ and Kaanders
JH: Activation of the PI3-K/AKT pathway and implications for
radioresistance mechanisms in head and neck cancer. Lancet Oncol.
9:288–296. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shimura T: Acquired radioresistance of
cancer and the AKT/GSK3β/cyclin D1 overexpression cycle. J Radiat
Res. 52:539–544. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hay N and Sonenberg N: Upstream and
downstream of mTOR. Genes Dev. 18:1926–1945. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu Y, Chen LH, Yuan YW, Li QS, Sun AM and
Guan J: Activation of AKT is associated with metastasis of
nasopharyngeal carcinoma. Tumour Biol. 33:241–245. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li HF, Kim JS and Waldman T:
Radiation-induced Akt activation modulates radioresistance in human
glioblastoma cells. Radiat Oncol. 4:432009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xia S, Zhao Y, Yu S and Zhang M: Activated
PI3K/Akt/COX-2 pathway induces resistance to radiation in human
cervical cancer HeLa cells. Cancer Biother Radiopharm. 25:317–323.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang HH, Lipovsky AI, Dibble CC, Sahin M
and Manning BD: S6K1 regulates GSK3 under conditions of
mTOR-dependent feedback inhibition of Akt. Mol Cell. 24:185–197.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Albert JM, Kim KW, Cao C and Lu B:
Targeting the Akt/mammalian target of rapamycin pathway for
radiosensitization of breast cancer. Mol Cancer Ther. 5:1183–1189.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shinohara ET, Cao C, Niermann K, Mu Y,
Zeng F, Hallahan DE and Lu B: Enhanced radiation damage of tumor
vasculature by mTOR inhibitors. Oncogene. 24:5414–5422. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cao C, Subhawong T, Albert JM, Kim KW,
Geng L, Sekhar KR, Gi YJ and Lu B: Inhibition of mammalian target
of rapamycin or apoptotic pathway induces autophagy and
radiosensitizes PTEN null prostate cancer cells. Cancer Res.
66:10040–10047. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nassim R, Mansure JJ, Chevalier S, Cury F
and Kassouf W: Combining mTOR inhibition with radiation improves
antitumor activity in bladder cancer cells in vitro and in vivo: A
novel strategy for treatment. PLoS One. 8:e652572013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Abraham RT and Wiederrecht GJ:
Immunopharmacology of rapamycin. Annu Rev Immunol. 14:483–510.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Prevo R, Deutsch E, Sampson O, Diplexcito
J, Cengel K, Harper J, O'Neill P, McKenna WG, Patel S and Bernhard
EJ: Class I PI3 kinase inhibition by the pyridinylfuranopyrimidine
inhibitor PI-103 enhances tumor radiosensitivity. Cancer Res.
68:5915–5923. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Suárez C, Rodrigo JP, Rinaldo A,
Langendijk JA, Shaha AR and Ferlito A: Current treatment options
for recurrent nasopharyngeal cancer. Eur Arch Otorhinolaryngol.
267:1811–1824. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
LoPiccolo J, Blumenthal GM, Bernstein WB
and Dennis PA: Targeting the PI3K/Akt/mTOR pathway: Effective
combinations and clinical considerations. Drug Resist Updat.
11:32–50. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim MK, Kim TJ, Sung CO, Choi CH, Lee JW,
Kim BG and Bae DS: High expression of mTOR is associated with
radiation resistance in cervical cancer. J Gynecol Oncol.
21:181–185. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Romano G: The role of the dysfunctional
akt-related pathway in cancer: Establishment and maintenance of a
malignant cell phenotype, resistance to therapy, and future
strategies for drug development. Scientifica (Cairo).
2013:3171862013.PubMed/NCBI
|
|
22
|
Liu SC, Tsang NM, Chiang WC, Chang KP,
Hsueh C, Liang Y, Juang JL, Chow KP and Chang YS: Leukemia
inhibitory factor promotes nasopharyngeal carcinoma progression and
radioresistance. J Clin Invest. 123:5269–5283. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chung J, Kuo CJ, Crabtree GR and Blenis J:
Rapamycin-FKBP specifically blocks growth-dependent activation of
and signaling by the 70 kd S6 protein kinases. Cell. 69:1227–1236.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Terada N, Takase K, Papst P, Nairn AC and
Gelfand EW: Rapamycin inhibits ribosomal protein synthesis and
induces G1 prolongation in mitogen-activated T lymphocytes. J
Immunol. 155:3418–3426. 1995.PubMed/NCBI
|
|
25
|
Baldin V, Lukas J, Marcote MJ, Pagano M
and Draetta G: Cyclin D1 is a nuclear protein required for cell
cycle progression in G1. Genes Dev. 7:812–821. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jirawatnotai S, Hu Y, Michowski W, Elias
JE, Becks L, Bienvenu F, Zagozdzon A, Goswami T, Wang YE, Clark AB,
et al: A function for cyclin D1 in DNA repair uncovered by protein
interactome analyses in human cancers. Nature. 474:230–234. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shimura T, Fukumoto M and Kunugita N: The
role of cyclin D1 in response to long-term exposure to ionizing
radiation. Cell Cycle. 12:2738–2743. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pawlik TM and Keyomarsi K: Role of cell
cycle in mediating sensitivity to radiotherapy. Int J Radiat Oncol
Biol Phys. 59:928–942. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fury MG, Lee NY, Sherman E, Ho AL, Rao S,
Heguy A, Shen R, Korte S, Lisa D, Ganly I, et al: A phase I study
of everolimus + weekly cisplatin + intensity modulated radiation
therapy in head-and-neck cancer. Int J Radiat Oncol Biol Phys.
87:479–486. 2013. View Article : Google Scholar : PubMed/NCBI
|