Introduction
Cancer is one of the leading causes of mortality
worldwide, and lung cancer is one of the leading causes of
cancer-associated mortality with a 5-year survival rate of ~16%
(1). Representing >85% of lung
cancer cases, non-small cell lung cancer (NSCLC) is the most common
type (2). Of patients with NSCLC,
>2/3 are initially diagnosed at an advanced stage, at which
point palliative chemotherapy is the primary option. Although
30–40% of patients may respond to cytotoxic chemotherapy, the
majority eventually suffer from disease progression (3). The survival rate of patients with
advanced lung adenocarcinoma improved in the early 2000′s, most
probably due to the emergence of inhibitors of epidermal growth
factor receptor, anaplastic lymphoma kinase and vascular
endothelial growth factor (VEGF) (4).
Angiogenesis, a process mainly mediated by VEGF, has
been demonstrated to be crucial for tumor growth, invasion and
metastasis (5); although small tumors
are able to obtain nutrients and oxygen through diffusion, the
formation of new vasculature is critical for tumor expansion and
metastasis (6). The associations
between various measures of tumor aggressiveness and increases in
intratumoral microvessel density illustrate the essential role of
angiogenesis in tumor growth and metastasis (7).
The role of VEGF in angiogenesis has been well
established (8–10). A range of evidence has suggested that
VEGF and its receptors exhibit altered activity in various human
cancer types (11). In general terms,
VEGF expression correlates negatively with clinical outcome
(12–14).
In 1971, it was proposed by Judah Folkman (15) that inhibiting angiogenesis may be an
effective approach to anticancer therapy. More than 30 years after
this original hypothesis, the first proof of anti-angiogenic
therapy in NSCLC came with the approval of bevacizumab, a
monoclonal antibody directed against human VEGF (16). Since then, a series of antibody-based
agents against VEGF have been developed, the majority of which are
undergoing clinical trials (16,17),
including trials of patients with NSCLC (18). Despite promising clinical effects,
resistance to these agents has been reported (19). Investigations into the mechanisms
underlying resistance to anti-VEGF antibodies are warranted in
order for the successful advancement of these promising therapeutic
agents.
Myeloid-derived suppressor cells (MDSCs) are a group
of myeloid cells comprising precursors of macrophages,
granulocytes, dendritic cells and myeloid cells at earlier stages
of differentiation (20–23). In mice, these cells are broadly
defined by their dual expression of Gr-1 and cluster of
differentiation (CD) 11b. Evidence has demonstrated that
tumor-infiltrating MDSCs are present in the peripheral blood of
patients with different types of cancer, including lung, breast,
and head and neck cancer (24,25). These
MDSCs have been suggested to contribute to the development of
resistance to several forms of treatment, including anti-angiogenic
agents that target VEGF receptor (VEGFR) signaling (26–32).
The myeloid differentiation antigen Gr-1 consists of
two epitopes, recognized by anti-lymphocyte antigen (Ly) 6G and
anti-Ly6C antibodies, which divide
CD11b+Gr-1+ MDSCs into Ly6G+
granulocytes and Ly6C+ monocytes (33). These two subpopulations may have
different functions in infectious diseases and cancer (34–36).
Ly6Chi monocytes have been reported to function as
transient accessory cells to enhance angiogenesis and remodeling of
existing small vessels into larger conduits upon their onsite
‘education’ by VEGF (37). There are
two well-established polarized phenotypes of tumor-associated
macrophages (TAMs): Classically activated macrophages (M1) and
alternatively activated macrophages (M2) (38,39). It is
generally accepted that M2 macrophages function in the moderation
of inflammatory responses, promoting angiogenesis and contributing
to tissue remodeling, all of which have been suggested to promote
tumor progression (40–42). Ly6Chi inflammatory
monocytes can also differentiate into M2 macrophages in autoimmune
encephalomyelitis (43) or promote M2
macrophage polarization in acute tissue injury (44).
To better understand the mechanisms underlying
resistance to anti-VEGF antibodies, an ideal model of anti-VEGF is
required. The current study aimed to develop a model of acquired
resistance to B20, a monoclonal antibody against VEGF, in nude mice
following chronic exposure to B20. Subsequently, the migration of
CD11b+Ly6C+ monocytes into the tumor tissue
was detected. These investigations may provide the basis for
strategies to improve the efficacy of anti-VEGF antibody
treatments.
Materials and methods
Preparation of monoclonal antibody
B20
The monoclonal antibody B20 is the mouse equivalent
of bevacizumab (Avastin®), which is able to bind to
mouse and human VEGF (45). B20
antibody was obtained from Genentech, Inc. (San Francisco, CA, USA)
and administered intraperitoneally (150 mg/mouse) every 3 days.
Cell culture and reagents
The human NSCLC cell line A549 was purchased from
the American Type Culture Collection (Manassas, VA, USA) and
cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented
with 10% heat-inactivated fetal bovine serum, 4 mol/l glutamine, 50
U/ml penicillin and 50 mg/ml streptomycin. The cell line was
cultured at 37°C in 5% CO2.
Xenotransplantation experiments
A total of 15 female BALB/c nude mice (age, 4–5
weeks; weight, 200–220 g) were purchased from the Experimental
Animal Center of the Third Military Medical University (Chongqing,
China). They were housed in a laminar flow room with specific
pathogen-free conditions at a temperature of 22±2°C and <40%
humidity, with free access to food and water. A549 cells in the
exponential growth stage were trypsinized, washed twice with
serum-free DMEM and suspended in PBS. The cells
(2×106/0.1 ml) were mixed with 0.1 ml of Matrigel (BD
Biosciences, Franklin Lakes, NJ, USA) and injected subcutaneously
into the lower right flank of the nude mice, establishing a tumor
xenograft model. Tumor growth was monitored and measured with a
vernier caliper, and tumor volume (V) was calculated based on the
formula previously described (46):
V=lengthx(width2)2
Mice inoculated with A549 cells alone were used as
the F0 control group (n=6). The F1 group (n=3) was injected with
A549 cells and treated with B20 twice per week; tumor volume was
monitored over time, and the mice were sacrificed on day 29
post-inoculation. Thereafter, the tumor explants were harvested
from the F1 group, cut into fragments of 2–3 mm in diameter and
transplanted into mice in the F2 generation (n=3), with B20
administered as for the F1 mice. Tumor volumes in the F2 mice were
observed, and the mice were sacrificed on day 21 post-inoculation.
Tumor explants from the previous generation were similarly inserted
into F3 (n=3) mice, with B20 administered, and then F4 mice (n=3),
without B20 treatment. Thereby, a total of five groups of mice were
used in the present study, with each group composed of 3–6 animals.
Following sacrifice of the mice, the volumes of the tumors were
observed in the five groups and the growth curves were drawn. All
animal procedures were conducted with the approval of the Ethical
Committee of Third Military Medical University.
Immunofluorescence microscopy
For immunofluorescence analysis, tumor tissue was
embedded in paraffin and cut into slices of 7-µm thickness for
immunostaining using a Leica RM 2125 rotary microtome (Leica
Microsystems, Inc., Buffalo Grove, IL, USA). The sections were
dewaxed at 60°C, serially immersed in solutions of decreasing
alcohol concentration and subsequently boiled in 10 mM sodium
citrate (pH 6.2) for 30 min for antigen retrieval. The tissue was
then incubated in 3% hydrogen peroxide for 5 min, blocked with 1%
bovine serum albumin and 5% goat serum (both Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) at room temperature for 1 h.
Following 3 washes in cold PBS, the tissue was incubated overnight
at 4°C with primary antibody, followed by incubation with a
secondary antibody at room temperature for 2 h. The nuclei were
stained with DAPI solution (1:1,000; Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany). The primary antibodies used in the present
study included anti-mouse antibodies directed against CD31 (cat.
no., sc-376764), CD11b (cat. no., sc-20050) and Ly6C (cat. no.,
sc-271811; all 1:300 dilution; Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA). DyLight 594 AffiniPure goat anti-mouse IgG (cat.
no., A23410) and DyLight 488 AffiniPure goat anti-mouse IgG (cat.
no., A23210; both 1:1,000 dilution; Abbkine Scientific Co., Ltd.,
Wuhan, China) were used as secondary antibodies. Immunofluorescence
images were captured using a Nikon TE-2000E laser confocal
microscope (magnification, ×200, ×400; Nikon Corporation, Tokyo,
Japan). For analysis, images at ×200 magnification of non-necrotic
and viable tumor regions were obtained. At least 4 sections per
tumor and 3 animals per group were analyzed. Image-Pro Plus
software (MediaCybernetics, Inc., Rockville, MD, USA) was used to
determine area densities or co-localization, as described
previously (47).
Statistical analysis
The statistical significance of each set of
experimental results was assessed using an analysis of variance or
a Student's t-test. All statistical analyses were performed using
SPSS v17.0 (SPSS Inc., Chicago, IL, USA). All data are presented as
the mean ± standard deviation. P<0.05 was considered to indicate
a statistically significant difference.
Results
Tumor growth curves
To determine whether resistance to the anti-VEGF
antibody was acquired in a xenograft model, A549 cells were
injected into the mice and the antibody administered generation by
generation. Tumor growth curves were produced for each group and
the tumor growth rates between different groups were compared
(Fig. 1). At the endpoint of
observation (day 21), the tumor volume was significantly larger in
the F0 group compared with in F1, F2, and F3 groups (all
P<0.01), suggesting continuous inhibition of tumor growth by the
anti-VEGF B20 antibody. Although the antibody reduced the growth of
xenograft tumors, the tumors grew progressively in F1, F2 and F3
groups during the course of the experiment; complete inhibition of
tumor growth was not observed. In the F4 group, the tumor volume
was significantly larger compared with that in the F1 and F2 groups
(P<0.05), and was almost the same as in F0, reflecting the
removal of anti-VEGF antibody-mediated inhibition. Overall, these
findings indicated that the NSCLC xenograft model of acquired
resistance to anti-VEGF antibody had not been successfully
established.
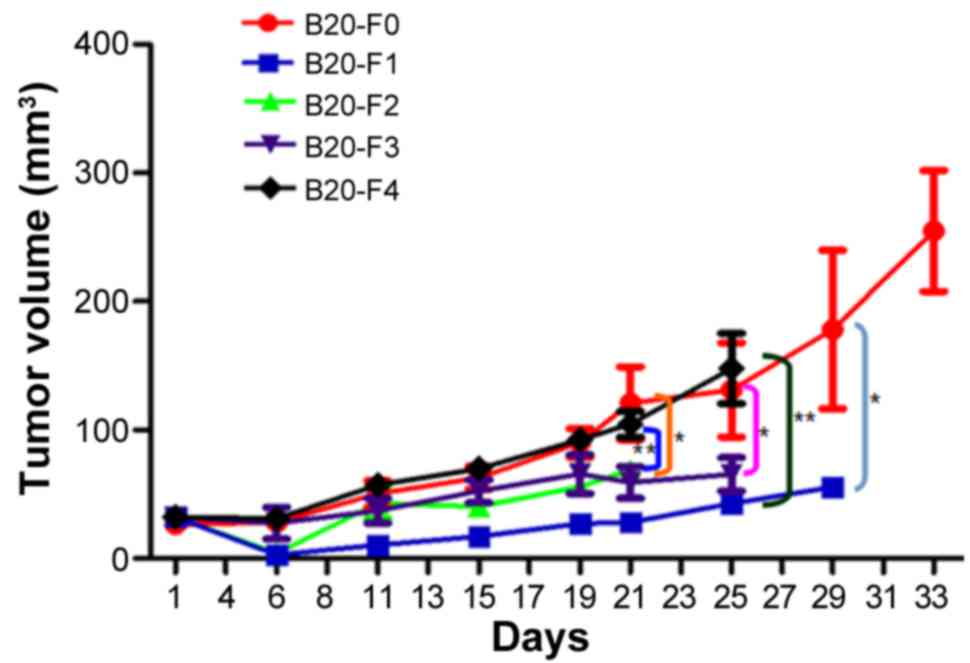 | Figure 1.Tumor growth curves of non-small cell
lung cancer xenografts. In the F1-F3 groups, the tumor sizes were
similar, indicating the continuous inhibitory effects of anti-VEGF
antibody on tumor growth. The tumor growth rate was significantly
lower in the F3 group compared with in the F0 (control) group
(P<0.01), suggesting that resistance to the B20 antibody was not
successfully acquired. The tumor growth rate in the F4 group was
similar to that in F0, indicating the withdrawal of inhibition by
the anti-VEGF antibody. *P<0.01, F0 vs. F1/F2/F3; **P<0.05,
F4 vs. F1/F2; n=3-6. VEGF, vascular endothelial growth factor; F0,
mice injected with A549 cells only; F1, mice injected with A549
cells and treated with B20 twice weekly; F2, mice transplanted with
F1 tumor explant and treated with B20 twice weekly; F3, mice
transplanted with F2 tumor explant and treated with B20 twice
weekly; F4, mice transplanted with F3 tumor explant with no
treatment. |
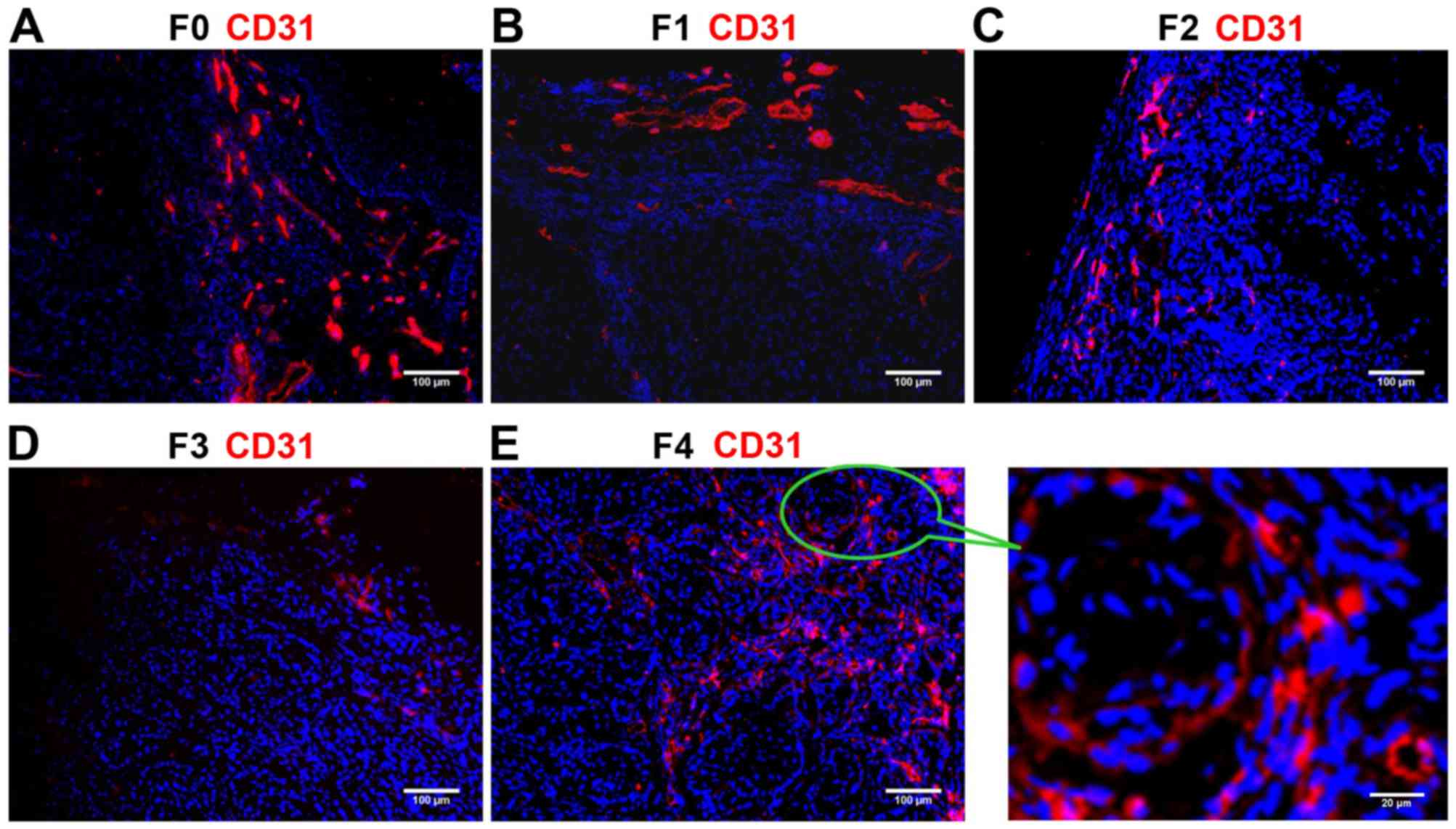
Detection of CD31 staining
The ratio of endothelial cells (CD31-positive, red)
to all cells in the field of vision (DAPI-positive, blue) was
calculated to evaluate the vessel density and degree of
angiogenesis. Immunostaining revealed that CD31 expression was
higher in the F0 (control) and F4 groups compared with that in the
F1-F3 groups (Fig. 2). CD31
expression progressively reduced from F1 (Fig. 2B) to F2 (Fig. 2C) to F3 (Fig. 2D), indicating the decreasing degree of
angiogenesis and the inhibitory effects of the anti-VEGF antibody.
The ratio of CD31/nucleus continuously decreased from the F1 to F2
to F3 groups, and was significantly lower in each of these three
groups compared with in the F0 and F4 groups (all P<0.01;
Table I). These results suggested
that the B20 anti-VEGF antibody effectively inhibited blood vessel
formation in the F1, F2 and F3 groups. Withdrawal of the drug from
F4 group partially reversed the suppression of angiogenesis, but
the lower vessel density in the F3 group compared with that in the
F0 control indicated unsuccessful establishment of the anti-VEGF
resistance model.
 | Table I.CD31 and CD11b ratios in non-small
cell lung cancer xenografts. |
Table I.
CD31 and CD11b ratios in non-small
cell lung cancer xenografts.
| Group | CD31 ratio | CD11b ratio |
|---|
| F0 |
0.18±0.01a |
0.16±0.02a |
| F1 | 0.11±0.01 | 0.10±0.01 |
| F2 | 0.07±0.02 | 0.09±0.03 |
| F3 | 0.05±0.01 | 0.06±0.01 |
| F4 |
0.16±0.02a |
0.15±0.03a |
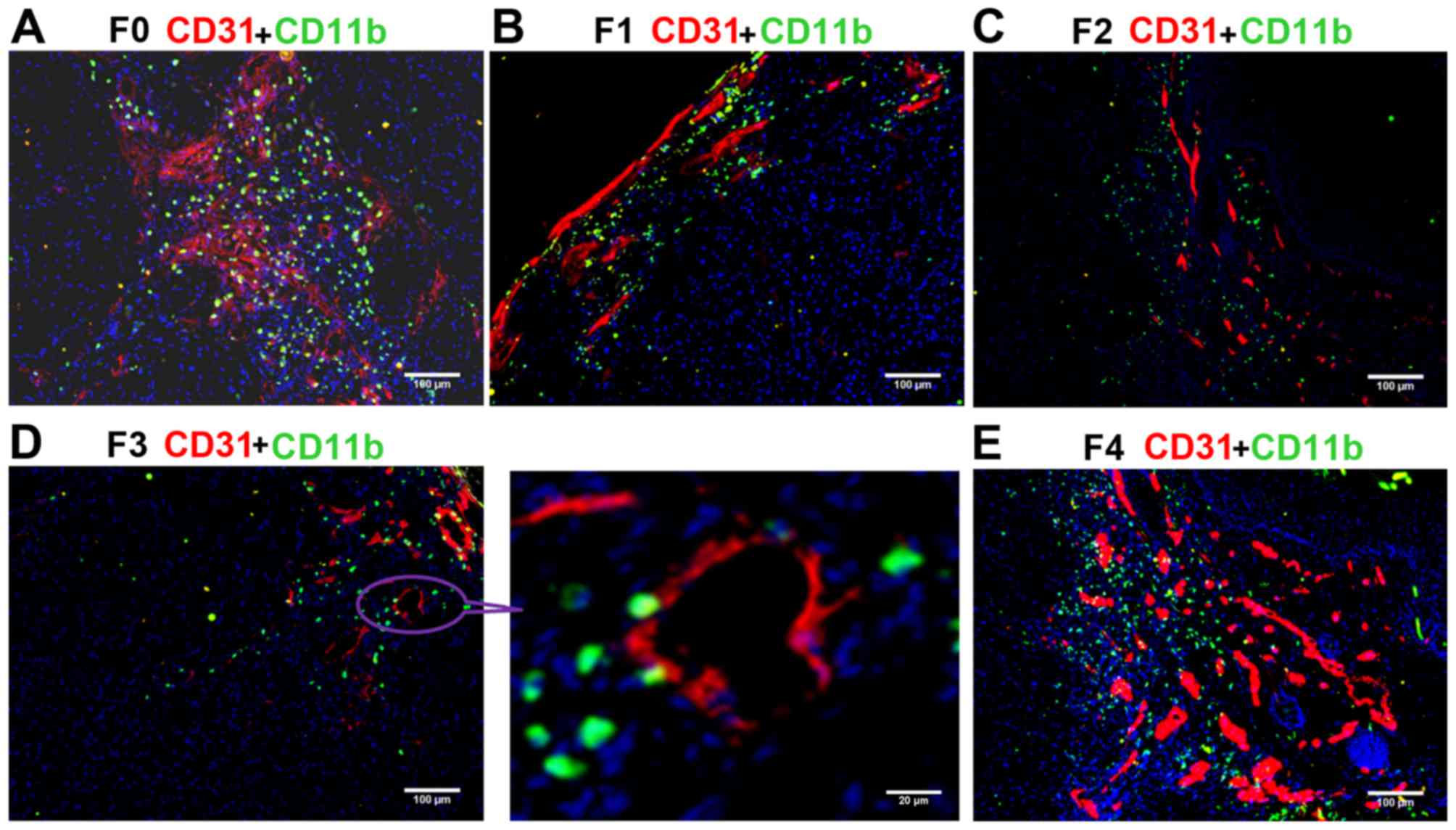
Assessment of the migration of
CD11b+ myeloid cells
The ratio of CD11b+ myeloid cells (green)
to cells in the field of vision (blue) was calculated. The blood
vessels were simultaneously marked by CD31. Confocal microscopy
demonstrated that the number of CD11b+ myeloid cells was
markedly higher in the F0 (control) and F4 groups compared with in
the other groups (Fig. 3), and
continuously decreased from F1 (Fig.
3B) to F2 (Fig. 3C) to F3
(Fig. 3D), suggesting the inhibition
of MDSC migration by the anti-VEGF antibody. The ratio of
CD11b/nucleus (CD11b ratio) progressively reduced from the F1 to F2
to F3 groups, and the CD11b ratio was significantly lower in each
of these three B20-treated groups compared with in the F0 and F4
groups (all P<0.01; Table I). The
changes observed in the number of CD11b+ cells were
consistent with those of CD31+ endothelial cells. These
results indicated that anti-VEGF antibody effectively decreased the
recruitment of MDSCs to the tumor tissue, thus inhibiting the
vessel formation in F1, F2, and F3 groups. The lower presence of
CD11b+ myeloid cells in the F3 compared with the F0
group demonstrated that drug resistance was not successfully
induced.
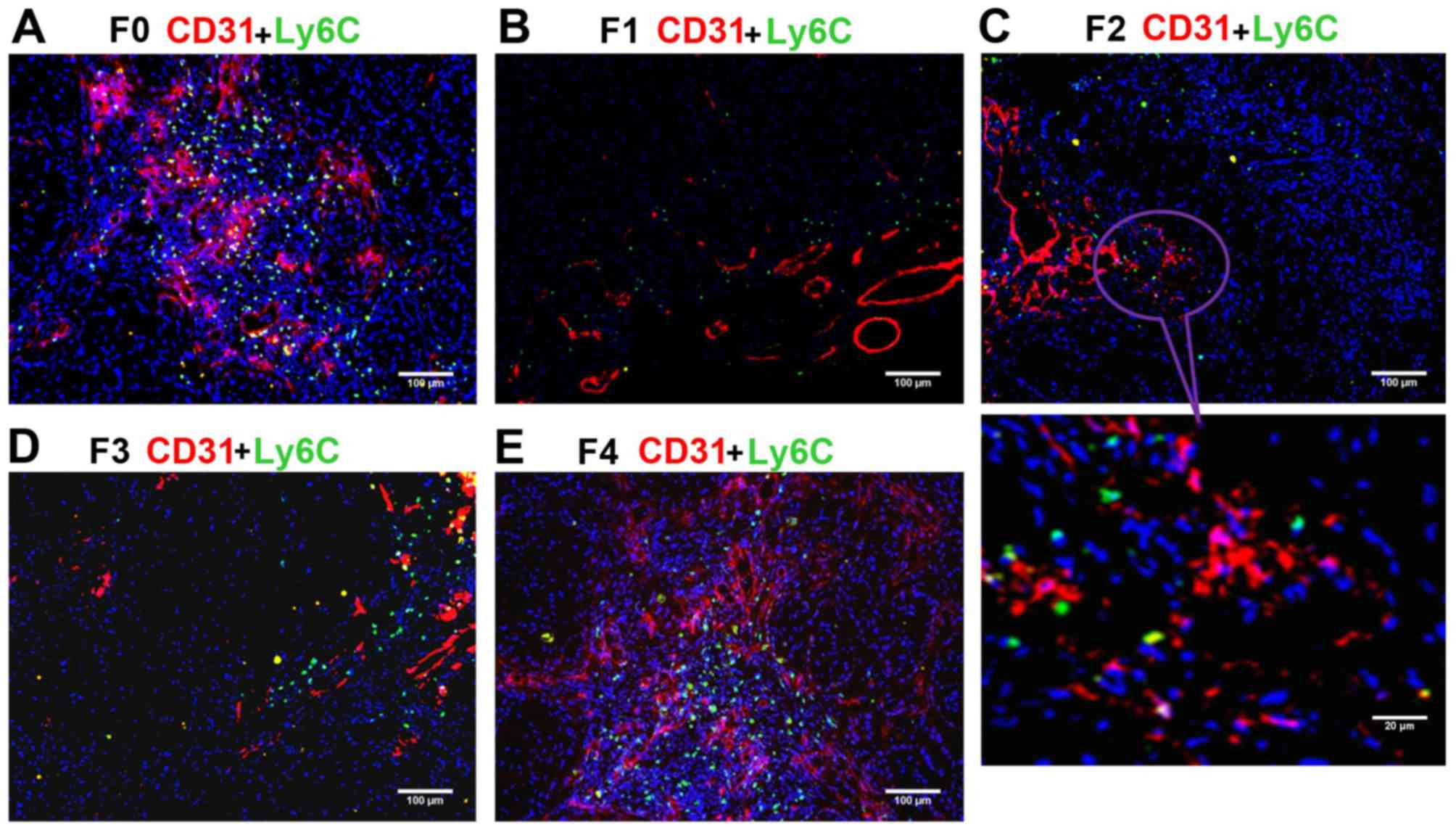
Assessment of Ly6C+ subset
of CD11b+ MDSCs
The anti-Ly6C antibody was used to mark the
Ly6C+ subset of CD11b+ MDSCs, which may serve
an essential role in promoting angiogenesis and tumor progression.
The ratio of Ly6C+ cells (green) to all cells in the
field of vision (blue) was calculated. The blood vessels were
simultaneously marked by staining of CD31. Under a confocal
microscope, the largest numbers of Ly6C+ monocytes were
present in the F0 (control) and F4 groups (Fig. 4), and the number of Ly6C+
monocytes decreased from F1 (Fig. 4B)
to F2 (Fig. 4C) to F3 (Fig. 4D), indicating inhibitory effects of
the anti-VEGF antibody on Ly6C+ monocyte migration and
angiogenesis. Consistent with the trend for CD11b+
cells, the percentage of Ly6C+ monocytes in the field of
vision (Ly6C ratio) continuously decreased from the F1 to F2 to F3
groups, and was significantly lower in each of these three groups
compared with in the F0 and F4 groups (P<0.01; Table II). These data indicated that
anti-VEGF antibody repressed the migration of MDSCs, including the
Ly6C+ subpopulation, to the tumor tissue, and that drug
resistance had not been acquired. To further determine the
migration tendency of Ly6C+CD11b+ monocytes,
the ratio of Ly6C+ monocytes to CD11b+ MDSCs
(Ly6C/CD11b) was calculated. Notably, it was identified that the
Ly6C/CD11b ratio was highest in the F3 group and significantly
higher in F3 compared with in F0, F1, and F4 groups (all P<0.01)
(Table II). These findings suggested
that the migration tendency of the Ly6C+ subset
relatively increased with the increasing mouse generation compared
with the other subsets in the whole CD11b+ population.
Thus, if the generations of nude mice or the tumor growth period
(between inoculation and harvesting) is increased, it is possible
that the xenograft model of acquired resistance to anti-VEGF
antibody may be successfully established.
 | Table II.Ly6C and Ly6C/CD11b ratios in
non-small cell lung cancer xenografts. |
Table II.
Ly6C and Ly6C/CD11b ratios in
non-small cell lung cancer xenografts.
| Group | CD31 ratio | Ly6C ratio |
Ly6C/CD11b |
|---|
| F0 |
0.18±0.03a |
0.06±0.01a | 0.38±0.05 |
| F1 | 0.10±0.02 | 0.05±0.01 | 0.44±0.10 |
| F2 | 0.06±0.02 | 0.04±0.01 | 0.51±0.13 |
| F3 | 0.04±0.01 | 0.04±0.00 |
0.68±0.11b |
| F4 |
0.17±0.02a |
0.06±0.01a | 0.40±0.10 |
Discussion
Molecular inhibition of VEGF/VEGFR signaling is
currently being investigated as a promising cancer treatment
strategy. For lung cancer, one study has demonstrated that
angiogenesis inhibitors are superior to non-angiogenesis inhibitors
with regard to objective response, disease control,
progression-free survival and overall survival rates in patients
with advanced NSCLC (18). The
advantages of anti-angiogenesis therapy have mostly been
demonstrated with antibody-based agents (48). Monoclonal antibodies against VEGF,
such as bevacizumab, have demonstrated efficacy when used alone or
in combination with chemotherapy (49). However, modest effects have been
reported and drug resistance has been widely observed in
preclinical and clinical trials (17,50,51). Thus,
efforts to better understand the mechanisms underlying acquired
resistance to anti-VEGF antibodies and potential strategies to
overcome resistance are warranted.
In the present study, the aim was to establish an
NSCLC xenograft model of acquired resistance to anti-VEGF antibody
in nude mice, in order to provide a tool for mechanism
investigation and future research, and then to detect the
expression of CD31 and the migration of
CD11b+Ly6C+ monocytes. In the
xenotransplantation experiments, tumor growth rate in the last
generation of drug administration (F3) was lower compared with that
in the control (F0). Following immunostaining, the expression of
CD31 was revealed to be lower in the F3 group compared with that in
the F0 group. These data indicated that the drug resistance model
had not been successfully established.
It was hypothesized that these negative data may
result from the changing microenvironment in distinct generations
of mice. It has been recognized that the development of resistance
to anti-angiogenesis agents is largely associated with the tumor
microenvironment, including stromal cells, extracellular
matrix-components, TAMs, and autocrine and paracrine signaling
factors (52). Tumor cells
communicate with components of their microenvironment via a complex
network of growth factors, cytokines and chemokines. TAMs support
lung cancer progression by inducing cancer cell motility and
metastasis, and angiogenesis (52,53). As a
result, changing the microenvironment of the tumor leads to updated
recruitment of accessory cells and molecules, thus decreasing the
tumor growth rate in xenografts. Therefore, elongation of the
period between tumor inoculation and harvesting may compensate for
the change in microenvironment. In the present study, the mice were
sacrificed on day 29 post-inoculation in F1 group, on day 21 in F2
group, day 25 in F3 group, and day 25 in F4 group. However, in a
similar study, Gyanchandani et al (54) generated a head and neck squamous cell
carcinoma xenograft model of acquired resistance to bevacizumab, in
which the time for a generation was ≥56 days. In the study by
Curtarello et al (55), mice
were maintained for ≥45 days after tumor inoculation to induce
resistance to bevacizumab in ovarian and breast cancer cells. In
the present study, the Ly6C/CD11b ratio increased from the F1 to
the F3 group. A larger number of generations may promote the
successful acquisition of resistance to anti-VEGF antibody by tumor
cells. The short time and low generation numbers of the present
study were its limitations.
However, the ratio of Ly6C/CD11b was higher in the
F3 group compared with that in the other groups, suggesting an
enhanced migration tendency of the Ly6C+ subset. This
subset is a population of cells that are able to polarize into M2
macrophages and serve a role in promoting angiogenesis (38,39). The
primary functions of M2 macrophages are limitation of the immune
response and promotion of tumor invasion, growth and metastasis via
the secretion of inhibitory cytokines and the prevention of T cells
from exerting antitumor effects (56). Although Ly6C+ and
Ly6G+ MDSC numbers are equally increased in
tumor-bearing mice (36), the
Ly6C+ subset has a greater tendency to polarize into M2
macrophages following proper stimulation. In contrast to these
reports, Ly6Chi monocytes are preferentially recruited
to inflamed tissues in a C-C motif chemokine receptor-2-dependent
manner and generate inflammatory macrophages, such as M1
macrophages, as described in myocardial infarction (57), muscle injury (58), and bacterial infection (59). Ly6Chi monocytes digest
damaged tissue, whereas Ly6Clo monocytes promote healing
via myofibroblast accumulation, angiogenesis and deposition of
collagen (57). It appears that
Ly6Chi monocytes cooperate with M1 macrophages in
inflammatory functions, whereas Ly6Clo monocytes work
together with M2 macrophages to achieve angiogenic functions
(60). Notably, Ly6Chi
monocytes can give rise to Ly6Clo monocytes under
steady-state conditions (61–63). Therefore, regardless of whether M2
macrophages derive from Ly6Chi or Ly6Clo
monocytes, increased recruitment of Ly6Chi monocytes
indicates enhanced angiogenesis.
Although Shojaei et al (30) did not provide definitive evidence of
macrophage involvement in tumor refractoriness following anti-VEGF
therapy, it was revealed that tumor relapse is affected by the
heterogeneous CD11b+Gr-1+ MDSCs; the
combination of anti-VEGF and anti-Gr-1 antibodies given to
tumor-bearing mice was more effective in preventing angiogenesis
and slowing tumor growth compared with either antibody alone. Since
the Gr-1 antibody recognizes Ly6C, a receptor expressed on
inflammatory monocytes, and Ly6G, it can be inferred that
monocytes/macrophages may be partially responsible for
refractoriness following anti-angiogenic therapy. Notably,
resistance to conventional chemotherapies did not involve
CD11b+Gr-1+ MDSCs in these models, suggesting
that myeloid cells specifically initiate refractoriness to
anti-angiogenic therapies (30).
Other studies have demonstrated upregulation of VEGF
expression in macrophages following radiotherapy in patients,
suggesting that increased levels of TAM-derived pro-angiogenic
factors can stimulate the formation of a new blood supply to
radio-resistant tumor cells (64). In
agreement with these data, Ahn et al (65) revealed the important contribution of
matrix metallopeptidase 9-expressing CD11b+ myeloid
cells to tumor revascularization and recovery following radiation.
Taken together, these findings indicate that inhibiting monocyte
recruitment to tumors or neutralizing the factors that they produce
in tumors, in combination with conventional therapeutic agents, may
have considerable therapeutic potential.
In conclusion, in the current study, the increased
migration tendency of CD11b+Ly6C+ myeloid
cells suggests the potential for successful resistance acquisition
and implies a possible contribution of these cells to tumor
refractoriness. Increasing the number of generations or the time
for post-inoculation tumor growth may generate an NSCLC model of
acquired resistance to the anti-VEGF antibody. The role of
CD11b+Ly6C+ monocytes in angiogenesis and
their association with M2 macrophages may have implications for
improving the efficacy of anti-VEGF therapies.
Acknowledgements
The present study was supported by the National
Science Foundation of China (grant nos. 30772108 and 81272910) and
the Chongqing Natural Science Foundation (grant no.
cstc2012jjA10096). The authors would like to thank Drs J. Martin
Brown and Sophia (School of Medicine, Stanford University,
Stanford, CA, USA) for their instructions, and Mr. Dian-Gang Chen
(Cancer Institute of People's Liberation Army, Xinqiao Hospital,
Third Military Medical University, Chongqing, China) for guidance
on experimental techniques.
Glossary
Abbreviations
Abbreviations:
|
NSCLC
|
non-small cell lung cancer
|
|
VEGF
|
vascular endothelial growth factor
|
|
VEGFR
|
vascular endothelial growth factor
receptor
|
|
MDSC
|
myeloid-derived suppressor cell
|
|
CD
|
cluster of differentiation
|
|
TAM
|
tumor-associated macrophage
|
References
|
1
|
Allemani C, Weir HK, Carreira H, Harewood
R, Spika D, Wang XS, Bannon F, Ahn JV, Johnson CJ, Bonaventure A,
et al: Global surveillance of cancer survival 1995–2009: Analysis
of individual data for 25,676,887 patients from 279
population-based registries in 67 countries (CONCORD-2). Lancet.
385:977–1010. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Stinchcombe TE and Socinski MA: Current
treatments for advanced stage non-small cell lung cancer. Proc Am
Thorac Soc. 6:pp. 233–241. 2009; View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Morgensztern D, Waqar S, Subramanian J,
Gao F and Govindan R: Improving survival for stage IV non-small
cell lung cancer: A surveillance, epidemiology, and end results
survey from 1990 to 2005. J Thorac Oncol. 4:1524–1529. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zetter BR: Angiogenesis and tumor
metastasis. Annu Rev Med. 49:407–424. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Carmeliet P: Angiogenesis in life, disease
and medicine. Nature. 438:932–936. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Weidner N: Intratumor microvessel density
as a prognostic factor in cancer. Am J Pathol. 147:9–19.
1995.PubMed/NCBI
|
|
8
|
Dvorak HF, Sioussat TM, Brown LF, Berse B,
Nagy JA, Sotrel A, Manseau EJ, Van de Water L and Senger DR:
Distribution of vascular permeability factor (vascular endothelial
growth factor) in tumors: Concentration in tumor blood vessels. J
Exp Med. 174:1275–1278. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim KJ, Li B, Winer J, Armanini M, Gillett
N, Phillips HS and Ferrara N: Inhibition of vascular endothelial
growth factor-induced angiogenesis suppresses tumour growth in
vivo. Nature. 362:841–844. 1993. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Toi M, Matsumoto T and Bando H: Vascular
endothelial growth factor: Its prognostic, predictive, and
therapeutic implications. Lancet Oncol. 2:667–673. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ferrara N: Vascular endothelial growth
factor as a target for anticancer therapy. Oncologist. 9 Suppl
1:S2–S10. 2004. View Article : Google Scholar
|
|
12
|
Hu P, Liu W, Wang L, Yang M and Du J: High
circulating VEGF level predicts poor overall survival in lung
cancer. J Cancer Res Clin Oncol. 139:1157–1167. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen P, Zhu J, Liu DY, Li HY, Xu N and Hou
M: Over-expression of survivin and VEGF in small-cell lung cancer
may predict the poorer prognosis. Med Oncol. 31:7752014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen J, Tang D, Wang S, Li QG, Zhang JR,
Li P, Lu Q, Niu G, Gao J, Ye NY and Wang DR: High expressions of
galectin-1 and VEGF are associated with poor prognosis in gastric
cancer patients. Tumor Biol. 35:2513–2519. 2014. View Article : Google Scholar
|
|
15
|
Folkman J: Tumor angiogenesis: Therapeutic
implications. N Engl J Med. 285:1182–1186. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Piperdi B, Merla A and Perez-Soler R:
Targeting angiogenesis in squamous non-small cell lung cancer.
Drugs. 74:403–413. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pallis AG and Syrigos KN: Targeting tumor
neovasculature in non-small-cell lung cancer. Crit Rev Oncol
Hematol. 86:130–142. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhou C, Wu YL, Chen G, Liu X, Zhu Y, Lu S,
Feng J, He J, Han B, Wang J, et al: BEYOND: A randomized,
double-blind, placebo-controlled, multicenter, phase III study of
first-line carboplatin/paclitaxel plus bevacizumab or placebo in
Chinese patients with advanced or recurrent nonsquamous
non-small-cell lung cancer. J Clin Oncol. 33:2197–2204. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tejpar S, Prenen H and Mazzone M:
Overcoming resistance to antiangiogenic therapies. Oncologist.
17:1039–1050. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sica A and Bronte V: Altered macrophage
differentiation and immune dysfunction in tumor development. J Clin
Invest. 117:1155–1166. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kusmartsev S and Gabrilovich DI: Role of
immature myeloid cells in mechanisms of immune evasion in cancer.
Cancer Immunol Immunother. 55:237–245. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rabinovich GA, Gabrilovich D and Sotomayor
EM: Immunosuppressive strategies that are mediated by tumor cells.
Annu Rev Immunol. 25:267–296. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Talmadge JE: Pathways mediating the
expansion and immunosuppressive activity of myeloid-derived
suppressor cells and their relevance to cancer therapy. Clin Cancer
Res. 13:5243–5248. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Almand B, Clark JI, Nikitina E, van Beynen
J, English NR, Knight SC, Carbone DP and Gabrilovich DI: Increased
production of immature myeloid cells in cancer patients: A
mechanism of immunosuppression in cancer. J Immunol. 166:678–689.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Young MR and Lathers DM: Myeloid
progenitor cells mediate immune suppression in patients with head
and neck cancers. Int J Immunopharmacol. 21:241–252. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ebos JM, Lee CR and Kerbel RS: Tumor and
host-mediated pathways of resistance and disease progression in
response to antiangiogenic therapy. Clin Cancer Res. 15:5020–5025.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kioi M, Vogel H, Schultz G, Hoffman RM,
Harsh GR and Brown JM: Inhibition of vasculogenesis, but not
angiogenesis, prevents the recurrence of glioblastoma after
irradiation in mice. J Clin Invest. 120:694–705. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yang L, Huang J, Ren X, Gorska AE, Chytil
A, Aakre M, Carbone DP, Matrisian LM, Richmond A, Lin PC and Moses
HL: Abrogation of TGF beta signaling in mammary carcinomas recruits
Gr-1+CD11b+ myeloid cells that promote metastasis. Cancer Cell.
13:23–35. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chan DA, Kawahara TL, Sutphin PD, Chang
HY, Chi JT and Giaccia AJ: Tumor vasculature is regulated by
PHD2-mediated angiogenesis and bone marrow-derived cell
recruitment. Cancer Cell. 15:527–538. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shojaei F, Wu X, Malik AK, Zhong C,
Baldwin ME, Schanz S, Fuh G, Gerber HP and Ferrara N: Tumor
refractoriness to anti-VEGF treatment is mediated by CD11b+Gr1+
myeloid cells. Nat Biotechnol. 25:911–920. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yang L, DeBusk LM, Fukuda K, Fingleton B,
Green-Jarvis B, Shyr Y, Matrisian LM, Carbone DP and Lin PC:
Expansion of myeloid immune suppressor Gr+CD11b+ cells in
tumor-bearing host directly promotes tumor angiogenesis. Cancer
Cell. 6:409–421. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shojaei F, Wu X, Zhong C, Yu L, Liang XH,
Yao J, Blanchard D, Bais C, Peale FV, van Bruggen N, et al: Bv8
regulates myeloid-cell-dependent tumour angiogenesis. Nature.
450:825–831. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Youn JI, Nagaraj S, Collazo M and
Gabrilovich DI: Subsets of myeloid-derived suppressor cells in
tumor-bearing mice. J Immunol. 181:5791–5802. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sawanobori Y, Ueha S, Kurachi M, Shimaoka
T, Talmadge JE, Abe J, Shono Y, Kitabatake M, Kakimi K, Mukaida N
and Matsushima K: Chemokine-mediated rapid turnover of
myeloid-derived suppressor cells in tumor-bearing mice. Blood.
111:5457–5466. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Dietlin TA, Hofman FM, Lund BT, Gilmore W,
Stohlman SA and van der Veen RC: Mycobacteria-induced Gr-1+ subsets
from distinct myeloid lineages have opposite effects on T cell
expansion. J Leukoc Biol. 81:1205–1212. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Movahedi K, Guilliams M, Van den Bossche
J, Van den Bergh R, Gysemans C, Beschin A, De Baetselier P and Van
Ginderachter JA: Identification of discrete tumor-induced
myeloid-derived suppressor cell subpopulations with distinct T
cell-suppressive activity. Blood. 111:4233–4244. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Avraham-Davidi I, Yona S, Grunewald M,
Landsman L, Cochain C, Silvestre JS, Mizrahi H, Faroja M,
Strauss-Ayali D, Mack M, et al: On-site education of VEGF-recruited
monocytes improves their performance as angiogenic and arteriogenic
accessory cells. J Exp Med. 210:2611–2625. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gordon S: Alternative activation of
macrophages. Nat Rev Immunol. 3:23–35. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Gordon S and Taylor PR: Monocyte and
macrophage heterogeneity. Nat Rev Immunol. 5:953–964. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Murdoch C, Muthana M, Coffelt SB and Lewis
CE: The role of myeloid cells in the promotion of tumour
angiogenesis. Nat Rev Cancer. 8:618–631. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Mantovani A, Allavena P, Sica A and
Balkwill F: Cancer-related inflammation. Nature. 454:436–444. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Comito G, Giannoni E, Segura CP,
Barcellos-de-Souza P, Raspollini MR, Baroni G, Lanciotti M, Serni S
and Chiarugi P: Cancer-associated fibroblasts and M2-polarized
macrophages synergize during prostate carcinoma progression.
Oncogene. 33:2423–2431. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Denney L, Kok WL, Cole SL, Sanderson S,
McMichael AJ and Ho LP: Activation of invariant NKT cells in early
phase of experimental autoimmune encephalomyelitis results in
differentiation of Ly6Chi inflammatory monocyte to M2 macrophages
and improved outcome. J Immunol. 189:551–557. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Chu HX, Broughton BR, Kim HA, Lee S,
Drummond GR and Sobey CG: Evidence that Ly6C(hi) monocytes are
protective in acute ischemic stroke by promoting M2 macrophage
polarization. Stroke. 46:1929–1937. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Jalali S, Chung C, Foltz W, Burrell K,
Singh S, Hill R and Zadeh G: MRI biomarkers identify the
differential response of glioblastoma multiforme to anti-angiogenic
therapy. Neuro Oncol. 16:868–879. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Naito S, von Eschenbach AC, Giavazzi R and
Fidler IJ: Growth and metastasis of tumor cells isolated from a
human renal cell carcinoma implanted into different organs of nude
mice. Cancer Res. 46:4109–4115. 1986.PubMed/NCBI
|
|
47
|
Ahn GO, Tseng D, Liao CH, Dorie MJ,
Czechowicz A and Brown JM: Inhibition of Mac-1 (CD11b/CD18)
enhances tumor response to radiation by reducing myeloid cell
recruitment. Proc Natl Acad Sci USA. 107:pp. 8363–8368. 2010;
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Hong S, Tan M, Wang S, Luo S, Chen Y and
Zhang L: Efficacy and safety of angiogenesis inhibitors in advanced
non-small cell lung cancer: A systematic review and meta-analysis.
J Cancer Res Clin Oncol. 141:909–921. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Blakely C and Jahan T: Emerging
antiangiogenic therapies for non-small-cell lung cancer. Expert Rev
Anticancer Ther. 11:1607–1618. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Giuliano S and Pagès G: Mechanisms of
resistance to anti-angiogenesis therapies. Biochimie. 95:1110–1119.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Sitohy B, Nagy JA and Dvorak HF:
Anti-VEGF/VEGFR therapy for cancer: Reassessing the target. Cancer
Res. 72:1909–1914. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Wood SL, Pernemalm M, Crosbie PA and
Whetton AD: The role of the tumor-microenvironment in lung
cancer-metastasis and its relationship to potential therapeutic
targets. Cancer Treat Rev. 40:558–566. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Saintigny P and Burger JA: Recent advances
in non-small cell lung cancer biology and clinical management.
Discov Med. 13:287–297. 2012.PubMed/NCBI
|
|
54
|
Gyanchandani R, Alves MV Ortega, Myers JN
and Kim S: A proangiogenic signature is revealed in FGF-mediated
bevacizumab-resistant head and neck squamous cell carcinoma. Mol
Cancer Res. 11:1585–1596. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Curtarello M, Zulato E, Nardo G, Valtorta
S, Guzzo G, Rossi E, Esposito G, Msaki A, Pastò A, Rasola A, et al:
VEGF-targeted therapy stably modulates the glycolytic phenotype of
tumor cells. Cancer Res. 75:120–133. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Mira E, Carmona-Rodríguez L, Tardáguila M,
Azcoitia I, González-Martín A, Almonacid L, Casas J, Fabriás G and
Mañes S: A lovastatin-elicited genetic program inhibits M2
macrophage polarization and enhances T cell infiltration into
spontaneous mouse mammary tumors. Oncotarget. 4:2288–2301. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Nahrendorf M, Swirski FK, Aikawa E,
Stangenberg L, Wurdinger T, Figueiredo JL, Libby P, Weissleder R
and Pittet MJ: The healing myocardium sequentially mobilizes two
monocyte subsets with divergent and complementary functions. J Exp
Med. 204:3037–3047. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Arnold L, Henry A, Poron F, Baba-Amer Y,
van Rooijen N, Plonquet A, Gherardi RK and Chazaud B: Inflammatory
monocytes recruited after skeletal muscle injury switch into
antiinflammatory macrophages to support myogenesis. J Exp Med.
204:1057–1069. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Auffray C, Fogg D, Garfa M, Elain G,
Join-Lambert O, Kayal S, Sarnacki S, Cumano A, Lauvau G and
Geissmann F: Monitoring of blood vessels and tissues by a
population of monocytes with patrolling behavior. Science.
317:666–670. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Anzai A, Anzai T, Nagai S, Maekawa Y,
Naito K, Kaneko H, Sugano Y, Takahashi T, Abe H, Mochizuki S, et
al: Regulatory role of dendritic cells in postinfarction healing
and left ventricular remodeling. Circulation. 125:1234–1245. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Varol C, Landsman L, Fogg DK, Greenshtein
L, Gildor B, Margalit R, Kalchenko V, Geissmann F and Jung S:
Monocytes give rise to mucosal, but not splenic, conventional
dendritic cells. J Exp Med. 204:171–180. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Ancuta P, Liu KY, Misra V, Wacleche VS,
Gosselin A, Zhou X and Gabuzda D: Transcriptional profiling reveals
developmental relationship and distinct biological functions of
CD16+ and CD16- monocyte subsets. BMC Genomics. 10:4032009.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Hettinger J, Richards DM, Hansson J, Barra
MM, Joschko AC, Krijgsveld J and Feuerer M: Origin of monocytes and
macrophages in a committed progenitor. Nat Immunol. 14:821–830.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
McDonnell CO, Bouchier-Hayes DJ, Toomey D,
Foley D, Kay EW, Leen E and Walsh TN: Effect of neoadjuvant
chemoradiotherapy on angiogenesis in oesophageal cancer. Br J Surg.
90:1373–1378. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Ahn GO and Brown JM: Matrix
metalloproteinase-9 is required for tumor vasculogenesis but not
for angiogenesis: Role of bone marrow-derived myelomonocytic cells.
Cancer Cell. 13:193–205. 2008. View Article : Google Scholar : PubMed/NCBI
|