Introduction
Nasopharyngeal carcinoma (NPC) is a distinctive type
of head and neck malignant neoplasm that occurs globally, but is
geographically distributed to a higher extent within South China
and South East Asia. NPC is highly associated with Epstein-Barr
virus (EBV) infection (1–5).
A number of different cell types can be infected by
EBV, including B cells and epithelial cells (6,7). Following
the natural infection with EBV, the virus executes a distinct
program of gene expression in the lytic or latent cycles to
establish a persistent infection. Nuclear antigen-1 (NA1) protein
is expressed during latent and lytic cycles, binding to a
replication origin within the viral genome. It also mediates
replication and partitioning of the episome during the division of
the host cell, and is essential for the maintenance of the viral
genome in latency (8–11). Since NA1 protein is expressed in
EBV-infected cells, including cancer cells or cells in the cancer
microenvironment, it may act as an antigen to induce immune
responses if the microenvironment is suitable for the induction of
humoral responses directed at NA1 (7).
To elicit humoral immune responses to induce the
production of NA1 antibodies in the tumor local environment, the
elements essential for a humoral response should all be present.
These elements include antigens (NA1 in this case),
antigen-presenting cells (APCs), T helper cells and B cells
(7). Since numerous immune cells
infiltrate in the NPC tumor tissues, we hypothesized that the local
tumor microenvironment may be adequate for the production of
antibodies directed at NA1. Furthermore, since EBV is mainly
transmitted via saliva, NA1 antibody originating in the local
microenvironment may be secreted into the serum and saliva of
patients with NPC (12). To confirm
these hypotheses, serum samples and nasopharyngeal tissues were
collected from patients with chronic inflammation with lymphoid
hyperplasia and from patients with NPC, and antibodies directed at
NA1 were detected. The results showed that NA1 antibodies were
detected in the serum and saliva samples of patients with NPC, and
that the local production of the antibodies could be completed in
part in the local tumor microenvironment.
Patients and methods
Patients
The research protocol was approved by the
Institutional Review Board of West China University Hospital,
Sichuan University (Chengdu, China). All patients provided written
informed consent and agreed to be involved in the present study.
Between September 2011 and April 2012, 39 patients (34 male, 5
female; mean age, 46.9; age range 22–69) with pathologically
confirmed NPC from West China University Hospital were enrolled in
the present study. Each study patient was evaluated by flexible
fiberoptic endoscopic examination, magnetic resonance imaging scans
of the head and neck, chest X-ray, bone scan and ultrasound of the
abdomen prior to treatment. X-ray computed tomography scans of the
chest/abdomen were performed when clinically indicated. The
histology of the tumor was evaluated according to the World Health
Organization classification (13).
Tumors were staged by Tumor-Node-Metastasis classification and
clinical staging according to the American Joint Committee on
Cancer 2009 cancer staging classification (13). NPC patient characteristics are
presented in Table I.
 | Table I.Clinical characteristics of patients
with NPC in the present study. |
Table I.
Clinical characteristics of patients
with NPC in the present study.
| Characteristics | Patients with
NPC |
|---|
| Total, n | 39 |
| Gender, n |
|
| Male | 34 |
|
Female | 5 |
| Mean age (range),
years | 46.9 (22–69) |
| Histology, n |
|
| Squamous
cell carcinoma, poorly differentiated, non-keratinized Carcinoma,
undifferentiated, non-keratinized | 1 |
| Tumor stage, n |
|
| I | 2 |
| II | 8 |
| III | 11 |
| IV | 18 |
| Depth of tumor
invasion, n |
|
| T1 | 14 |
| T2 | 6 |
| T3 | 6 |
| T4 | 13 |
| N stage, n |
|
| N0 | 2 |
| N1 | 16 |
| N2 | 14 |
| N3 | 7 |
| M stage, n |
|
| M0 | 31 |
| M1 | 8 |
| Metastases, n |
|
| Male | 7 |
|
Female | 1 |
| Metastasis region,
n |
|
| Lung | 2 |
| Bone | 4 |
| Multiple
regions (liver, lung, bone, lymph node) | 2 |
A total of 20 healthy individuals (15 females and 5
males) were recruited for the present study (mean age, 24.8 years;
range, 20–34 years).
Sample collection
Blood and saliva samples were collected from the
healthy volunteers and from the patients with NPC prior to
treatment. Vein blood (1 ml) was collected into a sterile
EDTA-containing vacutainer tube from each patient. Samples were
then centrifuged at 400 × g and 4°C for 10 min. The serum was
collected, aliquoted and stored at −80°C until use. At least 1 ml
saliva was collected using a sterile container. Samples were then
centrifuged at 400 × g and 4°C for 10 min. The serum was collected,
aliquoted and stored at −80°C until use.
ELISA
Titers of antibodies directed at NA1 [immunoglobulin
A (IgA)] in the saliva and serum samples were determined in
duplicate by ELISA using commercial kits (anti-EBNA1 ELISA kit;
catalog no. 03-01-60402) purchased from Zhongshan Bio-Tech Co.,
Ltd. (Guangzhou, China). Samples with antibody levels higher than
the detection limits were diluted and measured again. In the
Laboratory of Molecular and Translational Medicine, West China
Second University Hospital, Sichuan University, Chengdu, China),
the intra-assay and inter-assay coefficients of variation were ≤10%
for all assays. The experiments were conducted by following the
manufacturer's suggested procedures. Two wells of blank controls
(sample dilution buffer), two negative controls and two positive
controls (as provided in the kit) were included on each plate.
Briefly, diluted saliva and serum samples were added to each well
(100 µl in each well) of a 96-well plate. Following incubation at
room temperature (RT) for 2 h on a microplate agitator rotating at
25 g, the plate was then washed with 1X PBS wash buffer with 0.05%
Tween-20. Horseradish peroxidase (HRP)-conjugated anti-human IgA
antibody from the kit (100 µl) was added to each well. Following
incubation at RT for 20 min, the plate was then washed with 1X PBS
wash buffer. Color development was conducted by addition of 100 µl
tetramethylbenzidine substrate solution and incubation at RT for 10
min. Once the reaction was stopped, optical absorbance (OA) was
immediately read on a spectrophotometer (Infinite M200; Tecan
Trading AG, Männedorf, Switzerland) using 450 nm as the primary
wavelength and 630 nm as the secondary wavelength. OA value at a
wavelength of 450/630 nm for each test was determined.
Immunohistochemistry
The immunohistochemical (IHC) staining was performed
on paraffin-embedded nasopharyngeal tissue sections from 39 NPC
patients. As shown in goat anti-human CD80 polyclonal antibody
(1:100; IgG; catalog no. AF140; R&D Systems, Inc., Minneapolis,
MN, USA), goat anti-human CD86 polyclonal antibody (1:100; IgG;
catalog no. AF-141-NA; R&D Systems, Inc.), mouse anti-human CD4
monoclonal antibody (1:100; IgG1; catalog no. MAB379; R&D
Systems, Inc.), rabbit anti-human CD3 polyclonal antibody (1:100;
IgG; catalog no. ab5690; Abcam, Cambridge, UK), mouse anti-human
CD19 monoclonal antibody (1:100; IgG2a, κ; catalog no. ab31947;
Abcam), mouse anti-human human leukocyte antigen-antigen D related
(1:100; HLA-DR) monoclonal antibody (IgG1; catalog no. ab20181;
Abcam), mouse anti-human IgA monoclonal antibody (1:300; IgG1;
catalog no. GTX17839; GeneTex, Inc., Irvine, CA, USA) or mouse
anti-human EBNA1 antibody (1:300; IgG1; catalog no. GTX40777;
GeneTex) were used as the primary antibodies for IHC staining. A
negative control was set up using isotype control of goat serum
(Zhongshan Golden Bridge Biotechnology Co., Ltd., Beijing, China),
mouse IgG1, rabbit IgG, mouse IgG2a or mouse κ as the substitute
for the primary antibody, respectively, according to the source of
the primary antibody. All antibodies were diluted in PBS containing
0.1% bovine serum albumin (Roche Applied Science, Rotkreuz,
Switzerland) and 0.01% sodium azide. Briefly, 4-µm tissue sections
were deparaffinized for 15 min in xylene, hydrated in gradient
ethyl alcohol of 100, 95, 85, 80 and 75%, and then incubated with
3% hydrogen peroxidase for 10 min at room temperature to block
endogenous peroxidase. Following antigen retrieval in 0.1 mol/l
citrate buffer (pH 6.0) for 15 min at 95°C in a pressure cooker,
sections were cooled to RT and washed three times with PBS, and
then incubated with the aforementioned diluted primary antibodies
at 4°C overnight. Subsequently, sections were rewarmed to RT for 30
min and washed three times with PBS and then incubated with
HRP-conjugated goat anti-rabbit/mouse secondary antibody (1:100;
catalog no. ab6720; Dako; Agilent Technologies, Inc., Santa Clara,
CA, USA) for 40 min at 37°C. Diaminobenzidine was used at room
temperature for 3 min for color development. Finally, the sections
were counterstained with Mayer's hematoxylin and mounted in
Permount (ZSGB-BIO, Beijing, China). Subsequently, light microscopy
observation (magnification, ×4,000; 5 fields of view) was
performed. The reactivity of cell type and location in the
immunostained tissues were determined.
Statistical analysis
OA values at wavelength 450/630 nm of NA1 IgA in
saliva and serum samples are expressed as the mean value ± standard
deviation. The statistical significance of the findings was
assessed by one-way analysis of variance using Prism 5 from
GraphPad Software (GraphPad Software, Inc., La Jolla, CA, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
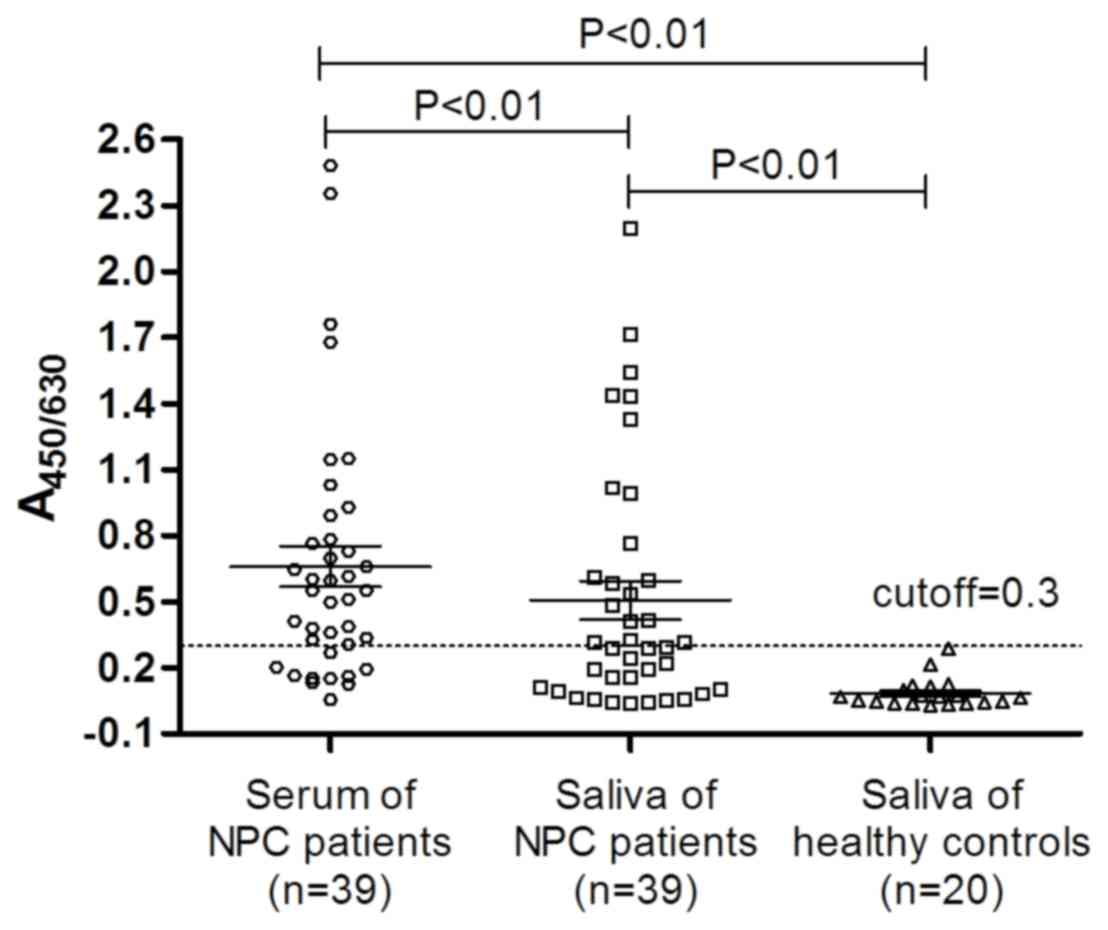
Anti-NA1 in the serum and saliva
As shown in Fig. 1,
antibody titers directed at NA1 were elevated in the saliva and
serum of the majority of NPC patients, and anti-NA1 antibody titer
in the serum was significantly higher than that of in the saliva of
NPC patients (P<0.01). The cut-off value was defined as
A450/630 of 0.3. Compared with that of the saliva of the
healthy control, the anti-NA1 antibody titer in the saliva of the
NPC patients was significantly higher (P<0.01). Anti-NA1
antibody titer was determined to be negative in the saliva of the
20 healthy volunteers; however, 43.6% (17/39) of the patients with
NPC were positive for the antibody. Furthermore, 74.4% (29/39) of
the patients with NPC were positive for anti-NA1 antibodies in the
sera.
Expression of HLA-DR, CD80, CD86, CD3,
CD4, CD19, IgA, EBNA1 in the nasopharyngeal tissue
As shown in and Fig.
2, HLA-DR, CD80, CD86, CD3, CD4, CD19, IgA and EBNA1 antibodies
were used as the primary antibodies for IHC staining. A negative
control was set up using an isotype control of goat serum, mouse
IgG1, rabbit IgG, mouse IgG2a or mouse κ as the substitute for each
primary antibody, respectively, according to the source of the
primary antibody.
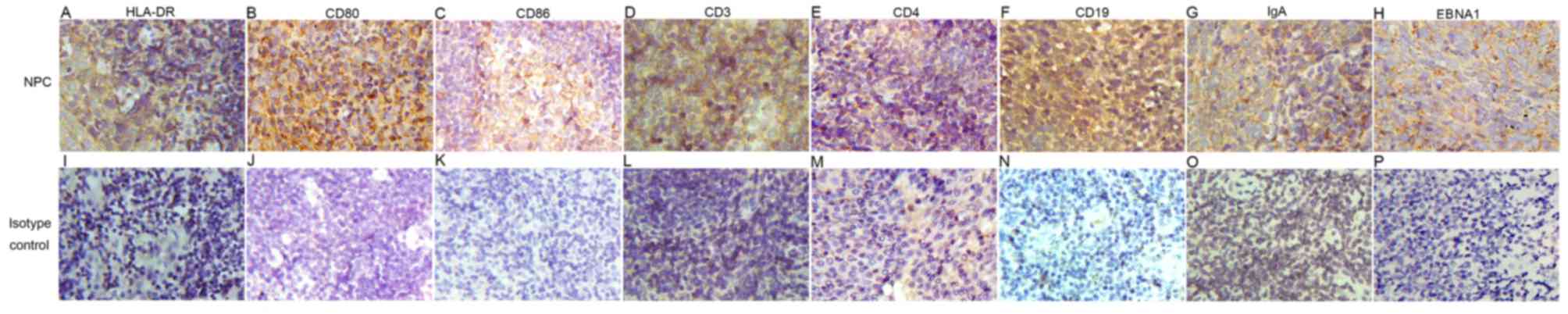 | Figure 2.Expression of (A) HLA-DR, (B) CD80,
(C) CD86, (D) CD3, (E) CD4, (F) CD19, (G) IgA and (H) EBNA1 was
detected in the nasopharyngeal tissue by immunohistochemistry. When
the primary antibody was replaced with the isotype control of (I)
goat serum, (J) mouse IgG1, (K) rabbit IgG, (L) mouse (M) IgG2a or
(N-P) κ, the positive signals were not observed (original
magnification, ×4,000). HLA-DR, human leukocyte antigen-antigen D
related; CD, cluster of differentiation; IgA, immunoglobulin;
EBNA1, Epstein-Barr virus nuclear antigen-1. |
When the control antibodies were used, no positive
signals were noted on tissue sections from patients with NPC. In
infiltrating cells, the expression of HLA-DR, CD80 and CD86 was
detected at the membrane of dendritic cells, the expression of CD3
and CD4 was detected at the membrane of T lymphocytes, the
expression of CD19, IgA was detected at the membrane of B
lymphocytes and the expression of EBNA1 protein was highly
expressed at the membrane of the tumor cells in NPC tissue
specimens (Fig. 2).
Discussion
The level of serum antibodies directed at the EBNA1
antigen during latent and lytic cycles is elevated in patients with
NPC and has been used as a serological marker for NPC diagnosis
(14,15). Combined detection of anti-NA1,
anti-early antigen (EA), anti-viral capsid antigen and
anti-replication and transcription activator increases the
sensitivity and has been considered as a useful serological method
for NPC screening and diagnosis (16–20).
Salivary IgA antibody titers were previously reported to be
increased in response to EA, gp340 or NA1-p107 in healthy
individuals compared with patients with NPC. However, these
salivary parameters were poor in sensitivity and specificity for
NPC screening and diagnosis (12,21,22). To
the best of our knowledge, the present study is the first to report
that anti-NA1 antibodies are elevated in the saliva and serum of
patients with NPC. Although the detection of anti-NA1 antibody in
saliva is non-invasive, quick and convenient, it has a limited
value for clinical use due to its low sensitivity (12,21,22).
The present study investigated whether anti-NA1
antibodies could be induced in the local tumor environment. EBNA1
is the only viral protein consistently expressed during all forms
of latency and in all EBV-associated malignancies (9,10). It is
potentially a universal target for immune recognition of
EBV-infected normal or malignant cells (10). The series of essential components for
a humoral immune response, including NA1-expressed cells, APCs
(HLA-DR+, CD80+ and CD86+), T helper cells (CD3+ and CD4+) and B
cells (CD19+ and IgA+), were all detected by IHC staining in the
local tumor environment. This indicated that anti-NA1 antibodies
may be induced in the local tumor environment. The current study
provides a novel insight into the use of EBNA1 antibodies and
indicates that the tumor microenvironment contributes to the
production of EBNA1 antibodies. Further study into the role of
EBNA1 antibodies in the pathogenesis of EBV-related inflammation
and NPC would, however, be useful to expand upon the present
data.
Acknowledgements
The present study was supported by the Special
Research Foundation of Doctoral Priority to the Development of
Field Project (grant no. 20110181130013).
References
|
1
|
Chen JN, Ding YG, Feng ZY, Li HG, He D, Du
H, Wu B and Shao CK: Association of distinctive Epstein-Barr virus
variants with gastric carcinoma in Guangzhou, southern China. J Med
Virol. 82:658–667. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Deng Z, Hasegawa M, Matayoshi S, Kiyuna A,
Yamashita Y, Maeda H and Suzuki M: Prevalence and clinical features
of human papillomavirus in head and neck squamous cell carcinoma in
Okinawa, southern Japan. Eur Arch Otorhinolaryngol. 268:1625–1631.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Garandawa HI, Ahmad BM and Nggada HA:
Nasopharyngeal cancer in North-Eastern Nigeria: Clinical trends.
Niger J Clin Pract. 12:379–382. 2009.PubMed/NCBI
|
|
4
|
Popat SR, Liavaag PG, Morton R, McIvor N,
Irish JC and Freeman JL: Epstein Barr virus genome in
nasopharyngeal carcinomas from New Zealand. Head Neck. 22:505–508.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rathaur RG, Chitale AR and Banerjee K:
Epstein-Barr virus in nasopharyngeal carcinoma in Indian patients.
Indian J Cancer. 36:80–90. 1999.PubMed/NCBI
|
|
6
|
Valencia SM and Hutt-Fletcher LM:
Important but differential roles for actin in trafficking of
Epstein-Barr virus in B cells and epithelial cells. J Virol.
86:2–10. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shannon-Lowe C, Adland E, Bell AI,
Delecluse HJ, Rickinson AB and Rowe M: Features distinguishing
Epstein-Barr virus infections of epithelial cells and B cells:
Viral genome expression, genome maintenance and genome
amplification. J Virol. 83:7749–7760. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gaur N, Gandhi J, Robertson ES, Verma SC
and Kaul R: Epstein-Barr virus latent antigens EBNA3C and EBNA1
modulate epithelial to mesenchymal transition of cancer cells
associated with tumor metastasis. Tumour Biol. 36:3051–3060. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kang MS and Kieff E: Epstein-Barr virus
latent genes. Exp Mol Med. 47:e1312015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Palser AL, Grayson NE, White RE, Corton C,
Correia S, Ba Abdullah MM, Watson SJ, Cotten M, Arrand JR, Murray
PG, et al: Genome diversity of Epstein-Barr virus from multiple
tumor types and normal infection. J Virol. 89:5222–5237. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tierney RJ, Shannon-Lowe CD, Fitzsimmons
L, Bell AI and Rowe M: Unexpected patterns of Epstein-Barr virus
transcription revealed by a high throughput PCR array for absolute
quantification of viral mRNA. Virology. 474:117–130. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Foong YT, Cheng HM, Sam CK, Dillner J,
Hinderer W and Prasad U: Serum and salivary IgA antibodies against
a defined epitope of the Epstein-Barr virus nuclear antigen (EBNA)
are elevated in nasopharyngeal carcinoma. Int J Cancer.
45:1061–1064. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Finger PT: 7th Edition, AJCC-UICC
Ophthalmic Oncology Task Force: The 7th edition AJCC staging system
for eye cancer: An international language for ophthalmic oncology.
Arch Pathol Lab Med. 133:1197–1198. 2009.PubMed/NCBI
|
|
14
|
Cheng HM, Foong YT, Sam CK, Prasad U and
Dillner J: Epstein-Barr virus nuclear antigen 1 linear epitopes
that are reactive with immunoglobulin A (IgA) or IgG in sera from
nasopharyngeal carcinoma patients or from healthy donors. J Clin
Microbiol. 29:2180–2186. 1991.PubMed/NCBI
|
|
15
|
Tedeschi R, Pin E, Martorelli D, Bidoli E,
Marus A, Pratesi C, Bortolin MT, Zanussi S, Vaccher E, Dolcetti R
and De Paoli P: Serum antibody response to lytic and latent
Epstein-Barr virus antigens in undifferentiated nasopharyngeal
carcinoma patients from an area of nonendemicity. Clin Vaccine
Immunol. 14:435–441. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tang JW, Rohwäder E, Chu IM, Tsang RK,
Steinhagen K, Yeung AC, To KF and Chan PK: Evaluation of
Epstein-Barr virus antigen-based immunoassays for serological
diagnosis of nasopharyngeal carcinoma. J Clin Virol. 40:284–288.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Paramita DK, Fachiroh J, Haryana SM and
Middeldorp JM: Evaluation of commercial EBV RecombLine assay for
diagnosis of nasopharyngeal carcinoma. J Clin Virol. 42:343–352.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Karray H, Ayadi W, Fki L, Hammami A, Daoud
J, Drira MM, Frikha M, Jlidi R and Middeldorp JM: Comparison of
three different serological techniques for primary diagnosis and
monitoring of nasopharyngeal carcinoma in two age groups from
Tunisia. J Med Virol. 75:593–602. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cho WC: Nasopharyngeal carcinoma:
Molecular biomarker discovery and progress. Mol Cancer. 6:12007.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ai P, Wang T, Zhang H, Wang Y, Song C,
Zhang L, Li Z and Hu H: Determination of antibodies directed at EBV
proteins expressed in both latent and lytic cycles in
nasopharyngeal carcinoma. Oral Oncol. 49:326–331. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yao QY, Rowe M, Morgan AJ, Sam CK, Prasad
U, Dang H, Zeng Y and Rickinson AB: Salivary and serum IgA
antibodies to the Epstein-Barr virus glycoprotein gp340: incidence
and potential for virus neutralization. Int J Cancer. 48:45–50.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nadala EC, Tan TM, Wong HM and Ting RC:
ELISA for the detection of serum and saliva IgA against the BMRFI
gene product of Epstein-Barr virus. J Med Virol. 50:93–96. 1996.
View Article : Google Scholar : PubMed/NCBI
|