Introduction
Pancreatic cancer is a lethal malignancy, with a
mortality rate of >90%; it is ranked fourth in terms of
cancer-related mortality (1–3). Although the treatment of pancreatic
cancer involves chemotherapy, patients typically receive treatment
in the terminal stage, owing to a lack of effective detection
methods for the early-stage disease (3). Gemcitabine is the chemotherapeutic agent
used for the treatment of pancreatic cancer, and the effect of
gemcitabine is marked, with suppression of tumor growth, leading to
the prolonging of patient survival, but only for a limited number
of months (4). In order to enhance
the effect of gemcitabine, studies have focused on combination
chemotherapy, but only a limited number of agents have been
screened, therefore a novel combination chemotherapy agent is
required. Owing to the rapid development of gemcitabine resistance
in pancreatic cancer, the effect of gemcitabine is limited. The aim
of the present study was to investigate the underlying molecular
mechanisms of gemcitabine resistance, and identify a suitable
target combination chemotherapy agent that may promote the effect
of gemcitabine for the treatment of pancreatic cancer.
Human equilibrative nucleoside transporter 1 (hENT1)
is able to carry pyrimidine nucleosides and purine into cells
(5). Previous studies have identified
that hENT1 serves an important role in gemcitabine resistance
(6,7).
Patients with pancreatic cancer with increased hENT1 expression
levels have an increased lifespan compared with those with lower
hENT1 expression under gemcitabine treatment (8). These results suggest that hENT1 may be a
target of gemcitabine resistance in pancreatic cancer, therefore
hENT1 agonist combination chemotherapy may promote the effect of
gemcitabine. Furthermore, ribonucleoside diphosphate reductase
(RRM) 1 and 2 are target molecules of gemcitabine, as, when RRM1
and RRM2 expression levels are low, patients with pancreatic cancer
exhibit increased sensitivity to gemcitabine chemotherapy (9). Previous studies have suggested that RRM1
and RRM2 expression levels are increased in patients with
gemcitabine resistance (10–12). Therefore, selecting suitable RRM1 and
RRM2 target agents may be a means to overcome gemcitabine
resistance.
Epithelial-mesenchymal transition (EMT) results in
the loss of cell-cell junctions and migratory and invasive
mesenchymal cell formation (13).
Transforming growth factor-β and Notch signaling pathways have been
demonstrated to mediate the gemcitabine-resistance-induced EMT
(13). Associations between EMT and
cancer aggressiveness have been demonstrated in pancreatic cancer,
and certain studies have suggested an association between EMT and
gemcitabine resistance in pancreatic cancer (14,15).
Therefore, preventing gemcitabine-resistance-induced EMT may be a
novel therapeutic strategy.
Astaxanthin (ASX) is a lipophilic compound,
exhibiting antioxidant, anti-inflammatory and immunomodulatory
characteristics. The anticancer ability of antioxidants has been a
focus of research, particularly the effect of oxidative stress and
metabolism. Powerful antioxidants may be novel and effective agents
for the treatment of carcinoma. Previous studies have demonstrated
that ASX was able to inhibit the proliferation of various types of
cancer cell through immunomodulatory and cell communication
modulation at gap junctions (16,17).
However, there has been limited research on ASX acting as a
combination chemotherapy agent with gemcitabine in the treatment of
human pancreatic cancer. The aim of the present study was to
investigate whether ASX was able to promote the effect of
gemcitabine, and suppress gemcitabine resistance and promote
gemcitabine-induced cell death in human pancreatic cancer cells
(HPCCs), namely Panc-1 and HTB-79. ASX was identified to promote
hENT1 expression levels and inhibit gemcitabine-resistance-induced
EMT through the hypoxia-inducible factor 1α (HIF-1α)/signal
transducer and activator of transcription 3 (STAT3)-TWIST1/ZEB1
signaling pathway in pancreatic cancer cells. Furthermore, ASX in
combination with gemcitabine was able to significantly suppress
tumor growth in a gemcitabine-resistant pancreatic cancer
cell-induced pancreatic tumor model. Therefore, ASX and gemcitabine
co-treatment provides a novel combination chemotherapy strategy for
targeting gemcitabine-resistant pancreatic cancer.
Materials and methods
Cell culture
The HPCCs Panc-1 and HTB-79 were purchased from the
American Type Culture Collection (Manassas, VA, USA). All cells
were cultured in Dulbecco's modified Eagle's medium containing 10%
fetal bovine serum (FBS) and 100 U/ml penicillin, at 37°C and 5%
CO2. All cell culture regents were purchased from Gibco;
Thermo Fisher Scientific, Inc. (Waltham, MA, USA). Panc-1 and
HTB-79 cells were exposed to gemcitabine at increasing
concentrations from 10 nM to generate gemcitabine-resistant cells.
After 2 weeks of adaptation, the concentration was doubled. The
final gemcitabine concentration to which the cells were adapted was
640 nM, and the cells were designated GR-Panc-1 cells and GR-HTB-79
cells.
Western blot analysis
For each sample, Panc-1 and HTB-79 cells were lysed
for 30 min in radioimmunoprecipitation assay lysis buffer (Beyotime
Institute of Biotechnology, Shanghai, China) on ice, and the cell
debris was centrifuged at 12,000 × g for 8 min at 4°C. The protein
concentration was determined using the bicinchoninic acid assay,
and 40 µg proteins were separated by SDS-PAGE (10–15% gels) and
blotted onto a polyvinylidene fluoride (PVDF) membrane. The PVDF
membrane was blocked in a solution of 5% non-fat dried milk in PBST
(0.05% Tween-20 in PBS) for 1 h at room temperature, and incubated
with antibodies at 4°C for 12 h. The PVDF membrane was washed three
times for 10 min in PBST, incubated with secondary antibodies at
37°C for 1 h, and washed again three times for 10 min in PBST.
GAPDH was used as a loading control. All western blotting reagents
were purchased from Beyotime Institute of Biotechnology. All the
antibodies were purchased from Santa Cruz Biotechnology, Inc.
(Dallas, TX, USA). The first antibodies were diluted at 1:500, and
the secondary antibodies were diluted at 1:5,000. The following
antibodies were used: hENT1 (cat. no. sc-48489; polyclonal, goat
anti-human), RRM1 (cat. no. sc-22786; monoclonal, rabbit
anti-human), RRM2 (cat. no. sc-137174; monoclonal, mouse
anti-human), GAPDH (cat. no. sc-293335; monoclonal, mouse
anti-human), Twist1 (cat. no. sc-134136, polyclonal, mouse
anti-human), ZEB1 (cat. no. sc-517272; monoclonal, mouse
anti-human), E-cadherin (cat. no. sc-33743; polyclonal, rabbit
anti-human), STAT3 (cat. no. sc-8059; monoclonal, mouse
anti-human), α-SMA (cat. no. sc-53142; monoclonal, mouse
anti-human), HIF-1α (cat. no. sc-13515; monoclonal, mouse
anti-human), mouse IgG (cat. no. sc-516176), rabbit IgG (cat. no.
sc-2794) and goat IgG (cat. no. sc-2419).
Cell viability assay
Each well of a 96-well plate was inoculated with
104 Panc-1 or HTB-79 cells and cells were allowed to
attach overnight, prior to treatment with drugs for 24 h. The
medium was removed and the cells were washed three times with PBS,
prior to the addition of 90 µl Dulbecco's modified Eagle's medium
(DMEM) and 10 µl Cell Counting kit-8 reagent (Beyotime Institute of
Biotechnology) to each well. Cells were incubated at 37°C for 1 h
before determining the optical density at 450 nm using a microplate
reader.
Trypan blue assay
Treated Panc-1 or HTB-79 cells suspensions were
obtained and a 1/9 volume of 0.4% (w/v) trypan blue solution was
added. The number of total cells and dead cells (those that did not
exclude the dye) were determined, and the total death rate was
calculated as (number of dead cells/number of total cells)
×100%.
Transfection experiment
Transient transfection of GR-Panc-1 cells with small
interfering RNAs (siRNAs) and plasmids was performed using
Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.),
the siRNAs were purchased from Shanghai Genechem Co., Ltd.
(Shanghai, China), and the sequences of siRNAs were as follows:
Control, 5′-UUCUCCGAACGUGUCACGUTT−3′; hENT1,
5′-AUGACAUUGUUGAAGAUGGCA-3′; ZEB1, 5′-GGACUGAAGUCAGGUAAGGCA-3′;
Twist1, 5′-AAACAUUUGUUUUAAGGAGAA-3′; STAT3,
5′-GCUAAGUCAGCUUCAUUGAGU−3′; and HIF-1α,
5′-GCAUUGCCAUCAGUCACGCUA-3′. The con-pcDNA3.1, RRM1-pcDNA3.1 and
RRM2-pcDNA3.1 plasmids were purchased from Shanghai Genechem Co.,
Ltd. The 6-wells plates were used for siRNA or plasmid transfection
assays. For the each well, 500 ng siRNAs or 2 µg plasmids were
added to 10 µl Lipofectamine 2000 according to the manufacturer's
protocol, and were incubated with GR-Panc-1 cells for 48 h. The
siRNAs or plasmids were then removed, and GR-Panc-1 cells were
treated with drugs for an additional 24 h. Subsequently, the cells
were collected for western blot analysis, as aforementioned.
Cell invasion assay
A BioCoat Matrigel invasion chamber system (Corning
Incorporated, Corning, NY, USA) was used to assay cell invasion.
Using a Transwell plate, the lower chamber was filled with culture
medium without cells, and the upper chamber was filled with cell
suspension and medium containing 10% FBS. The Transwell plate was
incubated at 37°C for 24 h. Cells that adhered to the upper chamber
surface were removed, and the cells that adhered to the lower
chamber surface were stained with 4% paraformaldehyde for 15 min,
rinsed with water and dried. The 0.5% crystal violet was extracted
with 50% ethanol containing 0.1 M sodium citrate, and the
absorbance at 600 nm was determined.
Xenograft model
A total of 30 male 6-week-old BALB/c nude mice
(weighing 18–22 g) were purchased from the Institute of Zoology
(Chinese Academy of Sciences, Beijing, China). All animal
experiments were performed according to the guidelines of the
Institutional Animal Care and Use Committee of Institute of Zoology
(Chinese Academy of Sciences, Beijing, China). All the animals were
fed in a pathogen-free environment, at 24–28°C. Ventilation was
required 10–20 times per hour, relative humidity was 50–60%, the
light/dark cycle was natural circadian light, the food was
sterilized by irradiation, and the water contained bacitracin (4
g/l) and neomycin (4 g/l). Each BALB/c nude mouse was
subcutaneously inoculated with 5×106 Panc-1 or GR-Panc-1
cells into the right and left hind footpads. At 2 days after
inoculation, the mice inoculated with Panc-1 cells were treated
with 10 mg/kg gemcitabine 3 times/day by intraperitoneal injection,
and the mice inoculated with GR-Panc-1 cells were treated with 10
mg/kg gemcitabine or co-treated with 500 mg/kg ASX and 10 mg/kg
gemcitabine 3 times/day by intraperitoneal injection, with the
injection of ASX occurring 2 h before that of gemcitabine. ASX and
gemcitabine were dissolved in saline. Tumor volumes were determined
weekly.
Terminal
deoxynucleotidyltransferase-mediated dUTP nick-end labeling (TUNEL)
assay
Tumors were immersed in 4% paraformaldehyde for 24 h
and dehydrated in 30% sucrose solutions, prior to
paraffin-embedding and cutting into sections (10 µm). The sections
were treated using an In Situ Cell Death Detection kit
(Roche Diagnostics, Indianapolis, IN, USA), according to the
manufacturer's protocol.
Statistical analysis
Results are presented as the mean ± standard
deviation of triplicate experiments and were performed with SPSS
17.0 (SPSS, Inc., Chicago, IL, USA). Two-way analysis of variance,
followed by Bonferroni post hoc testing, was used to compare
different groups. The unpaired Student t-test or Mann-Whitney U
test was used to compare two means. P<0.05 was considered to
indicate a statistically significant difference.
Results
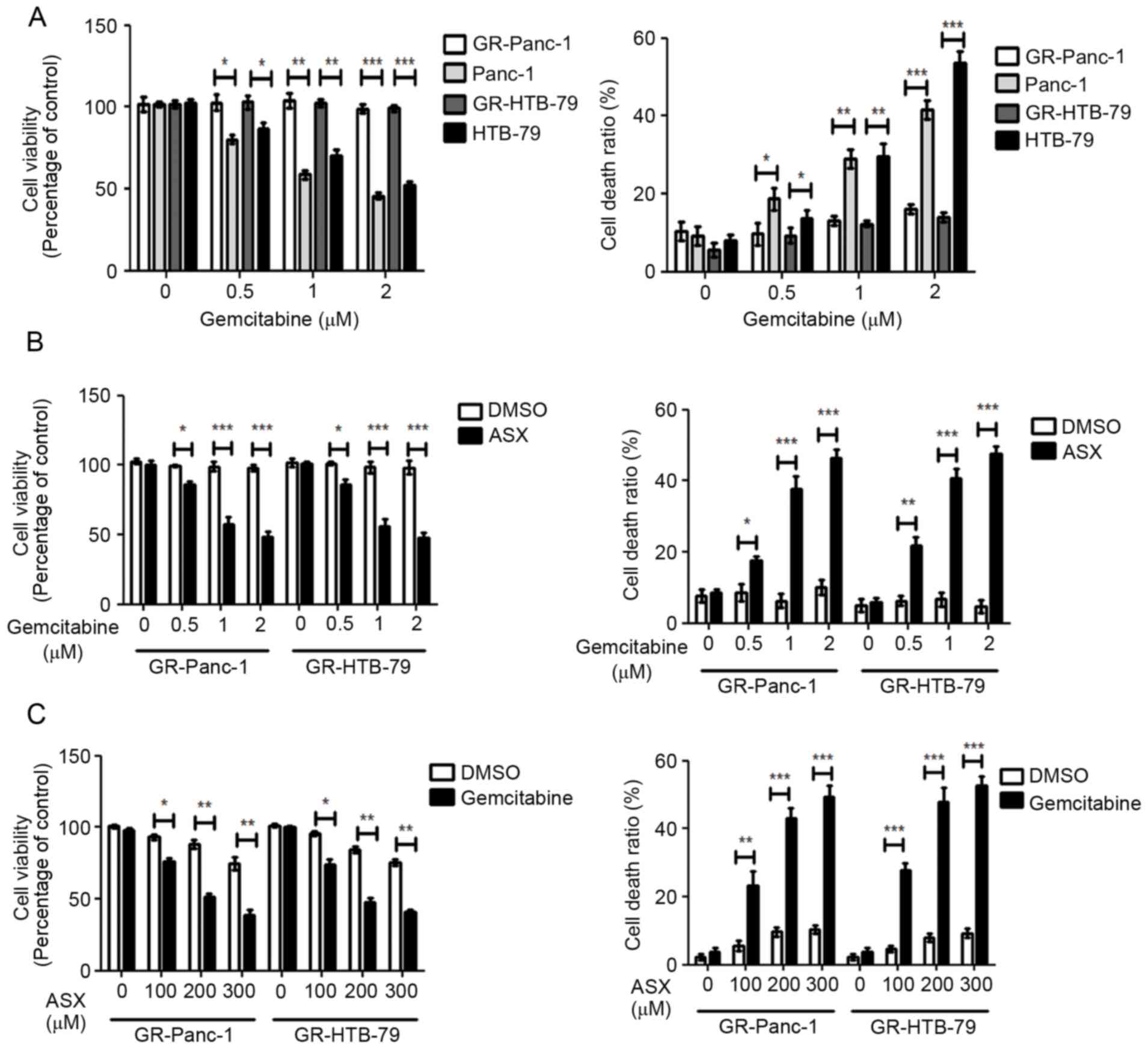
ASX enhances gemcitabine-induced
pancreatic cancer cell death
Using increasing gemcitabine concentrations starting
at 10 nM to induce Panc-1 and HTB-79 GR-HPCCs, designated GR-Panc-1
and GR-HTB-79. Panc-1 and HTB-79 cells were treated with various
concentrations of gemcitabine; cell viability was identified to
decrease with increasing gemcitabine concentration, and the cell
death ratio was identified to increase with increasing gemcitabine
concentration (Fig. 1A). In contrast,
GR-HPCCs were treated with the same concentrations of gemcitabine,
but no alteration in cell viability or cell death ratio was
observed with increasing gemcitabine concentration (Fig. 1A). When GR-HPCCs were treated with
various concentrations of gemcitabine in combination with 200 µM
ASX, the cell viability and cell death ratio were significantly
different compared with treatment with gemcitabine alone, which
occurred in a gemcitabine dose-dependent manner (Fig. 1B). When GR-HPCCs were treated with
various concentrations of ASX in combination with 1 µM gemcitabine,
the cell viability and cell death ratio were significantly
different compared with treatment with ASX alone, which occurred in
an ASX dose-dependent manner (Fig.
1C). These results indicated that ASX enhances
gemcitabine-induced GR-HPCC death.
ASX upregulates hENT1 and
downregulates RRM1 and RRM2 expression
It has been demonstrated previously that hENT1, RRM1
and RRM2 serve important roles in the anticancer efficiency of
gemcitabine (6,18,19). RRM1
and RRM2 are targets of gemcitabine: When RRM1 and RRM2 expression
levels are increased, patients exhibit only limited sensitivity to
gemcitabine chemotherapy, appearing gemcitabine-resistant.
Determining the expression level of hENT1, RRM1 and RRM2 in HPCCs
and GR-HPCCs treated with gemcitabine or gemcitabine in combination
with ASX, it was identified that the hENT1 expression level in
GR-HPCCs was decreased compared with that in HPCCs, and the RRM1
and RRM2 expression level in GR-HPCCs was increased compared with
that in HPCCs, ASX was able to enhance the hENT1 expression level
and inhibit the RRM1 and RRM2 expression level in GR-HPCCs
(Fig. 2A). By knocking down hENT1
using siRNA in GR-Panc-1 cells, it was identified that the cell
death ratio was decreased compared with cells transfected with
Con-siRNA, including cells co-treated with gemcitabine and ASX
(Fig. 2B). RRM1 and RRM2
overexpression was also able to inhibit the gemcitabine- and
ASX-induced cell death ratio in GR-Panc-1 cells (Fig. 2C and D). These results indicate that
ASX may enhance gemcitabine-induced GR-HPCC death by targeting
hENT1 and RRM1 and RRM2.
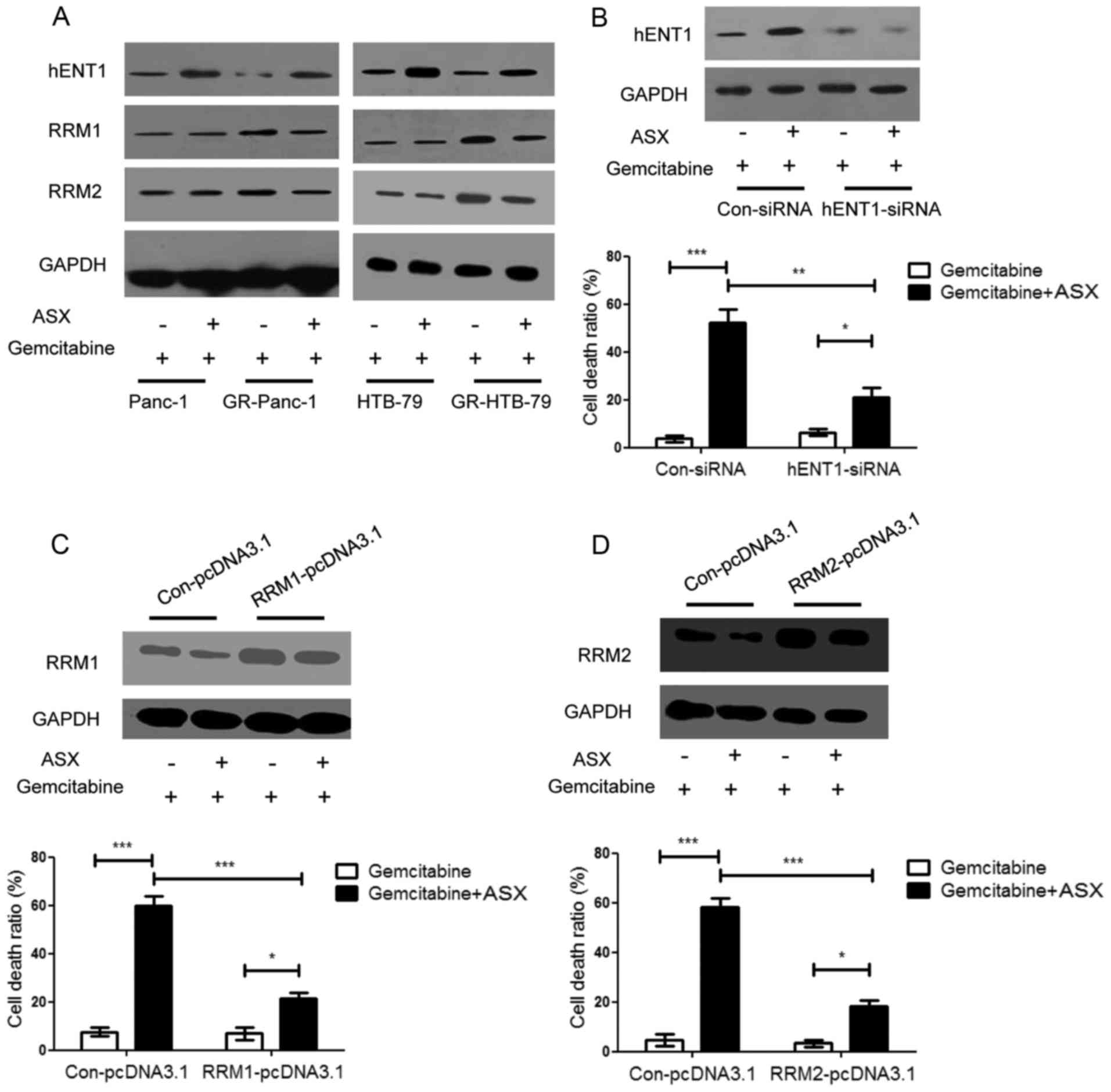 | Figure 2.ASX upregulates hENT1 and
downregulates RRM1 and RRM2 expression in GR-HPCCs, and increases
gemcitabine-induced GR-HPCC death through hENT1, RRM1 and RRM2
signaling. (A) HPCCs and GR-HPCCs were treated with 1 µM
gemcitabine alone or co-treated with 200 µM ASX (2 h pretreatment)
and 1 µM gemcitabine for 24 h. ASX was able to restore the decrease
in hENT1 protein expression and restore the RRM1 and RRM2 protein
expression levels in GR-HPCCs. (B) GR-Panc-1 cells were transfected
with control siRNA or hENT1-siRNA, and treated with 1 µM
gemcitabine alone or co-treated with 200 µM ASX (2 h pretreatment)
and 1 µM gemcitabine for 24 h. The cell death ratio was analyzed
using a trypan blue assay. GR-Panc-1 cells were transfected with
(C) RRM1 plasmid (RRM1-pcDNA3.1) or (D) RRM2 plasmid
(RRM2-pcDNA3.1) or control plasmid (Con-pcDNA3.1), and treated with
1 µM gemcitabine alone or co-treated with 200 µM ASX (2 h
pretreatment) and 1 µM gemcitabine for 24 h. The cell death ratio
was determined using a trypan blue assay. *P<0.05; **P<0.01;
***P<0.005. ASX, astaxanthin; hENT1, human equilibrative
nucleoside transporter 1; RRM, ribonucleoside diphosphate
reductase; GR-HPCCs, gemcitabine-resistant HPCCs; HPCCs, human
pancreatic cancer cells; DMSO, dimethyl sulfoxide. |
ASX suppresses the EMT phenotype in
GR-HPCCs
To investigate whether ASX affects the EMT phenotype
which was established to investigate gemcitabine resistance, the
expression epithelial (E-)cadherin, TWIST1, ZEB1 and α-smooth
muscle actin (α-SMA) were examined in HPCCs and GR-HPCCs treated
with gemcitabine or co-treated with gemcitabine and ASX. It was
identified that the expression levels of the mesenchymal cell
markers in GR-HPCCs were increased compared with those in HPCCs,
and that of the epithelial cell marker was decreased (Fig. 3A). Furthermore, ASX was able to
decrease mesenchymal cell marker expression and increase epithelial
cell marker expression in GR-HPCCs treated with gemcitabine
(Fig. 3A). TWIST1 and ZEB1 are
transcription factors that are able to mediate EMT, and are
overexpressed in a number of cancer EMT phenotypes (20,21). Thus,
it was hypothesized TWIST1 and ZEB1 were the key factors in the
ASX-mediated EMT phenotype in GR-HPCCs. In order to investigate
this hypothesis, TWIST1 was knocked down through siRNA transfection
in GR-Panc-1 cells, and the EMT phenotype was inhibited by
decreasing α-SMA and increasing E-cadherin expression, and hENT1
expression was increased. In addition, expression of RRM1 and RRM2
was inhibited in GR-Panc-1 cells with TWIST1 knocked down (Fig. 3B). Furthermore, the cell death ratio
was detected, and the results showed that the cell death ratio was
enhanced when TWIST1 was knocked down in GR-Panc-1 cells (Fig. 3C). Similarly, ZEB1 was knocked down in
GR-Panc-1 cells, the EMT and cell death ratio were detected, and
the results indicated that the EMT was inhibited (Fig. 3D). When ZEB1 was knocked down through
ZEB1-siRNA transfection, the expression of hENT1 was increased
while RRM1 and RRM2 expression was inhibited (Fig. 3D). In addition, the cell death ratio
also was improved in GR-Panc-1 cells when ZEB1 was knocked down
(Fig. 3E). Cell invasion assays
indicated that the invasion of GR-HPCCs was increased compared with
that of HPCCs, and ASX was able to inhibit GR-HPCC invasion
(Fig. 3F). These results suggested
that ASX is able to suppress the EMT phenotype and enhance GR-HPCC
sensitization to gemcitabine through the TWIST1 and ZEB1 signaling
pathway.
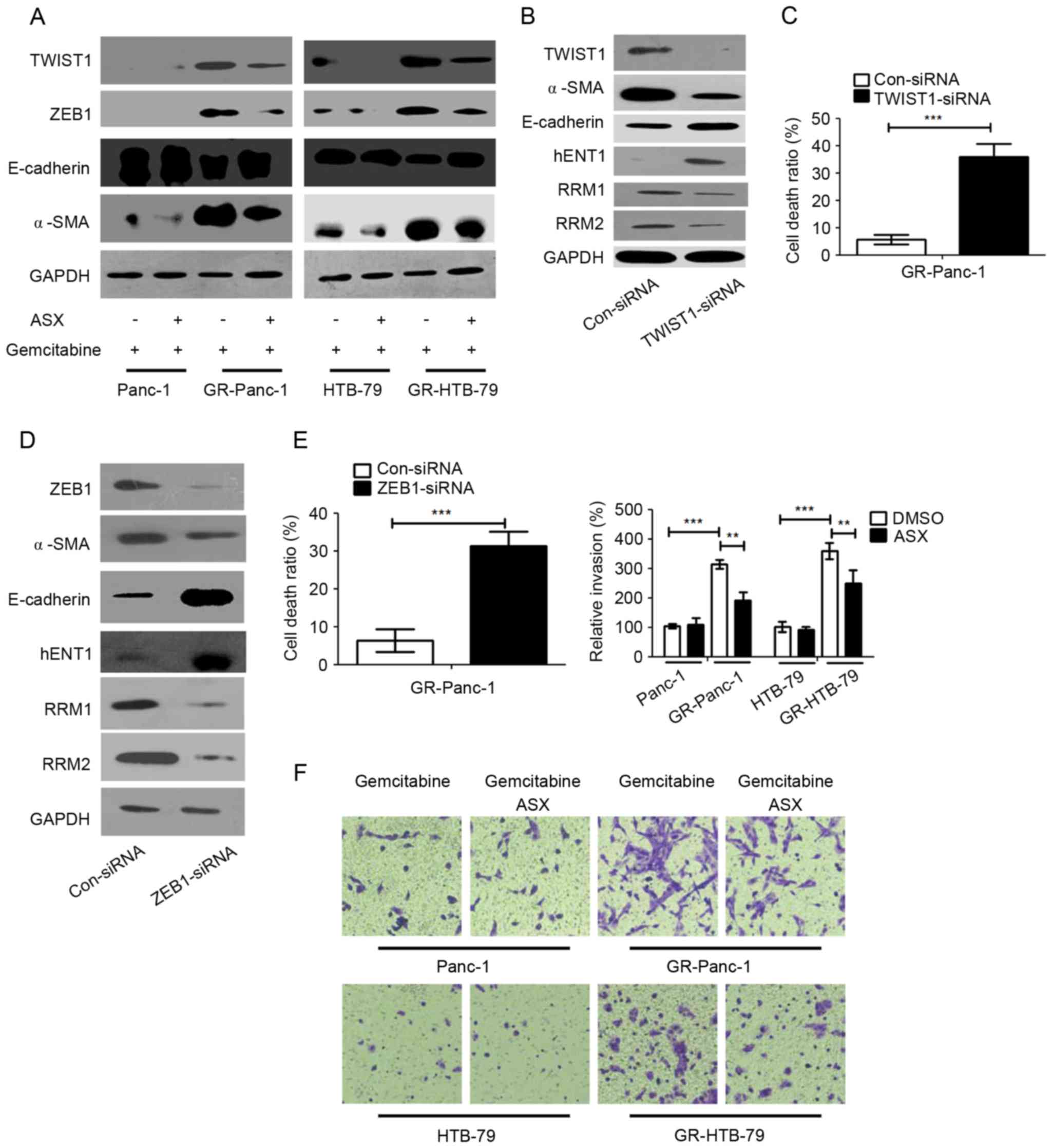 | Figure 3.ASX suppresses the EMT phenotype of
GR-HPCCs through TWIST1 and ZEB1 signaling, and recover the
activation of TWIST1 and ZEB1 in GR-HPCCs. (A) HPCCs and GR-HPCCs
were treated with 1 µM gemcitabine alone or co-treated with 200 µM
ASX (2 h pretreatment) and 1 µM gemcitabine for 24 h. (B) GR-Panc-1
cells were transfected with control siRNA (Con-siRNA) or
TWIST1-siRNA. (C) The cell death ratio was determined using a
trypan blue assay. (D) GR-Panc-1 cells were transfected with
control siRNA (Con-siRNA) or ZEB1-siRNA. (E) The cell death ratio
was determined using a trypan blue assay. (F) ASX suppresses the
invasive ability of GR-HPCCs. HPCCs and GR-HPCCs were treated with
1 µM gemcitabine alone or co-treated with 200 µM ASX (2 h
pretreatment) and 1 µM gemcitabine for 24 h. **P<0.01;
***P<0.005. ASX, astaxanthin; EMT, epithelial-mesenchymal
transition; GR-HPCCs, gemcitabine-resistant HPCCs; HPCCs, human
pancreatic cancer cells; siRNA, small interfering RNA; E-cadherin,
endothelial cadherin; α-SMA, α-smooth muscle actin; hENT1, human
equilibrative nucleoside transporter 1; RRM, ribonucleoside
diphosphate reductase. |
ASX resensitizes GR-HPCCs to
gemcitabine-induced cell death through the HIF-1α/STAT3 signaling
pathway
It has been demonstrated previously that HIF-1α and
STAT3 were mediators of TWIST1 and ZEB1 (22–24). The
aforementioned results indicated that ASX is able to suppress the
EMT phenotype and enhance GR-HPCC sensitization to gemcitabine
through the TWIST1 and ZEB1 signaling pathway. In order to
investigate the role of factors upstream of TWIST1 and ZEB1, HIF-1α
and STAT3 expression levels were determined in HPCCs and GR-HPCCs,
which were treated with gemcitabine or co-treated with gemcitabine
and ASX. It was identified that HIF-1α and STAT3 expression levels
in GR-HPCCs were increased compared with those in HPCCs (Fig. 4A). Furthermore, ASX was able to
inhibit HIF-1α and STAT3 expression (Fig.
4A). Knocking down STAT3 expression through siRNA transfection
could lead to TWIST1, ZEB1, RRM1 and RRM2 expression inhibition,
and an increase in hENT1 expression in GR-Panc-1 cells, and ASX
could regulate the expression levels for the aforementioned
molecules (Fig. 4B). In addition,
when STAT3 was knocked down in GR-Panc-1 cells, ASX could
effectively improve the gemcitabine-induced cell death ratio
(Fig. 4C). At the same time, HIF-1α
was also knocked down through siRNA transfection in GR-Panc-1
cells, and western blot analysis and trypan blue stain assays were
performed on the treated cells. The results suggested that TWIST1,
ZEB1, RRM1 and RRM2 expression levels were inhibited and hENT1
expression level was improved when HIF-1α was knocked down in
GR-Panc-1 cells, and ASX also could regulate the expression levels
for the aforementioned molecules (Fig.
4D). In addition, in GR-Panc-1 cells, when HIF-1α was knocked
down, ASX could effectively improve the gemcitabine-induced cell
death ratio (Fig. 4E). These results
indicated that ASX resensitizes GR-HPCCs to gemcitabine-induced
cell death through the HIF-1α/STAT3 signaling pathway.
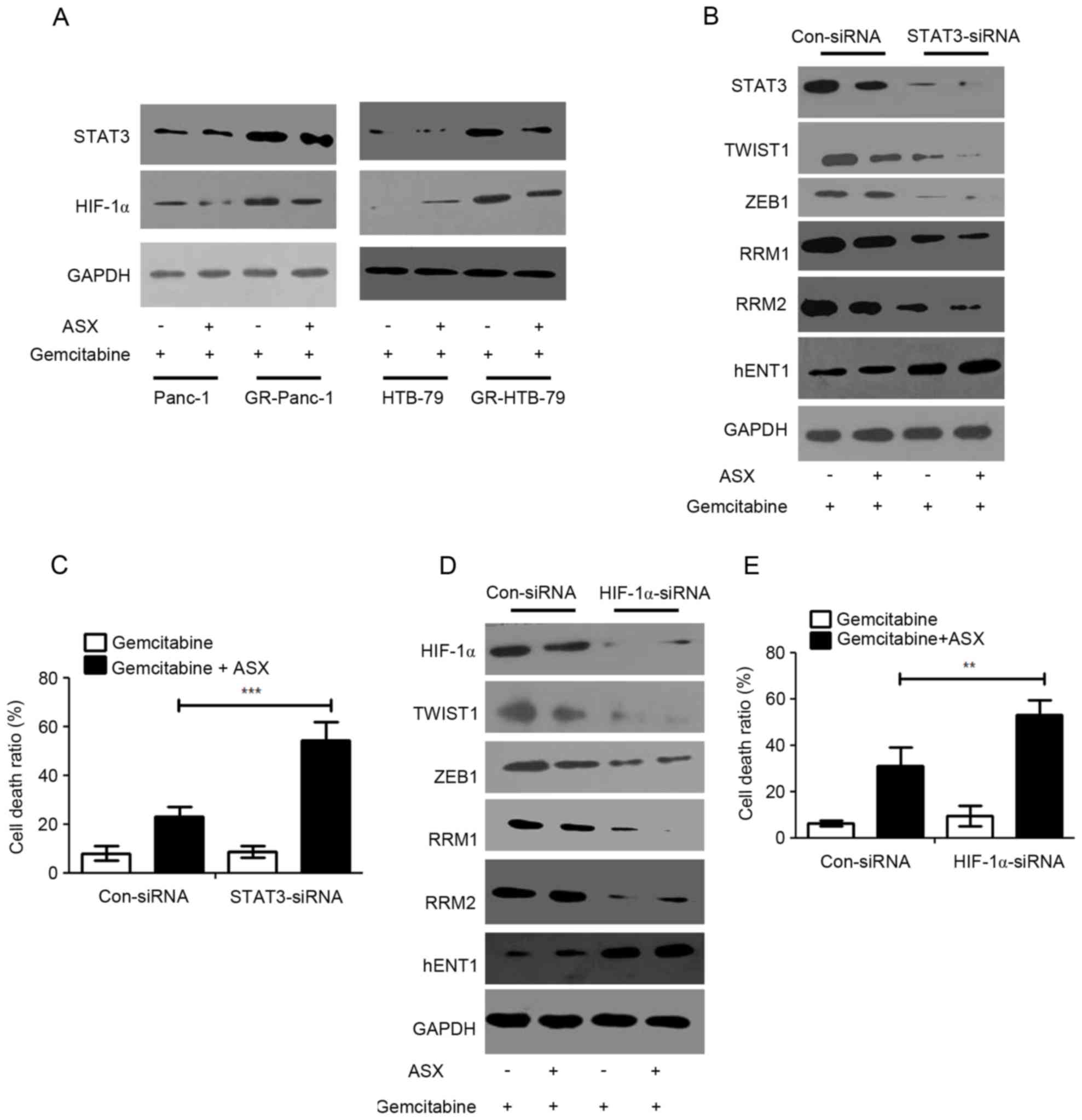 | Figure 4.ASX resensitizes GR-HPCCs to
gemcitabine-induced cell death through the HIF-1α/STAT3 signaling
pathway. (A) HPCCs and GR-HPCCs were treated with 1 µM gemcitabine
alone or co-treated with 200 µM ASX (2 h pretreatment) and 1 µM
gemcitabine for 24 h. (B) GR-Panc-1 was transfected with control
siRNA (Con-siRNA) or STAT3-siRNA, and treated with 1 µM gemcitabine
alone or co-treated with 200 µM ASX (2 h pretreatment) and 1 µM
gemcitabine for 24 h. (C) The cell death ratio was determined using
a trypan blue assay. (D) GR-Panc-1 was transfected with control
siRNA (Con-siRNA) or HIF-1α-siRNA, and treated with 1 µM
gemcitabine alone or co-treated with 200 µM ASX (2 h pretreatment)
and 1 µM gemcitabine for 24 h. (E) The cell death ratio was
determined using a trypan blue assay. **P<0.01; **P<0.01.
ASX, astaxanthin; GR-HPCCs, gemcitabine-resistant HPCCs; HIF-1α,
hypoxia-inducible factor 1α; HPCCs, human pancreatic cancer cells;
siRNA, small interfering RNA; STAT3, signal transducer and
activator of transcription 3; hENT1, human equilibrative nucleoside
transporter 1; RRM, ribonucleoside diphosphate reductase. |
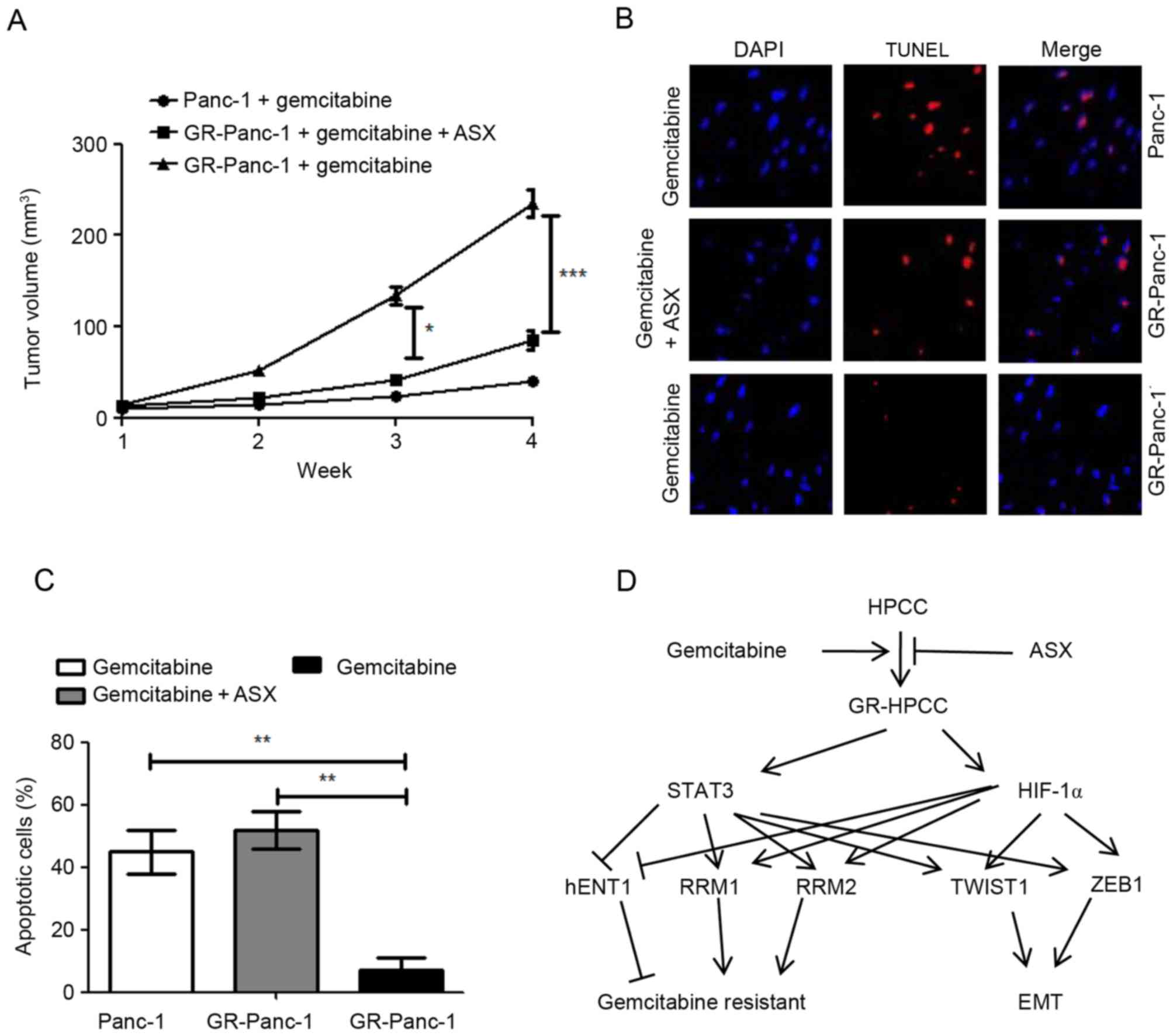
ASX inhibits the growth of
gemcitabine-resistant pancreatic tumors in vivo through
apoptosis
The aforementioned results indicated that ASX is
able to stimulate gemcitabine-induced cell death in GR-HPCCs in
vitro. In order to confirm this effect in vivo, a
pancreatic tumor xenograft model was used. Nude mice were
xenografted with Panc-1 or GR-Panc-1 cells. As expected, in the
presence of gemcitabine, the tumor volume induced by Panc-1 cells
was significantly decreased compared with that induced by GR-Panc-1
cells (Fig. 5A). For the tumor
induced by GR-Panc-1, co-treatment with ASX and gemcitabine led to
a significant decrease in the tumor volume compared with treatment
with gemcitabine alone (Fig. 5A). In
order to investigate the reason for the decrease in tumor volume,
TUNEL was used to detect apoptosis, with the results indicating
that gemcitabine was able to induce apoptosis in the xenografted
Panc-1 model, and that co-treatment with ASX and gemcitabine was
able to induce apoptosis in the xenografted GR-Panc-1 model
(Fig. 5B and C).
Discussion
In the present study, it was identified that ASX is
able to selective kill GR-HPCCs by increasing sensitivity to
gemcitabine. The results of the present study indicated that
GR-HPCCs exhibit an EMT phenotype, and GR-HPCCs exhibit a
downregulated hENT1 expression level and upregulated RRM1 and RRM2
expression levels via TWIST1 and ZEB1 mediated by the HIF-1α/STAT3
signaling pathway (Fig. 5D). More
importantly, in xenografted pancreatic tumors induced by GR-Panc-1
cells, co-treatment with ASX and gemcitabine was able to
significantly suppress tumor growth in comparison with treatment
with gemcitabine alone. Furthermore, it was identified that
co-treatment with ASX and gemcitabine suppressed tumor growth in
the xenografted GR-Panc-1 model by inducing apoptosis.
Previous studies investigated that hENT1 was a key
mediator of gemcitabine resistance in the clinic (5,8).
Additionally, RRM1 and RRM2, the targets of gemcitabine, were
demonstrated to be associated with gemcitabine resistance (8,10–12). Although hENT1, RRM1 and RRM2 are known
to associated with gemcitabine resistance, the underlying molecular
mechanism remains unknown. It has been investigated previously that
hENT1 was induced by peroxisome-proliferator-activated receptor
(PPAR)α and PPARγ in GR-HPCCs (25),
and RRM1 and RRM2 were mediated by the protein kinase B,
phosphoinositide 3-kinase, Ras/extracellular-signal-regulated
kinase (ERK) and mitogen-activated protein kinase/ERK kinase 1/2
signaling pathways (26–30). The results of the present study
indicated that TWIST1 and ZEB1, the transcription factors involved
in EMT, are able to decrease hENT1 expression and increase RRM1 and
RRM2 expression in GR-HPCCs treated with gemcitabine. Furthermore,
ASX is able to reverse the effect of TWIST1 and ZEB1 to hENT1, RRM1
and RRM2 by inhibiting TWIST1 and ZEB1 expression. ASX was able to
inhibit the EMT phenotype in GR-HPCCs treated with gemcitabine.
Furthermore, HIF-1α is a nucleoprotein with transcriptional
activity, which has a broad target gene spectrum, including hypoxia
adaptation, tumor growth and inflammation-associated genes. When
HIF-1α binds to target genes, transcriptional and
post-transcriptional regulation occurs and tumor growth may be
accelerated (31). STAT3 is able to
mediate growth factor receptors and signaling downstream of
cytokines; activated STAT3 is able to promote aerobic glycolysis
and downregulate mitochondrial activity, which may decrease
reactive oxygen species (ROS) generation and delay senescence, and
also promote survival and inhibit apoptosis in drug-resistant cells
(32). In the present study, the
participation of HIF-1α and STAT3 in GR-HPCC mediation was
investigated, together with the role of these factors in mediating
the expression of TWIST1 and ZEB1, mediators of hENT1, RRM1 and
RRM2 that contribute to EMT. Although HIF-1α and STAT3 exhibited
significant differences in expression between HPCCs and GR-HPCCs,
ASX was able to reverse the alterations in GR-HPCCs treated with
gemcitabine, and contribute to gemcitabine-induced cell death in
GR-HPCCs.
Using a tumor xenograft model to evaluate the effect
of ASX and gemcitabine co-treatment in vivo, it was
identified that single administration of gemcitabine was able to
suppress tumor growth in the gemcitabine-sensitive cell xenografted
model, but not suppress tumor growth in the gemcitabine-resistance
cell xenografted model; when co-treated with ASX and gemcitabine,
tumor growth was significantly inhibited in the
gemcitabine-resistance cell xenografted model, which supported the
in vitro results. Furthermore, using in situ
apoptosis detection, with ASX and gemcitabine co-treatment in the
gemcitabine-sensitive cell xenografted model, the suppression of
tumor growth occurred by enhancing apoptosis.
The results of the present study indicated that
HIF-1α/STAT3-TWIST1/ZEB1-EMT was a novel mechanism for gemcitabine
resistance in GR-HPCCs. Furthermore, it is suggested that
co-treatment with ASX and gemcitabine may be a novel and efficient
therapeutic strategy for gemcitabine-resistant pancreatic cancer by
targeting hENT1, RRM1 and RRM2, and inhibiting the
gemcitabine-induced EMT phenotype.
Glossary
Abbreviations
Abbreviations:
|
ASX
|
astaxanthin
|
|
EMT
|
epithelial-to-mesenchymal
transition
|
|
GR-HPCCs
|
gemcitabine-resistant human pancreatic
cancer cells
|
|
HPCCs
|
human pancreatic cancer cells
|
|
hENT1
|
human equilibrative nucleoside
transporter 1
|
|
RRM
|
ribonucleoside diphosphate
reductase
|
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Diener MK, Combs SE and Büchler MW:
Chemoradiotherapy for locally advanced pancreatic cancer. Lancet
Oncol. 14:269–270. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Akimoto M, Iizuka M, Kanematsu R, Yoshida
M and Takenaga K: Anticancer effect of ginger extract against
pancreatic cancer cells mainly through reactive oxygen
Species-mediated Autotic cell death. PLoS One. 10:e01266052015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Seicean A, Petrusel L and Seicean R: New
targeted therapies in pancreatic cancer. World J Gastroenterol.
21:6127–6145. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nordh S, Ansari D and Andersson R: hENT1
expression is predictive of gemcitabine outcome in pancreatic
cancer: A systematic review. World J Gastroenterol. 20:8482–8490.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Poplin E, Wasan H, Rolfe L, Raponi M,
Ikdahl T, Bondarenko I, Davidenko I, Bondar V, Garin A, Boeck S, et
al: Randomized, multicenter, phase II study of CO-101 versus
gemcitabine in patients with metastatic pancreatic ductal
adenocarcinoma: Including a prospective evaluation of the role of
hENT1 in gemcitabine or CO-101 sensitivity. J Clin Oncol.
31:4453–4461. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wattanawongdon W, Hahnvajanawong C, Namwat
N, Kanchanawat S, Boonmars T, Jearanaikoon P, Leelayuwat C,
Techasen A and Seubwai W: Establishment and characterization of
gemcitabine-resistant human cholangiocarcinoma cell lines with
multidrug resistance and enhanced invasiveness. Int J Oncol.
47:398–410. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jordheim LP and Dumontet C: Do hENT1 and
RRM1 predict the clinical benefit of gemcitabine in pancreatic
cancer? Biomark Med. 7:663–671. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yoneyama H, Takizawa-Hashimoto A, Takeuchi
O, Watanabe Y, Atsuda K, Asanuma F, Yamada Y and Suzuki Y: Acquired
resistance to gemcitabine and cross-resistance in human pancreatic
cancer clones. Anticancer Drugs. 26:90–100. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nakagawa N, Murakami Y, Uemura K, Sudo T,
Hashimoto Y, Kondo N and Sueda T: Combined analysis of intratumoral
human equilibrative nucleoside transporter 1 (hENT1) and
ribonucleotide reductase regulatory subunit M1 (RRM1) expression is
a powerful predictor of survival in patients with pancreatic
carcinoma treated with adjuvant gemcitabine-based chemotherapy
after operative resection. Surgery. 153:565–575. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bhutia YD, Hung SW, Krentz M, Patel D,
Lovin D, Manoharan R, Thomson JM and Govindarajan R: Differential
processing of let-7a precursors influences RRM2 expression and
chemosensitivity in pancreatic cancer: Role of LIN-28 and SET
oncoprotein. PLoS One. 8:e534362013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fisher SB, Fisher KE, Patel SH, Lim MG,
Kooby DA, El-Rayes BF, Staley CA III, Adsay NV, Farris AB III and
Maithel SK: Excision repair cross-complementing gene-1,
ribonucleotide reductase subunit M1, ribonucleotide reductase
subunit M2, and human equilibrative nucleoside transporter-1
expression and prognostic value in biliary tract malignancy.
Cancer. 119:454–462. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Namba T, Kodama R, Moritomo S, Hoshino T
and Mizushima T: Zidovudine, an anti-viral drug, resensitizes
gemcitabine-resistant pancreatic cancer cells to gemcitabine by
inhibition of the Akt-GSK3β-Snail pathway. Cell Death Dis.
6:e17952015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yi XP, Han T, Li YX, Long XY and Li WZ:
Simultaneous silencing of XIAP and survivin causes partial
mesenchymal-epithelial transition of human pancreatic cancer cells
via the PTEN/PI3K/Akt pathway. Mol Med Rep. 12:601–608. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cheng ZX, Wang DW, Liu T, Liu WX, Xia WB,
Xu J, Zhang YH, Qu YK, Guo LQ, Ding L, et al: Effects of the HIF-1α
and NF-κB loop on epithelial-mesenchymal transition and
chemoresistance induced by hypoxia in pancreatic cancer cells.
Oncol Rep. 31:1891–1898. 2014.PubMed/NCBI
|
|
16
|
Rao AR, Sindhuja HN, Dharmesh SM, Sankar
KU, Sarada R and Ravishankar GA: Effective inhibition of skin
cancer, tyrosinase, and antioxidative properties by astaxanthin and
astaxanthin esters from the green alga Haematococcus
pluvialis. J Agric Food Chem. 61:3842–3851. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nakao R, Nelson OL, Park JS, Mathison BD,
Thompson PA and Chew BP: Effect of dietary astaxanthin at different
stages of mammary tumor initiation in BALB/c mice. Anticancer Res.
30:2171–2175. 2010.PubMed/NCBI
|
|
18
|
Vena F, Li Causi E, Rodriguez-Justo M,
Goodstal S, Hagemann T, Hartley JA and Hochhauser D: The MEK1/2
inhibitor pimasertib enhances gemcitabine efficacy in pancreatic
cancer models by altering ribonucleotide reductase subunit-1
(RRM1). Clin Cancer Res. 21:5563–5577. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lai IL, Chou CC, Lai PT, Fang CS, Shirley
LA, Yan R, Mo X, Bloomston M, Kulp SK, Bekaii-Saab T and Chen CS:
Targeting the Warburg effect with a novel glucose transporter
inhibitor to overcome gemcitabine resistance in pancreatic cancer
cells. Carcinogenesis. 35:2203–2213. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
D'Angelo RC, Liu XW, Najy AJ, Jung YS, Won
J, Chai KX, Fridman R and Kim HR: TIMP-1 via TWIST1 induces EMT
phenotypes in human breast epithelial cells. Mol Cancer Res.
12:1324–1233. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Díaz-López A, Díaz-Martín J, Moreno-Bueno
G, Cuevas EP, Santos V, Olmeda D, Portillo F, Palacios J and Cano
A: Zeb1 and Snail1 engage miR-200f transcriptional and epigenetic
regulation during EMT. Int J Cancer. 136:E62–E73. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cho KH, Choi MJ, Jeong KJ, Kim JJ, Hwang
MH, Shin SC, Park CG and Lee HY: A ROS/STAT3/HIF-1α signaling
cascade mediates EGF-induced TWIST1 expression and prostate cancer
cell invasion. Prostate. 74:528–536. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang W, Shi X, Peng Y, Wu M, Zhang P, Xie
R, Wu Y, Yan Q, Liu S and Wang J: HIF-1α promotes
epithelial-mesenchymal transition and metastasis through direct
regulation of ZEB1 in colorectal cancer. PLoS One. 10:e01296032015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xiong H, Hong J, Du W, Lin YW, Ren LL,
Wang YC, Su WY, Wang JL, Cui Y, Wang ZH and Fang JY: Roles of STAT3
and ZEB1 proteins in E-cadherin down-regulation and human
colorectal cancer epithelial-mesenchymal transition. J Biol Chem.
287:5819–5832. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Montero TD, Racordon D, Bravo L, Owen GI,
Bronfman ML and Leisewitz AV: PPARα and PPARγ regulate the
nucleoside transporter hENT1. Biochem Biophys Res Commun.
419:405–411. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lei W, Feng XH, Deng WB, Ni H, Zhang ZR,
Jia B, Yang XL, Wang TS, Liu JL, Su RW, et al: Progesterone and DNA
damage encourage uterine cell proliferation and decidualization
through up-regulating ribonucleotide reductase 2 expression during
early pregnancy in mice. J Biol Chem. 287:15174–15192. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kaira K and Yamamoto N: Prognostic and
predictive factors in resected non-small-cell lung cancer. Expert
Opin Med Diagn. 4:373–381. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tassone P, Di Martino MT, Ventura M,
Pietragalla A, Cucinotto I, Calimeri T, Bulotta A, Neri P, Caraglia
M and Tagliaferri P: Loss of BRCA1 function increases the antitumor
activity of cisplatin against human breast cancer xenografts in
vivo. Cancer Biol Ther. 8:648–653. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
El-Khoueiry AB, Ramanathan RK, Yang DY,
Zhang W, Shibata S, Wright JJ, Gandara D and Lenz HJ: A randomized
phase II of gemcitabine and sorafenib versus sorafenib alone in
patients with metastatic pancreatic cancer. Invest New Drugs.
30:1175–1183. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kao YT, Hsu WC, Hu HT, Hsu SH, Lin CS,
Chiu CC, Lu CY, Hour TC, Pu YS and Huang AM: Involvement of p38
mitogen-activated protein kinase in acquired gemcitabine-resistant
human urothelial carcinoma sublines. Kaohsiung J Med Sci.
30:323–330. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yan Q, Chen P, Wang S, Liu N, Zhao P and
Gu A: Association between HIF-1α C1772T/G1790A polymorphisms and
cancer susceptibility: An updated systematic review and
meta-analysis based on 40 case-control studies. BMC Cancer.
14:9502014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Huang C and Xie K: Crosstalk of Sp1 and
Stat3 signaling in pancreatic cancer pathogenesis. Cytokine Growth
Factor Rev. 23:25–35. 2012. View Article : Google Scholar : PubMed/NCBI
|