Introduction
Breast cancer is the most common type of cancer in
women worldwide and the second most common type of cancer overall
(1–3).
Despite advances being made in therapeutic and diagnostic study,
only a fraction of treatment strategies are individualized, with
treatment based not only on a careful risk assessment for each
patient, but on specific clinicopathological features of the breast
cancer they suffer from (4–6). There are a number of established
predictive factors in breast cancer, the majority of which are also
therapeutic targets [including estrogen receptor, progesterone
receptor or human epidermal growth factor receptor 2 (Her2)]
(7). Therapeutic approaches are
primarily based on clinicopathological variables, including tumor
size, lymph node stage, histological grade, type and lymphovascular
invasion (8). However, there is a
limited choice of prognostic factors, which are essential for
decision-making, since these predict patient outcomes irrespective
of treatment (9). Furthermore, breast
cancer is one of the most heterogeneous diseases. There are a
growing number of aged patients (>75 years) with breast cancer,
and therefore there is an urgent requirement for personalized
therapies in order to avoid over- or under-treatment (10–12).
Multi-parameter gene expression analyses are evolving and
progressively suggesting promising markers (13–15). Thus,
it is important to study and propose methods for an effective and
efficient quantification of specific gene expression status
(16). At the same time, evidence of
statistical association between the gene expression and overall
survival (OS) and disease-free survival (DFS) are required for
designing antitumor management strategies and prognosis evaluation
(17,18).
Mammalian sterile 20-like kinase 1 (Mst1),
alternatively termed serine threonine kinase 4 (STK4), has been
known for decades, but regained significant attention when its role
as a tumor suppression gene in various entities of human cancers
was reported (19–22). Mst1 is a serine/threonine protein
kinase, which builds a complex with Mst2. Mst1/Mst2 are activated
by the phosphorylation of Thr183 and Thr180,
which leads to a feedback stimulation that regulates oxidant levels
through a number of mechanisms (23).
One of them is the regulation of cellular redox state (23). This specific mechanism may represent a
tumor suppressor function of Mst1/2 (23,24).
Mst1/2 kinases also affect immune cell activation, proliferation,
adhesion, migration, growth and apoptotic pathways, as they are
essential for the Hippo signaling pathway (25). Loss of Mst1 results in
hyper-proliferation and tumorigenesis (25). In addition, the Hippo pathway
interacts with other signaling pathways, including Wnt and Notch
pathways, known for their crucial roles in tumor pathogenesis
(26,27). Finally, the pro-apoptotic function of
Mst1 is also associated with pleckstrin homologydomain and
leucine-rich repeat protein phosphatases (28). These two proteins synergize to achieve
a greater potential of apoptosis (28). Phosphoinositide 3-kinase/protein
kinase Bacts as an inhibitor of Mst1 through phosphorylation of
threonine 120 (29).
Our previous study, for the first time, presented
data that supported the function of Mst1 as a prognostic factor in
human breast cancer, using immunostaining as the
Mst1-identification method (30).
This study demonstrated that Mst1-positive patients had a
significantly improved OS compared with Mst1-negative patients, and
that Mst1 is an independent prognostic factor in breast cancer. In
the present study, ELISA was performed to quantify Mst1
concentration in the serum of patients with breast cancer. The
methodological concept facilitates a direct translation into the
clinic, as it is easy, feasible, exact and less biased than
immunohistological estimations of the amount of Mst1 in tumor
cells. In addition, the sampling method is incomparably more
attractive for the daily routine and comfort of patients. The
present study demonstrated the prognostic significance of Mst1
expression for the rates of OS and DFS. The results of the present
study revealed the tumor suppressive function of Mst1, and
confirmed Mst1 as an independent prognostic factor in human breast
cancer.
Materials and methods
Ethical approval
The present study was performed at the Central
Laboratory and Department of Breast Surgery, Yangpu Hospital,
Tongji University School of Medicine (Shanghai, China), and was
approved by the local institutional review board. Written consent
forms were collected from all patients involved in the present
study. The ethics review board of Tongji University School of
Medicine approved the study design.
Study population
Blood samples used in the present study were
collected between January 2005 and December 2006 in the Department
of Breast Surgery, Yangpu Hospital, Tongji University School of
Medicine. In total, 98 women were included in the study, since they
completed the entire period of follow-up. All blood samples were
taken prior to any surgical interventions or antitumor treatment.
Data of patients, including age, tumor size, tumor stage,
histological grade, node status, histological type, molecular
subtypes, hormone receptor status and Her2 status were obtained
from the pathological reports. Table
I describes the baseline demographics of the study population.
The distribution of tumor grades and receptor status were
representative. The majority of the patients presented with
carcinoma of a ductal type with luminal subtype, grade 2. All
patients, the median age was 52 and the age range of patients was
35–73, were Chinese females and were followed until mortality or
the end of the follow-up period of 98 months.
 | Table I.Patients and tumor
characteristics. |
Table I.
Patients and tumor
characteristics.
| Variables | Patients, n
(%) |
|---|
| Age, years |
|
|
<50 | 27 (27.6) |
|
≥50 | 71 (72.4) |
| Tumor size, cm |
|
|
<2 | 42 (42.9) |
| ≥2 | 56 (57.1) |
| Tumor stage |
|
| T1 | 27 (27.6) |
| T2 | 52 (53.1) |
| T3 | 19 (19.3) |
| Histological
grade |
|
| G1 | 5 (5.1) |
| G2 | 78 (79.6) |
| G3 | 15 (15.3) |
| Lymph node
status |
|
|
Negative | 57 (58.2) |
|
Positive | 41 (41.8) |
| Histological
type |
|
|
Ductal | 87 (88.8) |
|
Others | 11 (11.2) |
| Molecular
subtypes |
|
|
Luminal | 68 (30.6) |
|
Others | 30 (69.4) |
| HR status |
|
|
Negative | 30 (30.6) |
|
Positive | 68 (69.4) |
| Her2 status |
|
|
Negative | 71 (72.4) |
|
Positive | 27 (27.6) |
| Mst1 status |
|
|
Negative | 13 (13.3) |
|
Positive | 85 (86.7) |
Indirect ELISA detection of Mst1 in the plasma of
patients. ELISA detection was used as an efficient and effective
method in order to assess the expression level of Mst1 in plasma
samples and associated them with the survival of patients. A total
of 98 human plasma samples were assayed by ELISA using Mst1/STK4
(C-term) antibodies. The Mst1/STK4 purified protein was included in
the assay system as a positive control for specificity and
sensitivity, as well as to create a calibration curve. Each assay
was repeated three times.
In brief, flat-bottom 96-well Costar plates were
coated with 100 µl per well of rabbit polyclonal antibody specific
for human Mst1/STK4 (cat. no. 3682; Cell Signaling Technology,
Inc., Danvers, MA, USA) at a concentration of 1 µg/ml in carbonate
buffer (15 mM Na2CO3, 35 mM
NaHCO3, pH 9.6) as previously described (31). Following an overnight incubation at
4°C, the plates were washed three times with PBS-Tween-20 (PBST;
1.47 mmol/l KH2PO4, 8.10 mmol/l
Na2HPO4, 136.89 mmol/l NaCl, 2.68 mmol/l KCl,
0.05% Tween 20), blocked with blocking buffer [1% bovine serum
albumin (BSA; w/v; cat. no. A3858; Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) in PBST] for 1 h at 37°C, followed by washing
with PBST three times. Subsequently, the clinical plasma samples
were diluted 5-fold (1:5) in sample diluent (1% BSA in PBST).
Pre-diluted samples (100 µl) was added into micro ELISA plate
wells. PBS served as a blank control. Following incubation for 2 h
at 37°C, the wells were again washed and filled with 100 µl of a
1:10,000 dilution of horseradish peroxidase-conjugated goat
anti-rabbit antibody (cat. no. 1662408EDU; Bio-Rad Laboratories,
Inc., Hercules, CA, USA). Following incubation for 1 h at 37°C,
plates were again washed with PBST, and wells were filled with 100
µl 3,3′,5,5′-tetramethylbenzidine substrate solution and incubated
for 15 min at room temperature in the dark. The reaction was
stopped by adding 50 µl 2 mol/l H2SO4 per
well. The optical density of each well was measured at a wavelength
of 450 nm in the ELISA plate reader. Calibration curves were
generated with log10 Mst1/STK4 purified protein
concentrations plotted along the x-axis. If the detected values
were higher than average, plasma samples were judged as
positive.
Statistical analysis
Data were analyzed by SPSS standard version 20.0
(IBM SPSS, Armonk, NY, USA). The Kaplan-Meier method was used to
estimate OS and DFS. A log rank test was used to compare the
survival curves. Multivariate analysis was performed by Cox
proportional hazards model. OS was calculated from the date of
diagnosis to the date of mortality or the last follow-up. DFS was
calculated from the date of surgery to the date of disease relapse.
The differences between groups were analyzed using an unpaired
two-tailed Student's t-test. All P-values were two-tailed.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Patient characteristics
Characteristics of the 98 patients enrolled in the
present study are summarized in Table
I. No patients succumbed and no patients withdrew during the
study period. The follow-up time was 98 months.
Mst1 levels in patients with breast
cancer
A total of 98 human plasma samples were assayed by
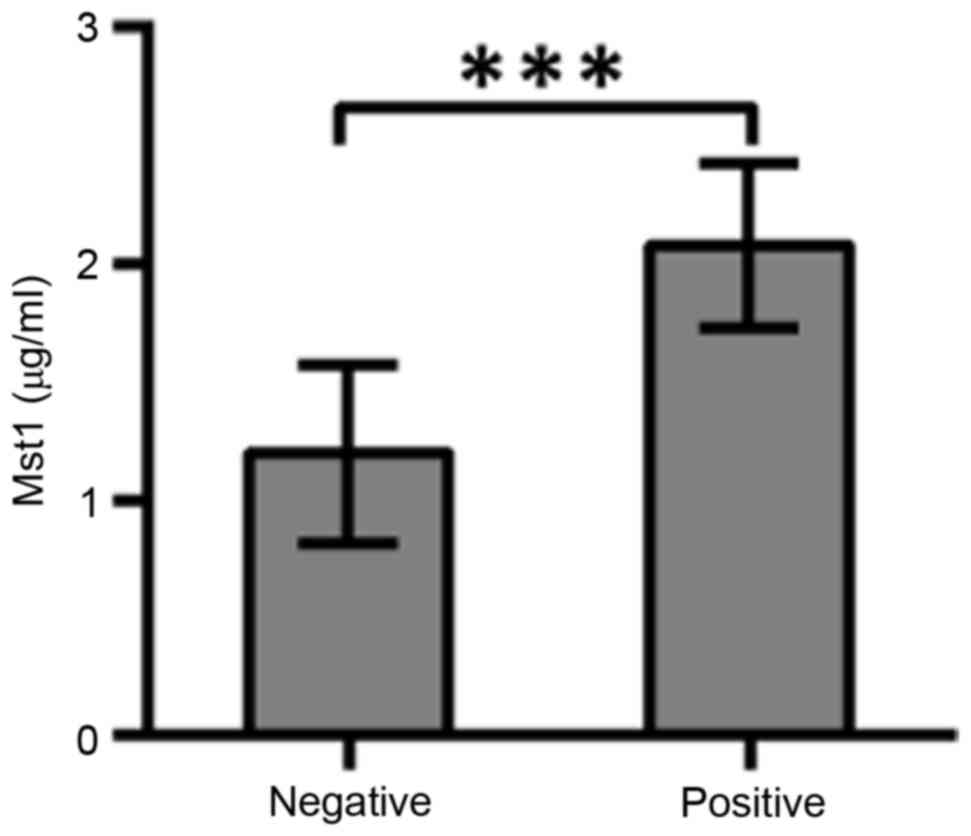
ELISA. The average Mst1 value of 98 human plasma samples was 1.8
µg/ml. Profiles of immunoglobulin IgG antibodies against human
plasma Mst1 antigens were estimated by indirect ELISA (Fig. 1). The average concentration of 1.8
µg/ml was used to discriminate the status of Mst1-positivevs.
Mst1-negative breast cancers. The IgG level of Mst1-positive vs.
Mst1-negative patients was significantly different (P<0.0001;
t=9.167). In total, 85 Mst1-positive and 13 Mst1-negative patients
with breast cancer were identified.
Association of Mst1 levels with OS and
DFS
To evaluate the significance of Mst1 as a clinical
prognostic factor in patients with breast cancer, the two groups of
patients were followed up, and the associations between OS, DFS and
Mst1 levels were investigated. Patients with positive expression of
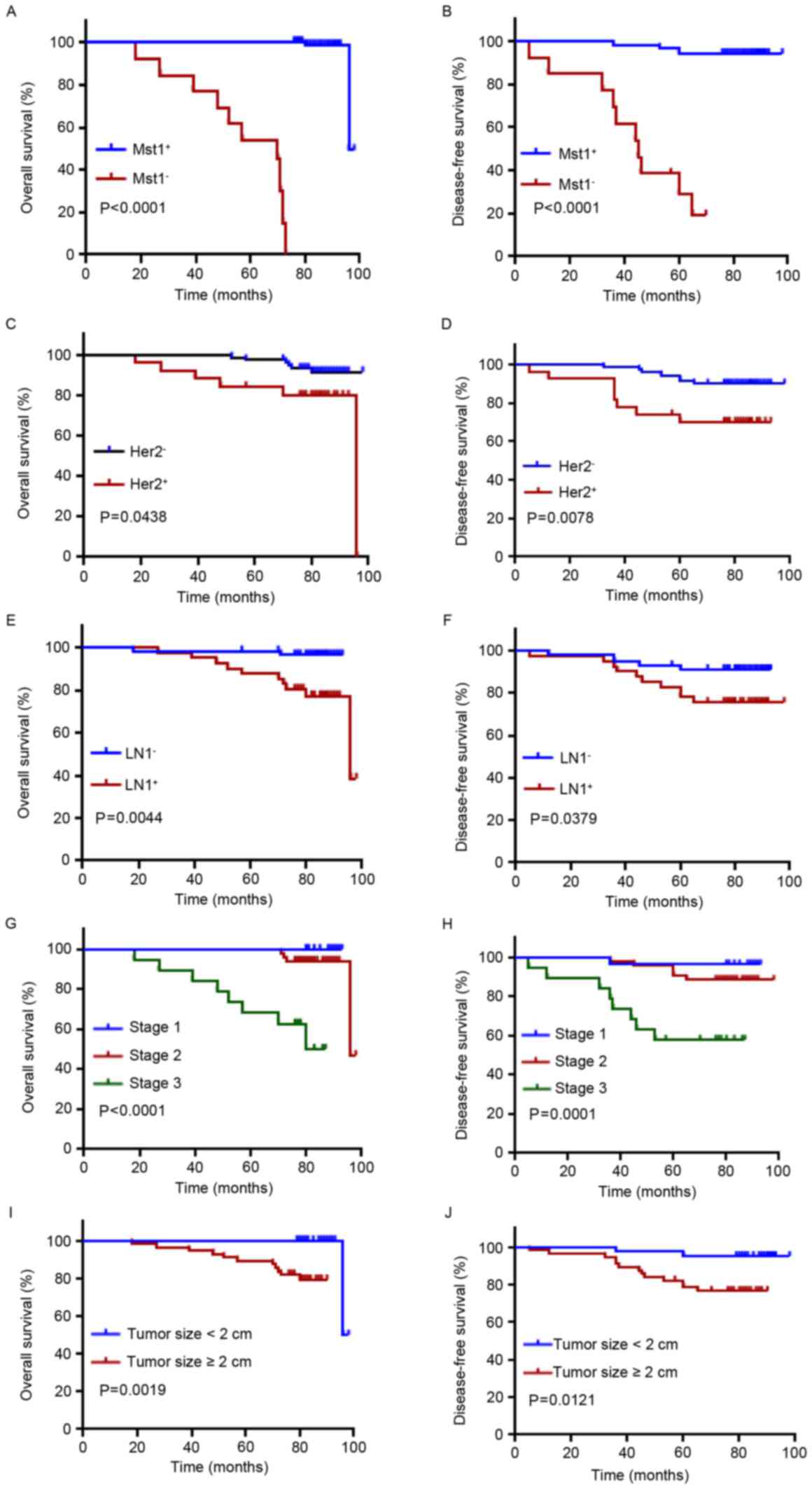
Mst1 had a significantly improved OS and DFS compared with patients
with negative Mst1 expression (P<0.0001; Fig. 2A and B). Univariate Cox analysis
indicated that Mst1 positivity had a significant difference in OS
in patients with breast cancer (P=0.010). In multivariate Cox
analysis, Mst1 positivity maintained significance as an independent
prognostic factor in breast cancer (P=0.002; Table II).
 | Table II.Univariate and multivariate analysis
of overall survival by the Cox proportional hazards model. |
Table II.
Univariate and multivariate analysis
of overall survival by the Cox proportional hazards model.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Clinicopathological
variables | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age | 0.999
(0.922–1.082) | 0.982 | 0.696
(0.388–1.247) | 0.224 |
| Tumor size | 15.987
(5.135–49.777) | 0.000 | 0.079
(0.000–233.758) | 0.534 |
| Tumor stage | 12.569
(3.514–44.961) | 0.000 | 9.002×103
(0.000–5.364×1034) | 0.801 |
| Histological
grade | 0.750
(0.205–2.743) | 0.663 | 0.081
(0.001–5.026) | 0.233 |
| Lymph node
status | 6.811
(1.471–31.545) | 0.014 |
7.377(0.581–93.673) | 0.123 |
| Histological
type | 1.768
(0.659–4.740) | 0.257 | 1.860
(0.185–18.743) | 0.598 |
| Molecular
subtypes | 1.347
(0.822–2.207) | 0.237 | 7.377
(0.581–93.673) | 0.239 |
| ER/PR status | 0.757
(0.222–2.587) | 0.657 | 0.002
(0.000–16.843) | 0.172 |
| Her2 status | 2.187
(0.793–6.033) | 0.130 | 5.215×103
(0.347–7.837×107) | 0.081 |
| Mst1 | 1.157
(1.065–1.257) | 0.010 |
1.445(1.251–1.670) | 0.002 |
Associations between OS, DFS and
clinicopathological features
As expected, OS and DFS were significantly improved
in patients with Her2-negative breast cancer (Fig. 2C, P=0.0438; Fig. 2D, P=0.0078), lymph node-negative
breast cancer (Fig. 2E, P=0.0044;
Fig. 2F, P=0.0379), stage 1 and 2
breast cancer (Fig. 2G, P<0.0001;
Fig. 2H, P=0.0001) and tumor size
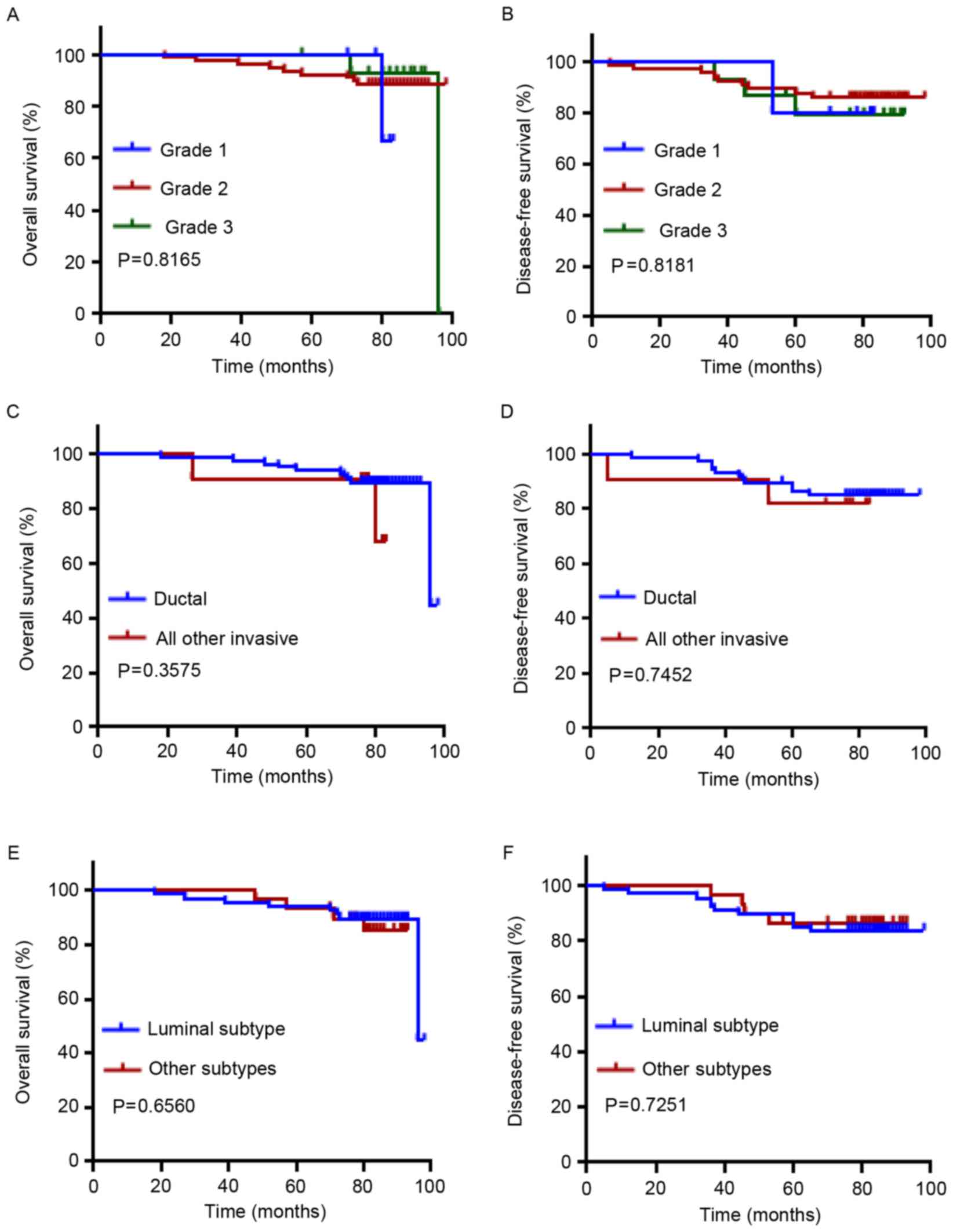
<2 cm (Fig. 2I, P=0.0019; Fig. 2J, P=0.0121). Classification of grades
and pathological types (ductal vs. all others) did not reveal a
prognostic significance (Fig. 3A-D).
In addition, no prognostic significance was observed in the
comparison of molecular subtypes of breast cancer (luminal vs.
others; Fig. 3E and F).
Discussion
Breast cancer is the most common type of
non-cutaneous cancer and the leading cause of cancer-associated
mortality among women (1–4). Along with demographical aging and cancer
risk factors associated with modern lifestyle, female breast cancer
incidence rates demonstrate a rising tendency (5). Screening techniques remain a crucial
part of prevention and reduction of breast cancer mortality
(1).
Diagnostic tumor markers are gaining increasing
importance as prognostic and predictive factors (32–34).
Current breast cancer markers, including hormone receptor status,
tumor-node-metastasisand grading are notsufficient, since breast
canceris complex, heterogeneous and alterable (35). Oncologists aim to identify high risk
individuals, detect cancer at an early stage, predict outcome,
monitor treatment and screen for disease recurrence. During tumor
progression, metastasis and anticancer therapy, molecular changes
result in various constellations of potential marker proteins
(33,36,37). To
date, comparatively few markers have been established (38). Therefore, it is crucial to identify
easily detectable, non-invasive, novel biological markers with
predictive power.
The results of the present study are promising for
the use of Mst1 level as an outcome predictor in patients with
breast cancer. Mst1 overexpression has been reported to inhibit the
growth of human non-small cell lung cancer in vitro and
in vivo, reduce intestinal stem cell proliferation and
colonic tumorigenesis, inhibit cell proliferation and induce
apoptosis of HepG2 cells, and induce cisplatin chemosensitivity in
hepatocellular carcinoma (20). In
colon cancer, nuclear Mst1 expression was associated with tumor
grade and shortened survival time (39). Loss of cytoplasmic Mst1 expression is
a marker of tumor progression in mismatch-repair-proficient as well
as mismatch-repair-deficient colorectal cancers (39). Methylation of the Mst1 promoter is
associated with a significantly decreased risk of tumor-associated
mortality in patients with soft tissue sarcomas, while alterations
of the Mst signaling pathway contribute to poor prognosis (40).
Mst1 is a member of the yeast Ste20-related kinase
family and a component of the Ras association domain family member
1-large tumor suppressor kinase 1tumor suppressor network (41,42).
Although its physiological function remains to be fully
established, it has been proposed as a tumor suppressor protein due
to its association with cell proliferation and apoptosis (43). Mst1 is also involved in diverse
biological processes, including cellular responses to oxidative
stress and longevity (43).
Deregulation of these fundamental developmental processes may lead
to cancer. The molecular mechanisms are known in Drosophila,
where the Hippo signaling pathway controls organ size by
restricting mitosis and promoting cell death. In mammals, Mst1, a
murine homolog of the Drosophila Hippo, contributes to size
control of certain organs, but not all (44).
In the present study, the Mst1 levels of 98 patients
with breast cancer with a follow-up period of 98 months were
analyzed, and the association of Mst1 levels with survival and
clinicopathological characteristics of patients were assessed. In
contrast to our previous study (30),
a more exact quantification method of ELISA was performed. Using
this method obtained objective numeric values (Mst concentration)
rather than biased immunohistochemistry-based observations on Mst1
amounts. Additionally, the plasma of patients was used as the
detection material. This sampling is easier and more feasible than
tumor tissues. To summarize, a novel, easy and effective way to
assess Mst1 levels in patients with breast cancer was proposed,
which may be further used to predict their prognosis and therapy
response.
A cut off was established, and patients were divided
into Mst1-positive and Mst1-negative groups. It was revealed that
Mst1 positivity was significantly associated with OS, and
Mst1-positive patients had an improved OS and DFS compared with
Mst1-negative patients. Multivariate analysis also indicated that
Mst1 positivity was an independent prognostic factor for breast
cancer.
The present, long-term, follow-up study demonstrated
that Mst1 expression has prognostic significance in patients with
breast cancer and may present potential opportunities for breast
cancer therapy in the future.
Acknowledgements
The present study was supported by the National Key
Basic Research Program of China (grant no. 2013CB531601), the
National Natural Science Foundation of China (grant no. 30972633),
the Scientific Research Foundation for the Returned Overseas
Chinese Scholars, State Education Ministry (grant no. 2015-311),
the Shanghai Health and Family Planning Commission Project (grant
nos. 20134298 and 201640253), the Shanghai Health and Family
Planning Commission Fund for Qing Nian Yi Shi Training Project
(grant no. 2014118), the Shanghai Yangpu District Science and
Technology Commission Project (grant no. 2016-2017), the Shanghai
Yangpu District Health and Family Planning Commission Project
(grant nos. 2011-2013 and 2016-2017), the Shanghai Yangpu District
Health and Family Planning Commission Fund for Bai Yi Deng Gao
Training Project (grant no. 2014-2016) and the Academic Leader in
Climbing Program from Yang-Pu Center Hospital (grant no.
YE2201608).
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Swanton C, Burrell RA and Futreal PA:
Breast cancer genome heterogeneity: A challenge to personalised
medicine? Breast Cancer Res. 13:1042011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jackson SE and Chester JD: Personalised
cancer medicine. Int J Cancer. 137:262–266. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tyrer J, Duffy SW and Cuzick J: A breast
cancer prediction model incorporating familial and personal risk
factors. Stat Med. 23:1111–1130. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yersal O and Barutca S: Biological
subtypes of breast cancer: Prognostic and therapeutic implications.
World J Clin Oncol. 5:412–424. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Garg M, Nagpal N, Sidhu DS and Singh A:
Effect of lump size and nodal status on prognosis in invasive
breast cancer: Experience from Rural India. J Clin Diagn Res.
10:PC08–PC11. 2016.PubMed/NCBI
|
|
9
|
Senkus E, Cardoso F and Pagani O: Time for
more optimism in metastatic breast cancer? Cancer Treat Rev.
40:220–228. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Marmé F and Schneeweiss A: Personalised
therapy in breast cancer. Onkologie. 35 Suppl 1:S28–S33. 2012.
View Article : Google Scholar
|
|
11
|
Andersen SL, Terry DF, Wilcox MA, Babineau
T, Malek K and Perls TT: Cancer in the oldest old. Mech Ageing Dev.
126:263–267. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lichtman SM: Guidelines for the treatment
of elderly cancer patients. Cancer Control. 10:445–453. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Baird RD and Caldas C: Genetic
heterogeneity in breast cancer: The road to personalized medicine?
BMC Med. 11:1512013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sabatier R, Gonçalves A and Bertucci F:
Personalized medicine: Present and future of breast cancer
management. Crit Rev Oncol Hematol. 91:223–233. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dawson SJ, Rueda OM, Aparicio S and Caldas
C: A new genome-driven integrated classification of breast cancer
and its implications. EMBO J. 32:617–628. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ellsworth RE, Decewicz DJ, Shriver CD and
Ellsworth DL: Breast cancer in the personal genomics era. Curr
Genomics. 11:146–161. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dancey JE, Bedard PL, Onetto N and Hudson
TJ: The genetic basis for cancer treatment decisions. Cell.
148:409–420. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
André F, Bachelot T, Commo F, Campone M,
Arnedos M, Dieras V, Lacroix-Triki M, Lacroix L, Cohen P, Gentien
D, et al: Comparative genomic hybridisation array and DNA
sequencing to direct treatment of metastatic breast cancer: A
multicentre, prospective trial (SAFIR01/UNICANCER). Lancet Oncol.
15:267–274. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Song H, Mak KK, Topol L, Yun K, Hu J,
Garrett L, Chen Y, Park O, Chang J, Simpson RM, et al: Mammalian
Mst1 and Mst2 kinases play essential roles in organ size control
and tumor suppression. Proc Natl Acad Sci USA. 107:1431–1436. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Qin F, Tian J, Zhou D and Chen L: Mst1 and
Mst2 kinases: Regulations and diseases. Cell Biosci. 3:312013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cinar B, Collak FK, Lopez D, Akgul S,
Mukhopadhyay NK, Kilicarslan M, Gioeli DG and Freeman MR: MST1 is a
multifunctional caspase-independent inhibitor of androgenic
signaling. Cancer Res. 71:4303–4313. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Abdollahpour H, Appaswamy G, Kotlarz D,
Diestelhorst J, Beier R, Schäffer AA, Gertz EM, Schambach A, Kreipe
HH, Pfeifer D, et al: The phenotype of human STK4 deficiency.
Blood. 119:3450–3457. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Praskova M, Khoklatchev A, Ortiz-Vega S
and Avruch J: Regulation of the MST1 kinase by autophosphorylation,
by the growth inhibitory proteins, RASSF1 and NORE1, and by Ras.
Biochem J. 381:453–462. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Graves JD, Gotoh Y, Draves KE, Ambrose D,
Han DK, Wright M, Chernoff J, Clark EA and Krebs EG:
Caspase-mediated activation and induction of apoptosis by the
mammalian Ste20-like kinase Mst1. EMBO J. 17:2224–2234. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Qin F, Tian J, Zhou D and Chen L: Mst1 and
Mst2 kinases: Regulations and diseases. Cell Biosci. 3:312013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rawat SJ, Creasy CL, Peterson JR and
Chernoff J: The tumor suppressor Mst1 promotes changes in the
cellular redox state by phosphorylation and inactivation of
peroxiredoxin-1 protein. J Biol Chem. 288:8762–8771. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Guo C, Zhang X and Pfeifer GP: The tumor
suppressor RASSF1A prevents dephosphorylation of the mammalian
STE20-like kinases MST1 and MST2. J Biol Chem. 286:6253–6261. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Warfel NA and Newton AC: Pleckstrin
homology domain leucine-rich repeat protein phosphatase (PHLPP): A
new player in cell signaling. J Biol Chem. 287:3610–3616. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Artemenko Y, Batsios P, Borleis J, Gagnon
Z, Lee J, Rohlfs M, Sanséau D, Willard SS, Schleicher M and
Devreotes PN: Tumor suppressor Hippo/MST1 kinase mediates
chemotaxis by regulating spreading and adhesion. Proc Natl Acad Sci
USA. 109:13632–13637. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lin X, Cai F, Li X, Kong X, Xu C, Zuo X
and Yang Q: Prognostic significance of mammalian sterile 20-like
kinase 1 in breast cancer. Tumour Biol. 34:3239–3243. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pan X, Zeng SL, Yu DH, Liang XL, Ji CD,
Pan BJ, Cai JL, Wang Y, Min Y, Fang W and Liao WQ: Variable domain
of the heavy chain of heavy-chain antibody of the Rv0733 antigen of
mycobacterium tuberculosis panned and identified from a nonimmune
llama VHH phage display library. Int J Clin Exp Pathol.
9:2869–2878. 2016.
|
|
32
|
Yoneda A, Lendorf ME, Couchman JR and
Multhaupt HA: Breast and ovarian cancers: A survey and possible
roles for the cell surface heparan sulfate proteoglycans. J
Histochem Cytochem. 60:9–21. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hirata Banin BK, Oda JM, Guembarovski Losi
R, Ariza CB, de Oliveira CE and Watanabe MA: Molecular markers for
breast cancer: Prediction on tumor behavior. Dis Markers.
2014:5131582014.PubMed/NCBI
|
|
34
|
Esteva FJ and Hortobagyi GN: Prognostic
molecular markers in early breast cancer. Breast Cancer Res.
6:109–118. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
35
|
Prat A, Parker JS, Karginova O, Fan C,
Livasy C, Herschkowitz JI, He X and Perou CM: Phenotypic and
molecular characterization of the claudin-low intrinsic subtype of
breast cancer. Breast Cancer Res. 12:R682010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kumar R: Breast cancer tumor markers. J
Solid Tumors. 2:432012. View Article : Google Scholar
|
|
37
|
Payne SJ, Bowen RL, Jones JL and Wells CA:
Predictive markers in breast cancer-the present. Histopathology.
52:82–90. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Penault-Llorca F and Viale G: Pathological
and molecular diagnosis of triple-negative breast cancer: A
clinical perspective. Ann Oncol. 23 Suppl 6:vi19–vi22. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Minoo P, Zlobec I, Baker K, Tornillo L,
Terracciano L, Jass JR and Lugli A: Prognostic significance of
mammalian sterile 20-like kinase 1 in colorectal cancer. Mod
Pathol. 20:331–338. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Seidel C, Schagdarsurengin U, Blümke K,
Würl P, Pfeifer GP, Hauptmann S, Taubert H and Dammann R: Frequent
hypermethylation of MST1 and MST2 in soft tissue sarcoma. Mol
Carcinog. 46:865–871. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Cinar B, Collak FK, Lopez D, Akgul S,
Mukhopadhyay NK, Kilicarslan M, Gioeli DG and Freeman MR: MST1 is a
multifunctional caspase-independent inhibitor of androgenic
signaling. Cancer Res. 71:4303–4313. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Oh HJ, Lee KK, Song SJ, Jin MS, Song MS,
Lee JH, Im CR, Lee JO, Yonehara S and Lim DS: Role of the tumor
suppressor RASSF1A in Mst1-mediated apoptosis. Cancer Res.
66:2562–2569. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Lehtinen MK, Yuan Z, Boag PR, Yang Y,
Villén J, Becker EB, DiBacco S, de la Iglesia N, Gygi S, Blackwell
TK and Bonni A: A conserved MST-FOXO signaling pathway mediates
oxidative-stress responses and extends life span. Cell.
125:987–1001. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Avruch J, Zhou D, Fitamant J, Bardeesy N,
Mou F and Barrufet LR: Protein kinases of the Hippo pathway:
Regulation and substrates. Semin Cell Dev Biol. 23:770–784. 2012.
View Article : Google Scholar : PubMed/NCBI
|