Introduction
Increased lymphatic vessel density (LVD) in the
peritumoral and/or intratumoral regions has been reported to be
associated with the presence of lymphatic vessel invasion (LVI),
resulting in regional lymph node metastases and poor prognosis, and
reflects tumor lymphangiogenesis in carcinomas of various sites
(1–7).
Endometrioid carcinoma is the most
frequently-occurring carcinoma of the uterine corpus. The incidence
of nodal metastases is relatively low and the presence or degree of
lymphovascular invasion was related to the nodalmetastases or worse
survival (8–11). However, an association between the LVD
and the frequency of LVI has not been reported. Histologically, the
lymphatic vessels with tumor invasion are usually scattered, not
continuous or numerous in the peritumoral region of the cases even
with nodal metastases. In the articles published so far regarding
the carcinomas of other sites (1–7), the
association has been examined by comparing the LVDs between two
groups, one with the presence of LVI and the other with the absence
of LVI. We think that if tumor lymphangiogenesis causes LVI by
increasing LVD, the LVD in an area including LVI should be higher
than in an area not including LVI within a case. So we would select
cases with nodal metastases and investigate the association in a
different, more detailed way, by examining the LVDs in two types of
hot spots, an LVI-present (LVI+) area and an LVI-absent
(LVI−) area, in both the inner- and outer-half
myometrium of the peritumoral region in a case. Since the
distribution of lymphatic vessels in the uterine corpus tends to be
irregular and random (12,13), the numbers of lymphatic vessels could
originally be different between the inner- and the outer-half
myometrium. We would also investigate the LVDs in the control
regionfor the comparison.
Second, we would examine whether the destruction of
the existing myometrium influences the LVD, by investigating the
LVD in the intratumoral region, which we define as a region where
residual muscular tissue is not recognizable with desmin
immnostaining. In the breast and prostate (14–16), the
LVDs have been reported to increase in the peritumoral region but
not in the intratumoral region, where the existing architecture is
destroyed by carcinoma.
In addition, we would investigate the LVDs in inner-
and outer-half myometrium of the peritumoral and control regions in
cases without nodal metastases as a negative control group.
By clarifying the questions mentioned above, we
would assess the prognostic significance of tumor lymphangiogenesis
and LVD in endometrioid carcinoma of the uterine corpus.
Materials and methods
Case selection
Out of the 222 cases diagnosed as endometrioid
carcinoma of the uterine corpus that underwent total abdominal
hysterectomy, bilateral salpingo-oophorectomy and lymph node
dissection (pelvic or pelvic and para-aortic lymph nodes) without
chemoradiation therapy at our institution between 2008 and 2015, 20
cases which also had endometrioid carcinoma in the ovary were
excluded. Twenty-four cases of the remaining 202 cases showed nodal
metastases. Nineteen of these 24 cases, where the carcinoma reached
the outer-half myometrium (N+ group), were selected. As
a negative control group, 29 cases in stage IB with more than 20
lymph nodes dissected showing no metastases (N− group),
were selected during the same period.
The age of the N+ group ranged from 38 to
74 with a mean of 58.4. The tumor grade was assessed according to
the FIGO system, and the cases consisted of G1, n=7, G2, n=8 and
G3, n=4. The age of the N− group ranged from 39 to 73
with a mean of 58.6. The cases consisted of G1, n=16, G2, n=8 and
G3, n=5.
Immunohistochemistry
Several paraffin blocks, containing lesions of the
deepest invasion, LVI and control areas for each case, were chosen
for immunohistochemical examination using antibodies against D2-40
(M3619, Dako, Glostrup, Denmark) and desmin (D33; Dako). A D2-40
antibody was used for the detection of lymphatic vessels and a
desmin antibody for the recognition of the existing muscular
tissue. Four-micrometer-thick sections were obtained from
paraffin-embedded blocks. Immunohistochemistry was conducted using
a BenchMark XT (Ventana Medical Systems Inc., Tucson, AZ, USA) and
an I-View DAB detection kit (Ventana Medical Systems Inc.) at a
dilution of 1:100 for both antibodies.
Quantification of lymphatic
vessels
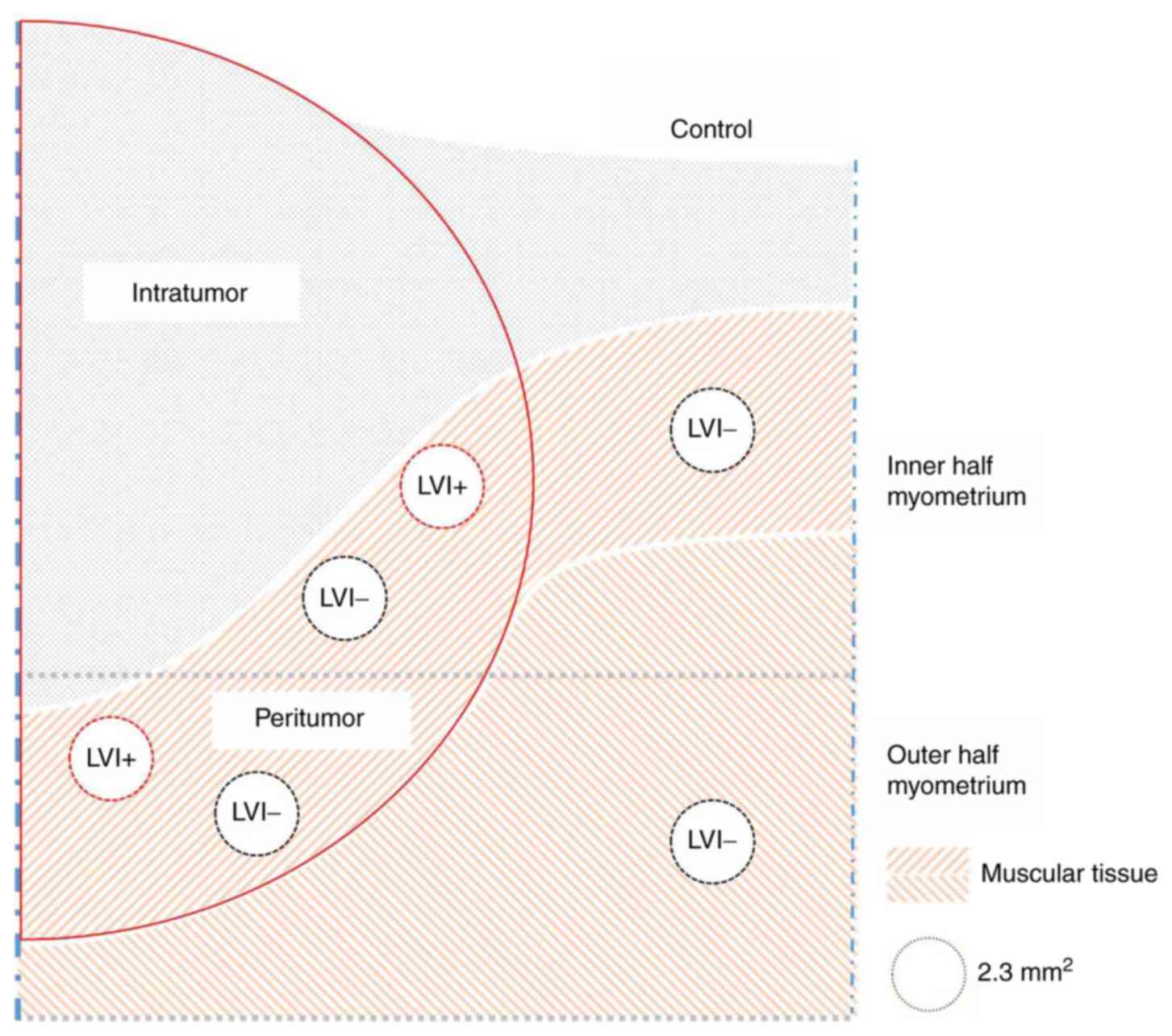
The three compartments, the intratumoral,
peritumoral and control compartments of the uterine corpus were
defined as follows. The intratumoral compartment was defined as the
area encompassing all the cancer glands. The stroma of the
intratumoral compartment was the region where the residual muscular
tissue was not recognizable with desmin-immunostaining. The
peritumoral compartment was a 2 mm-wide area around the intraumoral
compartment. The peritumoral compartment was defined as a region
where the existing muscular tissue was preserved. The control
compartment was an area beyond the peritumoral compartment.
A hot spot on a D2-40 immunostained slide was chosen
at 100× magnification through cell Sense Standard (a software
installed to the camera connected to the microscope, Olympus) and
the view of the chosen area (2.3 mm2) was printed out
for manual counting of lymphatic vessels. We refer to the number of
the lymphatic vessels per 2.3 mm2 as LVD. Hot spots were
chosen for the LVI+ and LVI− areas of both
the inner- and outer-half myometrium in the peritumoral
compartment, and similarly for the LVI− area in the
control compartment, which amounted to 6 spots examined per case
(Fig. 1). In addition, for the
LVI+ area, the number of lymphatic vessels with tumor
invasion (LVD with tumor invasion) was also counted.
For the intratumoral region, when the lymphatic
vessels were recognized, the LVD was similarly counted and if LVI
was present, the LVD with tumor invasion was also counted.
For the N− group, the LVDs in both inner-
and outer-half myometrium of the peritumoral and control
compartments were counted. Only one hot spot was examined in each
part of the myometrium of the peritumoral compartment regardless of
LVI.
Statistical analysis
The LVDs in the peritumoral and control compartments
were statistically compared within each group. And the LVDs in the
inner- and outer-half myometrium of the peritumoral regions were
compared respectively betweengroups, in which case the higher LVD
of each location of the peritumoral compartment in the
N+ group was chosen for the comparison regardless of the
LVI status. Statistical analyses were performed using Statcel 3.
Student's t-test, Welch t-test or Mann-Whitney's test was used for
the comparison. P<0.05 was considered to indicate a
statistically significant difference.
Spearman's rank correlation test was used to assess
the correlation between the LVD with tumor invasion and the LVD in
the LVI+ areas of the peritumoral compartment of the
N+ group.
Results
On Hematoxylin and eosin (H&E) slides, in the
endometrium all cases showed continuous expansive proliferation of
carcinoma with little stroma. In the myometrium, on the other hand,
G1 and G2 tumors generally showed a discontinuous, adenomyosis-like
pattern of infiltration with some desmoplastic stroma or muscular
tissue intervening. In contrast, G3 tumors showed an expansive
pattern of infiltration without intervening stroma as seen in the
endometrium.
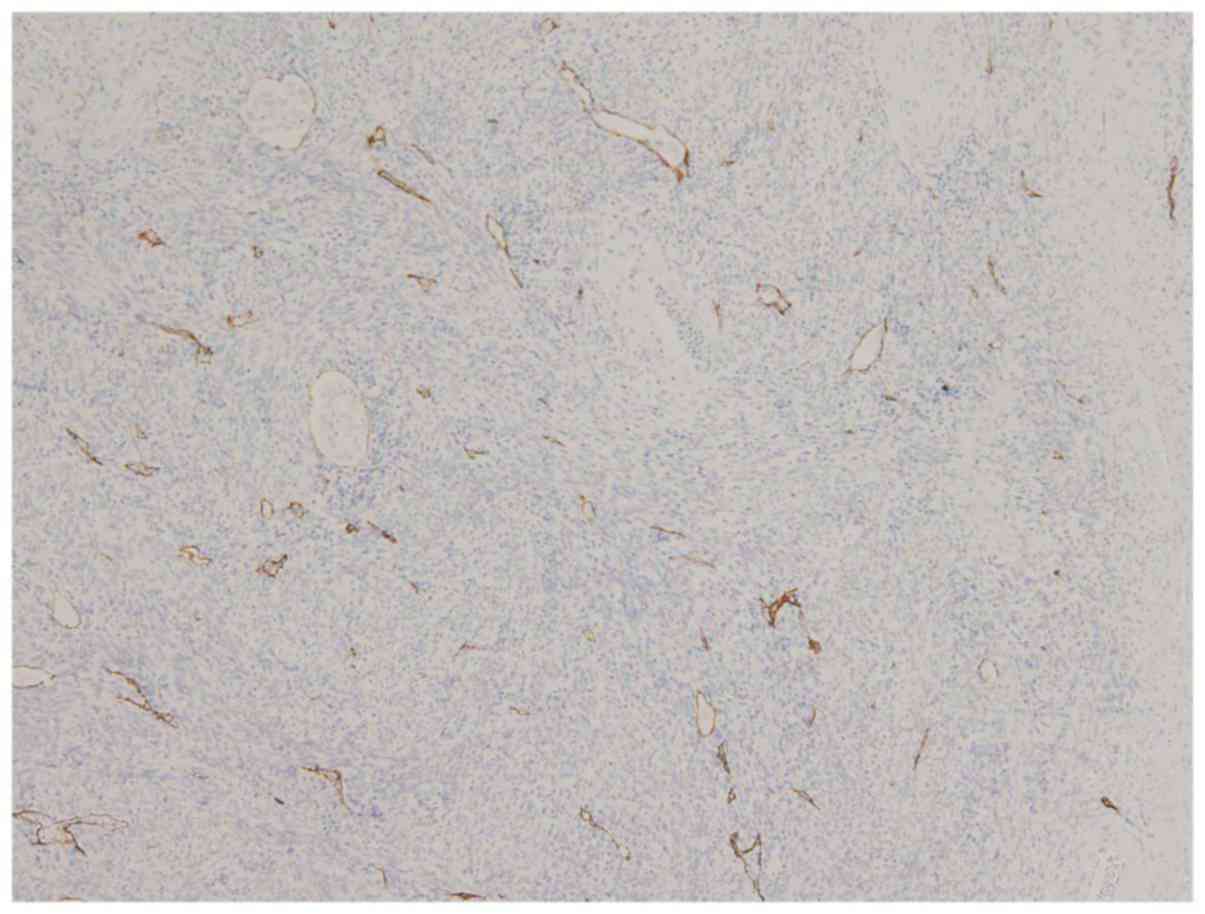
At least one LVI was suspected on H&E slides and
confirmed with D2-40 immunostaining in all 19 cases of the
N+ group. In the peritumoral compartment, the LVI was
present (Fig. 2) in both the inner-
and outer-half myometrium in 14 of the 19 cases, only in the
inner-half in one case, in the outer-half in 3 cases and in neither
of them in one case, which showed the LVI only in the intratumoral
compartment (Table I). The number of
cases available for examining the LVD of the inner-half myometrium
of the control compartment was 18 with one case showing ubiquitous
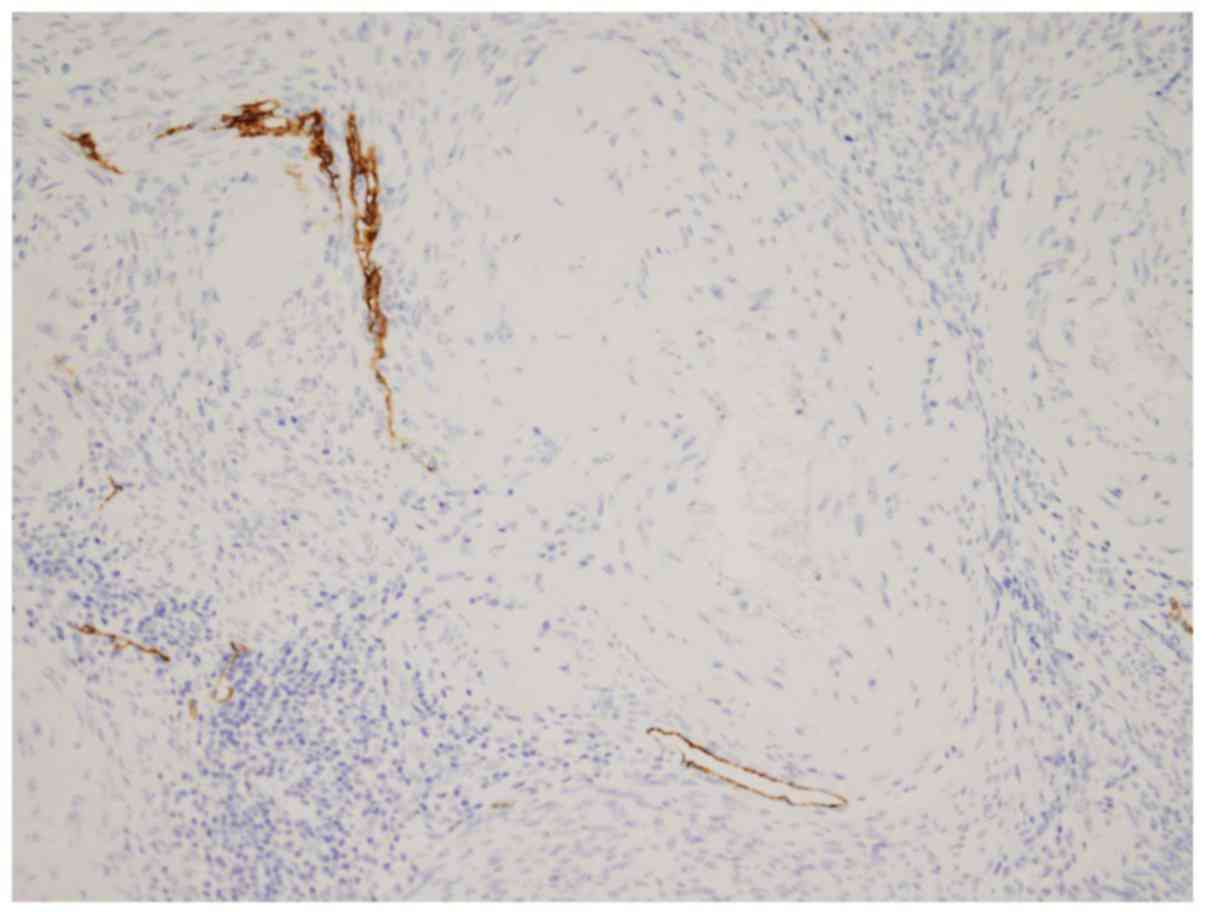
LVI in the area excluded. A close proximity between the lymphatic
vessels and small-to medium-sized arteries was noted in all
compartments (Fig. 3).
 | Table I.Location of LVI in the peritumoral
compartment of 19 cases in N+ group. |
Table I.
Location of LVI in the peritumoral
compartment of 19 cases in N+ group.
| Location | Presence of LVI |
|---|
| Inner-half
myometrium | + | + | − | − |
|
| Outer-half
myometrium | + | − | + | − |
|
| Number of cases | 14 | 1 | 3 | 1a | 19 |
In the N− group, even when LVI was
unrecognized on H&E stained slides, at least one LVI was
suspected on D2-40 immunostained slides in most of the cases. The
absence of LVI was confirmed in only 2 cases.
<LVDs of the N+ and the N−
groups> In the N+ group (Table IIA) the LVDs of the LVI+
and LVI− areas in the peritumoral and control
compartmentswere 69.53±30.53, 76.47±36.00 and 34.17±26.02 for the
inner-half myometrium and 33.24±23.06, 46.53±32.22 and 16.37±8.38
for the outer-half myometrium. The range of LVDs varied widely in
both compartments. The LVDs with tumor invasion were 8.07±7.25 and
6.94±9.98 for the inner- and the outer-half myometrium,
respectively. No correlation was found between the LVD with tumor
invasion and LVD in both the inner- and outer-half myometrium of
the peritumoral compartment (Spearman's rank correlation test).
 | Table II.LVDs of N+ and
N− groups. |
Table II.
LVDs of N+ and
N− groups.
| A, LVD of
N+ group |
|---|
|
|---|
|
| Mean ± standard
deviation (range) |
|---|
|
|
|
|---|
|
| Peritumoral
compartment | Control
compartment |
|---|
|
|
|
|
|---|
|
| Inner-half
myometrium | Outer-half
myometrium |
|
|
|---|
|
|
|
|
|
|
|---|
|
| LVI+ area
(n=15) | LVI− area
(n=19) | LVI+ area
(n=17) | LVI− area
(n=19) | Inner-half myometrium
(n=18) | Outer-half myometrium
(n=19) |
|---|
| LVD | 69.53±30.53
(11–111) | 76.47±35.97
(23–164) | 33.24±23.06
(6–99) | 46.53±32.22
(6–145) | 34.17±26.02
(0–101) | 16.37±8.38
(3–33) |
| LVD with tumor
invasiona | 8.07±7.25 (2–22) | Not applicable | 6.94±9.98 (1–43) | Not applicable | Not applicable | Not applicable |
|
| B, LVD of
N− group |
|
|
| Mean ± standard
deviation (range) |
|
|
|
|
| Peritumoral
compartment | Control
compartment |
|
|
|
|
|
| Inner half
myometrium |
| Outer half
myometrium | Inner half
myometrium |
| Outer half
myometrium |
| LVD | 63.21±36.24 |
| 41.86±27.21 | 46.10±59.38 |
| 18.21±9.53 |
|
| (15–139) |
| (0–91) | (0–295) |
| (3–40) |
In the N− group (Table IIB) the LVDs in the peritumoral and
control compartments were 63.21±36.24 and 46.10±59.38 for the
inner-half myometrium and 41.86±27.21 and 18.21±9.53 for the
outer-half myometrium.
The comparison of the LVDs within each group is
shown in Tables IIIA and IIIB. In the N+ group (Table IIIA), the LVDs between the
LVI+ and the LVI− areas of both the inner-
and outer-half myometrium were not different (P=0.56 for the
inner-half myometrium and 0.17 for the outer-half myometrium.
However, compared to the LVDs in the control compartment, those in
the peritumoral compartment increased, irrespective of the presence
of LVI (P=0.0011, 0.00025 for the LVI+ and the
LVI− areas of the inner-half myometrium, and 0.0099,
0.00079 for those of the outer-half myometrium). And the LVDs of
the inner-half myometrium were higher than those of the outer-half
myometrium in both the peritumoral and control compartments
(P=0.00062, 0.042, 0.00016, 0.010 for the peritumoral compartment,
and 0.012 for the control compartment).
 | Table III.Comparison of LVDs within each
group. |
Table III.
Comparison of LVDs within each
group.
| A, Statistically
significant differences in LVD in N+ group |
|---|
|
|---|
| Location |
|
|
|---|
|
|
|
|---|
| Peritumoral
compartment | Control
compartment | P-value | Statistical
test |
|---|
| Inner-half
myometrium |
|
|
|
|
LVI+ |
| 0.0011 | Student's
t-test |
|
LVI− |
| 0.00025 | Student's
t-test |
| Outer-half
myometrium |
|
|
|
|
LVI+ |
| 0.0099 | Welch's test |
|
LVI− |
| 0.00079 | Welch's test |
|
| Inner-half
myometrium | Outer-half
myometrium |
|
|
|
| Peritumoral
compartment |
|
|
|
|
LVI+ |
LVI+ | 0.00062 | Student's t
test |
|
|
LVI− | 0.042 | Student's t
test |
|
LVI− |
LVI+ | 0.00016 | Student's t
test |
|
|
LVI− | 0.010 | Student's t
test |
| Control
compartment |
|
|
|
|
|
|
| 0.012 | Welch's test |
|
| B, Statistically
significant differences in LVD in N− group |
|
| Location |
|
|
|
|
|
| Peritumoral
compartment | Control
compartment | P-value | Statistical
test |
|
| Inner-half
myometrium |
| 0.0075 | Mann-Whitney's
test |
| Outer-half
myometrium |
| 0.00014 | Mann-Whitney's
test |
|
| Inner-half
myometrium | Outer-half
myometrium |
|
|
|
| Peritumoral
compartment |
| 0.021 | Mann-Whitney's
test |
| Control
compartment |
| 0.032 | Mann-Whitney's
test |
In the N− group, as in the result of the
N+ group, LVDs of both inner- and outer-half myometrium
of the peritumoral compartment were higher than those of the
control compartment (P=0.0075 and 0.00014). Also, LVDs of the
inner-half myometrium were higher than those of the outer-half
myometrium in both the peritumoral and control compartments
(P=0.021 and 0.032).
In both the N+ and the N−
groups, the differences in LVD between the peritumoral and control
compartments were more statistically significant than those between
the inner- and outer-half myometrium.
The differences in LVD of the peritumoral
compartments between the groups were not significant (P=0.067 and
0.29 for inner- and outer-half myometrium).
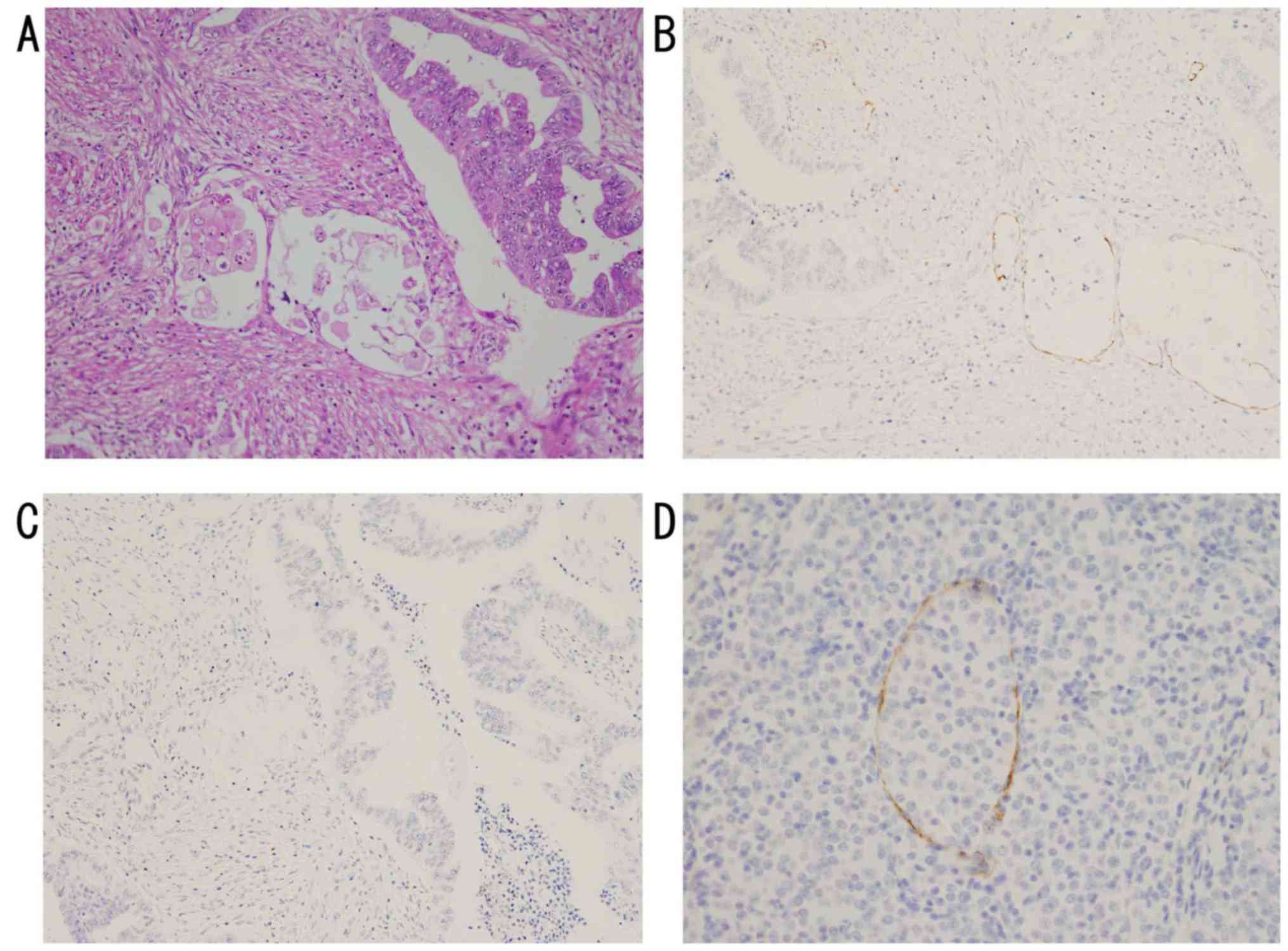
In the intratumoral compartment of the N+
group, no lymphatic vessels were found in 16 of the 19 cases, and
only 3 cases showed the presence of lymphatic vessels, 2 in the
desmin-negative stroma and one in the solidly proliferating area of
G3 tumor. All the 3 cases showed the LVI (Fig. 4). The ratio of the LVD with tumor
invasion to the LVD were 3 to 18 (G2), 3 to 11 (G3) and 1 to 1 (G1)
in the 3 cases (Table IV).
 | Table IV.LVD and LVD with tumor invasion in
the intratumoral compartment in N+ group. |
Table IV.
LVD and LVD with tumor invasion in
the intratumoral compartment in N+ group.
| Cases | LVD | LVD with tumor
invasion |
|---|
|
|---|
| 16 cases | 0 | Not applicable |
|---|
| 3 cases |
|
|
| Case 1
(G2) | 18 in stroma | 3 |
| Case 2
(G3) | 11 in tumor | 3 |
| Case 3
(G1) | 1 in stroma | 1 |
Discussion
In our study, twenty-four of the 202 cases (12%) had
nodal metastases. The rate was consistent with the relatively low
incidence reported so far, which ranged from 10 to 15% (11). The presence of LVI has been reported
to be associated with nodal metastases in one article (11). In our study, the LVI was recognized in
all 19 cases of the N+ group with D2-40 immunostaining,
and even in the N− group, the absence of LVI was
confirmed only in 2 of 29 cases. Therefore, not the presence of LVI
itself but the degree of LVI could be a possible predictor of nodal
metastases. There was no correlation between the LVD and frequency
of LVI, and the LVD did not differ with the presence of LVI in the
N+ group. However, tumor lymphangiogesis was suggested,
in that the LVDs in both the inner- and outer-half myometrium of
the peritumoral compartments were higher than those of the control
compartments. In both the N+ and N− groups.
Also, there was no difference in LVD of peritumoral compartments
between the groups. Therefore, although tumor lymphangiogenesis
increased LVD, the increased LVD was not related to the LVI and
nodal metastases.
One of the factors to determine the LVD other than
tumor lymphangiogenesis was the location in the myometrium. Not
only in the peritumoral compartment but also in the control
compartment, the LVDs in the inner-half myometrium were higher than
those in the outer-half myometrium in both groups. Lymphatic
vessels in uterine corpus showed an intimate association with
arteries (Fig. 3) (13). Anatomically, in the inner third of the
myometrium, abundant radial vessels from the subserosal arteries,
the arcuate arteries, branched into the endometrium (12). Therefore, the richer vasculature in
the inner-half myometrium should be one explanation for the higher
LVD. This tendency was retained in the carcinoma-harboring uterine
corpus regardless of the presence of LVI and nodal metastases.
Another influence on the LVD was the existing
myometrium. There were no lymphatic vessels where the existing
muscular tissue disappeared in 16 of 19 cases of the N+
group. The result was in contrast to the high intratumoral LVD of
gastric cancer, which generally had a high incidence of nodal
metastases (17). Tumor
lymphangiogenesis was also suggested, in that the remaining 3 cases
showing lymphatic vessels also presented LVI, though the frequency
(3/19) was low. In conclusion, the range of LVD varied widely in
the uterine corpus. Our result showed that tumor lymphangiogenesis
did not have an absolute impact on the LVD, LVI and nodal
metastases in the carcinoma. The location, inner- or outer-half, in
the myometrium, and the presence of the existing myometrium were
important influences on the LVD other than tumor lymphangiogesis.
The prognostic significance of the increased LVD caused by tumor
lymphangiogenesis in the peritumoral compartment was not evident in
endometrioid carcinoma of the uterine corpus in our study.
References
|
1
|
Franchi A, Gallo O, Massi D, Baroni G and
Santucci M: Tumor lymphangiogenesis in head and neck squamous cell
carcinoma: A morphometric study with clinical correlations. Cancer.
101:973–978. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dadras SS, Paul T, Bertoncini J, Brown LF,
Muzikansky A, Jackson DG, Ellwanger U, Garbe C, Mihm MC and Detmar
M: Tumor lymphangiogenesis: A novel prognostic indicator for
cutaneous melanoma metastasis and survival. Am J Pathol.
162:1951–1960. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dadras SS, Lange-Asschenfeldt B, Velasco
P, Nguyen L, Vora A, Muzikansky A, Jahnke K, Hauschild A, Hirakawa
S, Mihm MC and Detmar M: Tumor lymphangiogenesis predicts melanoma
metastasis to sentinel lymph nodes. Mod Pathol. 18:1232–1242. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Schoppmann SF, Bayer G, Aumayr K, Taucher
S, Geleff S, Rudas M, Kubista E, Hausmaninger H, Samonigg H, Gnant
M, et al: Prognostic value of lymphangiogenesis and lymphovascular
invasion in invasive breast cancer. Ann Surg. 240:306–312. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li S and Li Q: Cancer stem cells,
lymphangiogenesis, and lymphatic metastasis. Cancer Lett.
357:438–447. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lohela M, Bry M, Tammela T and Alitalo K:
VEGFs and receptors involved in angiogenesis versus
lymphangiogenesis. Curr Opin Cell Biol. 21:154–165. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Raica M and Ribatti D: Targeting tumor
lymphangiogenesis: An update. Curr Med Chem. 17:698–708. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mannelqvist M, Stefansson I, Salvesen HB
and Akslen LA: Importance of tumour cell invasion in blood and
lymphatic vasculature among patients with endometrial carcinoma.
Histopathology. 54:174–183. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hachisuga T, Kaku T, Fukuda K, Eguchi F,
Emoto M, Kamura T, Iwasaka T, Kawarabayashi T, Sugimori H and Mori
M: The grading of lymphovascular space invasion in endometrial
carcinoma. Cancer. 86:2090–2097. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Inoue Y, Obata K, Abe K, Ohmura G, Doh K,
Yoshioka T, Hoshiai H and Noda K: The prognostic significance of
vascular invasion by endometrial carcinoma. Cancer. 78:1447–1451.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Miyakuni Y, Matsumoto T, Arakawa A, Sonoue
H, Suzuki C and Takeda S: Lymphatic invasion according to D2-40
immunostaining is a predictor of nodal metastasis in endometrioid
adenocarcinoma of the uterine corpus. Pathol Int. 58:471–476. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hendrickson RM, Atkins AK and Kempson LR:
Uterus and Fallopian tubesHistology for Pathologist. Mills ES: 3rd.
Lippincott Williams & Wilkins; Philadelphia, PA: pp. 1016–1017.
2007
|
|
13
|
Rogers PA, Donoghue JF and Girling JE:
Endometrial lymphangiogensis. Placenta. 29 Suppl A:S48–S54. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Williams CS, Leek RD, Robson AM, Banerji
S, Prevo R, Harris AL and Jackson DG: Absence of lymphangiogenesis
and intratumoural lymph vessels in human metastatic breast cancer.
J Pathol. 200:195–206. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vleugel MM, Bos R, van der Groep P,
Greijer AE, Shvarts A, Stel HV, van der Wall E and van Diest PJ:
Lack of lymphangiogenesis during breast carcinogenesis. J Clin
Pathol. 57:746–751. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Roma AA, Magi-Galluzzi C, Kral MA, Jin TT,
Klein EA and Zhou M: Peritumoral lymphatic invasion is associated
with regional lymph node metastases in prostate adenocarcinoma. Mod
Pathol. 19:392–398. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Donizy P, Rudno-Rudzinska J, Halon A,
Dziegala M, Kabarowski J, Frejlich E, Dziegiel P, Kielan W and
Matkowski R: Intratumoral but not peritumoral lymphatic vessel
density measured by D2-40 expression predicts poor outcome in
gastric cancer-ROC curve analysis to find cut-off point. Anticancer
Res. 34:3113–3118. 2014.PubMed/NCBI
|