Introduction
It has been reported that spinal tumor cases account
for 5% of all primary bone tumors. They are divided into primary
and metastatic based on tumor origin, with the latter being more
common. The primary lesion of metastatic spinal tumor is usually
derived from lung, breast and prostate cancer (1–3). Matrix
metalloproteinases (MMPs), a proteolytic enzyme family, are
involved in the degradation of extracellular matrix (ECM) and
promote the metastasis of tumor cells to various peripheral
tissues. Most published studies have reported that p53 gene
mutation is associated with the formation and development of many
human tumors, and its abnormal expression leads to abnormal cell
proliferation and causes tumor formation and development (4–8).
Due to the particularity of the spinal tumor
anatomical location, spinal tumor tissue invasion and metastasis
directly damage the patient's vertebral and paraspinal tissue
structure, causing neurological dysfunction in cancer patients.
Recent studies have shown that the p53 and MMP-9 proteins are
associated with the occurrence, development and invasion of tumor
cells (5,6).
The aim of this study was to investigate whether the
expression of p53 and MMP-9 proteins was associated with metastatic
spinal tumor of lung cancer and examine its effect on patient
prognosis. This study provides new ideas for the prevention of
tumor cell metastasis in the human body.
Materials and methods
Materials
Experimental materials
In total, 100 tissue samples were obtained from
metastatic spinal tumor tissue of lung cancer, 75 samples were
obtained from non-metastatic lung cancer tissue and 30 cases were
obtained from para-cancerous tissue. All the samples were
pathologically diagnosed and all tissues were paraffin-embedded and
sectioned continuously (4 µm).
Main reagents
Rabbit anti-human MMP-9 monoclonal antibody (Beijing
Dingguo Changsheng Biotechnology Co. Ltd., Beijing, China), rabbit
anti-human p53 monoclonal antibody (BD Biosciences, Franklin Lakes,
NJ, USA), DAB chromogenic agent (Shanghai Haoran Biotechnology Co.,
Ltd., Shanghai, China), citrate buffer powder (Shanghai Haoran
Biotechnology Co., Ltd.), hematoxylin (Shanghai Rongbai Biological
Technology Co., Ltd., Shanghai, China) and eosin (Shanghai Rongbai
Biotechnology Co., Ltd.) were used for immunohistochemistry.
Experimental methods
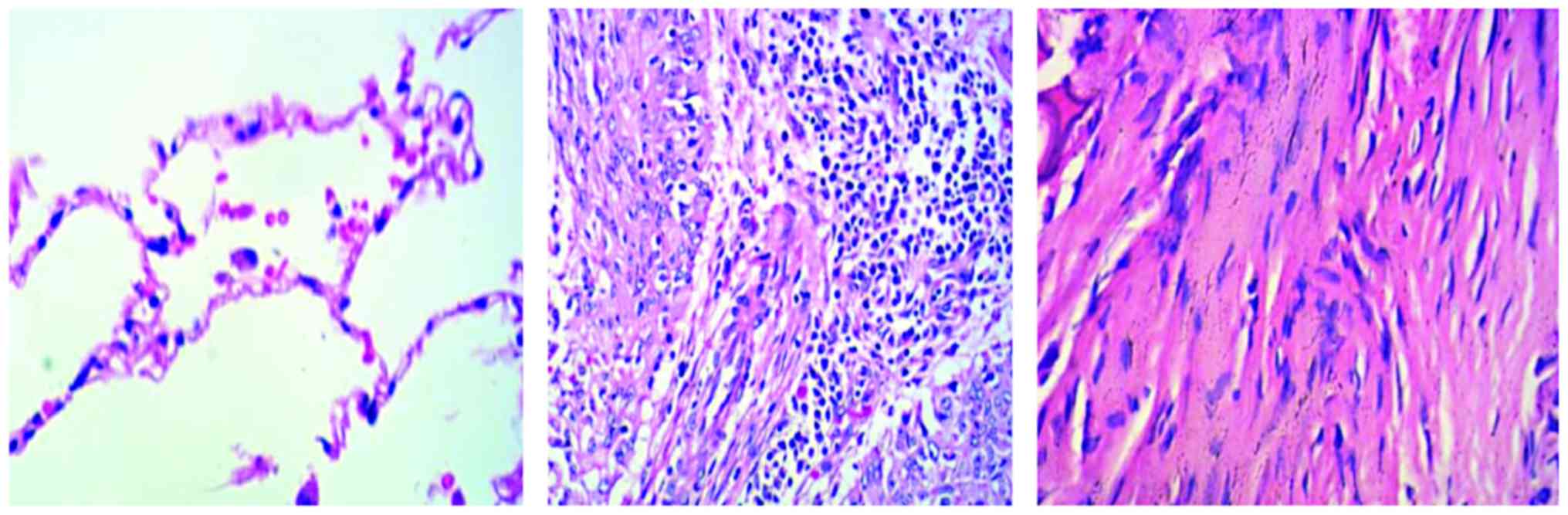
Hematoxylin and eosin (H&E) staining
Paraffin-embedded tissue sections were
deparaffinized and washed with ethanol. The sections were
thoroughly hydrated and stained with hematoxylin for 10 min and
then washed with H2O. The sections were differentiated
with 1% hydrochloric acid alcohol for 3 sec, washed and then placed
in saturated lithium carbonate for 3 sec until a blue color was
observed. After the sections were rinsed with running water for 20
min, they were dipped in 1% alcohol soluble eosin for 10 sec
followed by routine dehydration, transparency and mounting.
Immunohistochemical staining
Immunohistochemical staining was used in this study
using the following protocol: i) Paraffin-embedded tissue sections
were heated at 60°C for 2 h and then deparaffinized and rehydrated
with ethanol; ii) for antigen retrieval, the sections were inserted
into an iron frame and placed in a tin box full of citric acid. The
tin box was heated until the citric acid solution reached boiling
point, the voltage was lowered to maintain a rolling boil for 7
min, and then the solution was allowed to cool; iii) one drop of
H2O2 solution was added and then washed out
after 10 min; iv) sections were incubated with serum for 10 min; v)
sections were incubated with primary antibody for 10 min and then
washed with water 3 times; vi) sections were incubated with
secondary antibody for 10 min and then washed 3 times; vii)
streptomycin-biotin-peroxidase was added to the sections and washed
3 times after a 10 min incubation; viii) fresh DAB was added to
sections, which were placed in a dark room; and ix) sections were
washed, counterstained, mounted and observed.
Evaluation of experimental results
One hundred cells were counted in a randomly
selected visual field, with the average number of cells in the
visual field calculated as the number of positive cells expressing
the corresponding protein within the tissue. For the coloring score
scale, 0–2 points represented no coloring, weak coloring and strong
coloring, respectively. The positive rate of stained cells and
scores: 1–4 points represented the percentage of positive cells
1–25, 26–50, 51–75 and 76–100%, respectively. The two scores were
multiplied, with a final score of 1–2 points indicating a negative
expression and a score of 3–8 points indicating a positive
expression.
Statistical analysis
Data were analyzed with SPSS 17.0 (Beijing Xinmei
Jiahong Technology Co., Ltd., Beijing, China). The differences in
the positive rate between groups were analyzed with a χ2
test. The Spearman test was used to analyze the correlation between
two proteins. GraphPad Prism 5 was used to analyze the survival
rate. P<0.05 was considered to indicate a statistically
significant difference.
Results
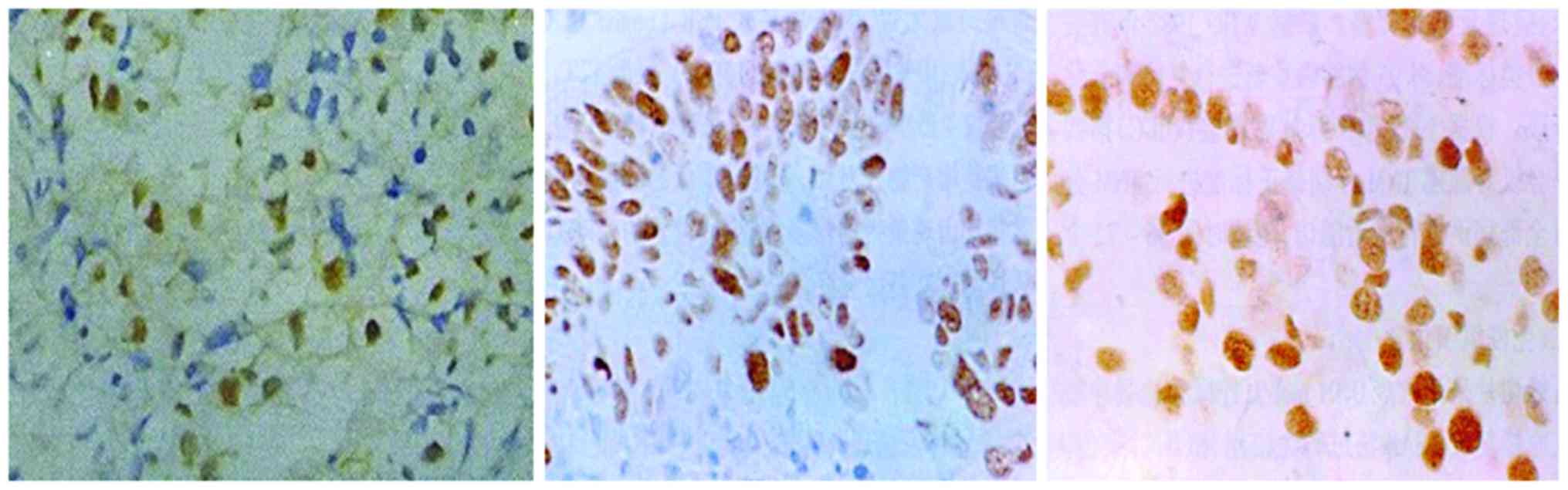
Expression of MMP-9 protein
The results showed that MMP-9 protein was almost
absent in para-cancerous tissue and present in non-metastatic lung
cancer tissue. By contrast, there was a large amount of MMP-9
protein expressed in metastatic spinal tumor tissue (Fig. 1).
The positive rate of MMP-9 protein was 20% in 6 of
the 30 para-cancerous tissue samples. Fifty of the 75
non-metastatic lung cancer tissue samples expressed MMP-9 protein
(positive rate 67%). In 100 lung cancer metastatic spinal tumor
tissue samples, 83 showed positive staining for MMP-9 protein.
The positive rates for MMP-9 protein expression in
metastatic spinal tumor tissue and non-metastatic lung cancer
tissue were significantly higher than that in para-cancerous tissue
and the difference was statistically significant (p<0.05)
(Table I).
 | Table I.Tissue MMP-9 protein expression. |
Table I.
Tissue MMP-9 protein expression.
|
|
| MMP-9 protein |
|
|
|
|---|
|
|
|
|
|
|
|
|---|
| Groups | Samples | + | − | Positive rate
(%) | χ2 | P-value |
|---|
| Para-cancerous tissue
(I) | 30 | 6 | 24 | 20 | 29.35 | <0.05 |
| Non-metastatic lung
cancer tissue (II) | 75 | 50 | 25 | 67a,c |
|
|
| Lung cancer
metastatic spinal tumor tissue (III) | 100 | 83 | 17 | 83b |
|
|
Expression of p53 protein
The results showed that p53 protein was expressed in
para-cancerous tissue and this expression was increased in
non-metastatic lung cancer tissue. In contrast, there was a large
amount of p53 protein expressed in metastatic spinal tumor tissue
(Fig. 2).
The positive rate for p53 protein was 16.7%, with 5
positives out of 30 para-cancerous tissue samples. Fifty-nine of
the 75 non-metastatic lung cancer tissue samples expressed the p53
protein (positive rate 78.7%). Of 100 lung cancer metastatic spinal
tumor tissue samples, 92 were positive for p53 protein
expression.
The positive expression rate for p53 protein in
metastatic spine tumor tissue and non-metastatic lung cancer tissue
was significantly higher than that in para-cancerous tissue. The
positive expression rate between groups was compared and difference
was statistically significant (p<0.01) (Table II).
 | Table II.p53 protein expression in three tissue
sample groups. |
Table II.
p53 protein expression in three tissue
sample groups.
|
|
| p53 protein |
|
|
|
|---|
|
|
|
|
|
|
|
|---|
| Groups | Samples | + | − | Positive rate
(%) | χ2 | P-value |
|---|
| Para-cancerous tissue
(I) | 30 | 5 | 25 | 16.7 | 34.28 | <0.01 |
| Non-metastatic lung
cancer tissue (II) | 75 | 59 | 13 | 78.7a,c |
|
|
| Lung cancer
metastatic spinal tumor tissue (III) | 100 | 92 | 8 | 92b |
|
|
Correlation of MMP-9 and p53 protein
expression levels in metastatic spinal tumor of lung cancer
There were 92 lung cancer metastatic tumor samples
presenting positive p53 expression. Of these, 80 showed a positive
expression of MMP-9 protein (double positive rate, 87%). The
expression of the two proteins showed a positive correlation
(Spearman correlation coefficient r =0.351, p<0.05) (Table III).
 | Table III.Correlation of MMP-9 and p53 protein
expression in lung cancer metastatic spinal tumor tissues. |
Table III.
Correlation of MMP-9 and p53 protein
expression in lung cancer metastatic spinal tumor tissues.
|
| p53 |
|
|
|
|---|
|
|
|
|
|
|
|---|
| MMP-9 | + | − | Sum | r (Spearman) | P-value |
|---|
| + | 80 | 3 | 83 | 0.351 | 0.028 |
| − | 12 | 5 | 17 |
|
|
| Sum | 92 | 8 | 100 |
|
|
Association of MMP-9 and p53 protein
expression with metastatic spinal tumor of lung cancer patient
prognosis
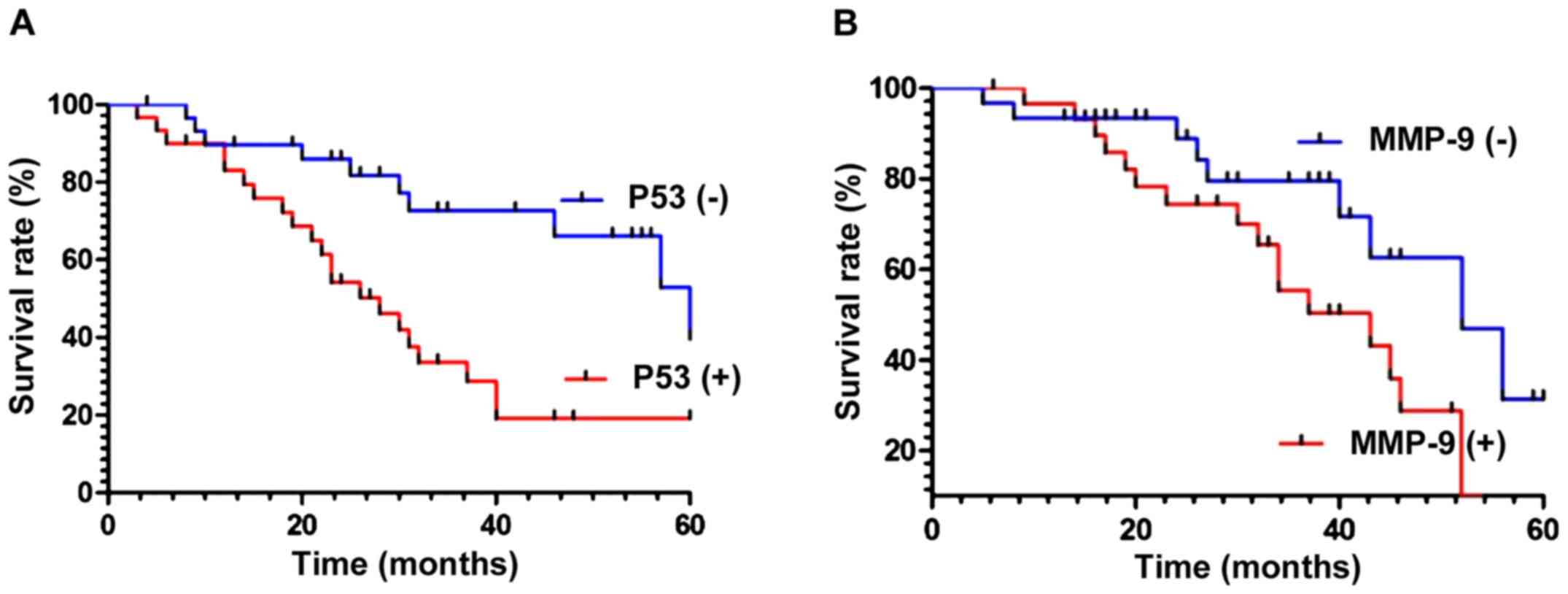
Survival rates in patients with a positive or
negative p53 expression were compared during follow-up and analyzed
using statistical software. The results showed that the survival
rate of patients with negative p53 expression was higher than that
of patients with a positive expression of p53 protein
(χ2=9.826, p<0.01) (Fig.
3A). Similarly, the survival rate of patients with negative
expression of MMP-9 protein was higher than that of patients with
positive MMP-9 expression (χ2=4.436, p<0.05)
(Fig. 3B).
Discussion
The spine is the most common bone metastatic site
for human tumor cells, with 60–85% of cancer patients showing
symptoms of bone metastases. During the bone metastasis process,
many changes in gene and protein expression occur. For instance,
MMP-9 and p53 are two cancer-related genes which likely play
important roles in tumor metastasis (9–13), but
their interactions need to be further elucidated.
In tumor cells, MMP-9 is usually produced in the
form of zymogen. Its main function is to degrade type-IV and -V
collagen in the ECM, thus aiding tumor cell metastasis, improving
capillary endothelial cell regeneration and neovascularization, and
promoting tumor cell in vivo occurrence and development.
At present, it has been found that p53 gene
is the most closely related gene in tumorigenesis and development,
and encodes p53 protein. Kornblum et al (14) found that p53 gene mutations are
the most significant genetic change during lung cancer and play a
crucial role in the occurrence and development of lung cancer. At
the same time, it was also found that tumors with p53 gene
abnormalities presented the strongest invasiveness and poorest
prognosis.
Immunohistochemical staining was used in this study,
with the results showing MMP-9 protein positive expression rate of
20% in para-cancerous tissue, 67% in non-metastatic lung cancer
tissue and 83% in metastatic spinal tumor tissue of lung cancer
(p<0.05). Therefore, MMP-9 protein expression can be used as an
indicator for predicting the degree of malignancy and
prognosis.
The positive or negative expression of MMP-9 and p53
proteins was statistically significant during the survival time
(p<0.05). The positive expression rate of p53 was 16.7% in
para-cancerous tissue, 78.7% in non-metastatic lung cancer tissue
and 92% in metastatic tumor tissue of lung cancer (p<0.01).
There was a positive correlation between the expression of MMP-9
and p53 proteins (p<0.05), suggesting that the two proteins
likely played a synergistic role in the development and progression
of lung cancer metastatic spinal tumor, with the oncogene p53
(wild-type) likely regulating the expression of MMP-9 through
negative feedback.
It has been reported widely (15–18) that
the p53 protein was expressed in a number of tumor cells. Previous
evidence suggested (19) that the p53
protein had no correlation with the age and survival of patients,
and that it was impossible to confirm a correlation between p53
protein expression and prognosis. It has also been suggested
indicated that an enhanced MMP-9 protein expression had a clear
positive correlation with the growth, development and metastasis of
breast cancer, non-small cell lung cancer, and pancreatic cancer
(1,5,20). One of
the conclusions of this study further supports the point of view
that the expression of this protein in lung cancer metastatic
spinal tumor may also have a corresponding function.
In the present study, MMP-9 and p53 protein
expression were positively correlated. In addition, the positive or
negative expression of MMP-9 correlated with 5-year survival.
Further studies regarding the correlation between MMP-9 and p53
expression in tumor cells will be useful to clarify the development
and treatment of metastatic spinal tumor in lung cancer.
References
|
1
|
Wang N and Stamenovic D: Mechanics of
vimentin intermediate filaments. J Muscle Res Cell Motil.
23:535–540. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ulirsch J, Fan C, Knafl G, Wu MJ, Coleman
B, Perou CM and Swift-Scanlan T: Vimentin DNA methylation predicts
survival in breast cancer. Breast Cancer Res Treat. 137:383–396.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kidd ME, Shumaker DK and Ridge KM: The
role of vimentin intermediate filaments in the progression of lung
cancer. Am J Respir Cell Mol Biol. 50:1–6. 2014.PubMed/NCBI
|
|
4
|
Otsuki S, Inokuchi M, Enjoji M, Ishikawa
T, Takagi Y, Kato K, Yamada H, Kojima K and Sugihara K: Vimentin
expression is associated with decreased survival in gastric cancer.
Oncol Rep. 25:1235–1242. 2011.PubMed/NCBI
|
|
5
|
Schveigert D, Valuckas KP, Kovalcis V,
Ulys A, Chvatovic G and Didziapetriene J: Significance of MMP-9
expression and MMP-9 polymorphism in prostate cancer. Tumori.
99:523–529. 2013.PubMed/NCBI
|
|
6
|
Schveigert D, Cicenas S, Bruzas S,
Samalavicius NE, Gudleviciene Z and Didziapetriene J: The value of
MMP-9 for breast and non-small cell lung cancer patients' survival.
Adv Med Sci. 58:73–82. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ureshino RP, Bertoncini CR, Fernandes MJ,
Abdalla FM, Porto CS, Hsu YT, Lopes GS and Smaili SS: Alterations
in calcium signaling and a decrease in Bcl-2 expression: Possible
correlation with apoptosis in aged striatum. J Neurosci Res.
88:438–447. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chernyatina AA, Nicolet S, Aebi U,
Herrmann H and Strelkov SV: Atomic structure of the vimentin
central α-helical domain and its implications for intermediate
filament assembly. Proc Natl Acad Sci USA. 109:13620–13625. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Czabotar PE, Lessene G, Strasser A and
Adams JM: Control of apoptosis by the BCL-2 protein family:
Implications for physiology and therapy. Nat Rev Mol Cell Biol.
15:49–63. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gregory MS, Repp AC, Holhbaum AM, Saff RR,
Marshak-Rothstein A and Ksander BR: Membrane Fas ligand activates
innate immunity and terminates ocular immune privilege. J Immunol.
169:2727–2735. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Alecu M, Coman G and Dănăilă L: High
levels of sFas and PBMC apoptosis before and after excision of
malignant melanoma - case report. Roum Arch Microbiol Immunol.
61:267–273. 2002.PubMed/NCBI
|
|
12
|
Huang SC, Tang MJ, Hsu KF, Cheng YM and
Chou CY: Fas and its ligand, caspases, and bcl-2 expression in
gonadotropin-releasing hormone agonist-treated uterine leiomyoma. J
Clin Endocrinol Metab. 87:4580–4586. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Metser U, Lerman H, Blank A, Lievshitz G,
Bokstein F and Even-Sapir E: Malignant involvement of the spine:
Assessment by 18F-FDG PET/CT. J Nucl Med. 45:279–284.
2004.PubMed/NCBI
|
|
14
|
Kornblum MB, Wesolowski DP, Fischgrund JS
and Herkowitz HN: Computed tomography-guided biopsy of the spine. A
review of 103 patients. Spine. 23:81–85. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sucu HK, Ciçek C, Rezanko T, Bezircioğlu
H, Erşahin Y, Tunakan M and Minoğlu M: Percutaneous
computed-tomography-guided biopsy of the spine: 229 procedures.
Joint Bone Spine. 73:532–537. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rades D, Dunst J and Schild SE: The first
score predicting overall survival in patients with metastatic
spinal cord compression. Cancer. 112:157–161. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Masala S, Anselmetti GC, Muto M, Mammucari
M, Volpi T and Simonetti G: Percutaneous vertebroplasty relieves
pain in metastatic cervical fractures. Clin Orthop Relat Res.
469:715–722. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tseng YY, Lo YL, Chen LH, Lai PL and Yang
ST: Percutaneous polymethylmethacrylate vertebroplasty in the
treatment of pain induced by metastatic spine tumor. Surg Neurol.
70:78–83. 2008. View Article : Google Scholar
|
|
19
|
Cho DC and Sung JK: Palliative surgery for
metastatic thoracic and lumbar tumors using posterolateral
transpedicular approach with posterior instrumentation. Surg
Neurol. 71:424–433. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sciubba DM, Gokaslan ZL, Black JH III,
Simmons O, Suk I, Witham TF, Bydon A and Wolinsky JP: 5-Level
spondylectomy for en bloc resection of thoracic chordoma: Case
report. Neurosurgery. 69:E248–E256. 2011.
|