Introduction
Alveolar soft-part sarcoma (ASPS) is a rare but
distinct soft-tissue tumor that accounts for <1% of all
sarcomas, and usually arises in the soft tissues of the extremities
(1). Its histogenesis is unclear, but
it has unique histopathological and electron microscopic features.
In a 1952 study by Christopherson et al (2), the patients studied were predominantly
young and female (median age at diagnosis, 22 years), which remain
characteristic features of ASPS. ASPS presents as a slowly growing
tumor and is usually overlooked due to lack of symptoms. Unlike the
majority of sarcomas, ASPS frequently metastasizes, primarily to
the lungs (in 42% of cases), bones (19%), brain (15%) and lymph
nodes (7%) (3). A number of studies
have been performed to identify the molecular mechanism underlying
the development of ASPS; however, the pathological mechanism
remains unknown (3–5). ‘Alveolar’ soft part sarcoma is diagnosed
on the basis of the histological features of the tumors (1,2). Thesite
of origin remains controversial with either myogenic or neurogenic
origin being proposed (1,3). The primary therapeutic option for ASPS
is complete resection with no microscopic residual tumor. The aim
of surgery is complete tumor excision. Adequate excision translates
into improved outcome in such patients. Radiotherapy, which
produces improved local control, is recommended following subtotal
surgical removal.
Brain metastasis of ASPS is rare, with only 14 case
reports published in the literature in English (4–7). For a
period of 6 years, between November 2008 and March 2015, eight
patients with ASPS with brain metastasis were treated at the
Department of Neurosurgery of Beijing Tian Tan Hospital. In the
present study, the clinical, pathological and prognostic features
of all eight cases were investigated.
Materials and methods
Between November 2008 and March 2015, eight patients
with ASPS with brain metastasis underwent surgery at the Department
of Neurosurgery of Beijing Tian Tan Hospital (Table I). Patients were identified from the
Beijing Tian Tan Hospital registry and all were treated for brain
metastatic ASPS in the same hospital. Relevant clinical (including
follow-up) data were collected through a chart review and telephone
interviews as necessary. The present study also analyzed all
available neuroimaging data and radiological reports. Magnetic
resonance imaging (MRI) with gadolinium contrast enhancement was
performed as standard radiological investigation prior to and
following treatment. MR images were evaluated for the predominant
signal intensity and homogeneity of the tumor on T1- and T2-weight
images. MR images obtained following intravenous gadolinium chelate
injection were evaluated for the degree and predominant pattern of
contrast enhancement. Tumor size was recorded according to the
measurement of the maximum diameter on MRI. Peritumoral brain edema
was evaluated by T2-weighted images or fluid-attenuated inversion
recovery sequences on MRI. The patients' neurological status was
recorded using the Karnofsky Performance Scale (KPS) score
(8). All diagnoses were reviewed at
the Department of Neuropathology at the Beijing Neurosurgical
Institute (Beijing, China) using the 2007 World Health Organization
classification of tumors of the central nervous system (9).
 | Table I.Clinical features of eight patients
with brain metastatic alveolar soft-part sarcoma. |
Table I.
Clinical features of eight patients
with brain metastatic alveolar soft-part sarcoma.
| Case number | Sex/age, years | Initial Symptom | Duration of symptoms,
months | Extent of
Resection | Blood Supply | Primary site | Pulmonary
metastases | RT | CT | Preoperative KPS | KPS at last
follow-up | Recurrent tumor | Mortality | Duration of
follow-up, months |
|---|
| 1 | M/22 | Headache | 5 | GTR | Rich | Arm | Yes | No | Yes | 90 | 50 | 24 months PO | No | 69 |
| 2 | F/15 | Left hemiparesis | 0.25 | GTR | Rich | Chest | Yes | No | No | 90 | 100 | 33 months PO | No | 35 |
| 3 | F/26 | Headache | 2 | GTR | Rich | Thigh | No | Yes | No | 80 | 90 | No | No | 32 |
| 4 | M/32 | Headache | 0.33 | GTR | Rich | Thigh | Yes | Yes | No | 90 | 100 | No | No | 31 |
| 5 | M/25 | Head mass | 1 | GTR | Medium | Crus | Yes | No | No | 90 | 40 | 18 months PO | 20 months after
surgery | 20 |
| 6 | M/33 | Headache and
vomiting | 1 | GTR | Rich | Thigh | Yes | No | No | 80 | 50 | 12 months PO | No | 25 |
| 7 | M/26 | Head mass | 3 | GTR | Rich | Thigh | No | No | No | 90 | 100 | No | No | 14 |
| 8 | M/23 | Headache | 1 | GTR | Rich | Abdomen | No | No | No | 80 | 100 | No | No | 6 |
Pathological examination
All specimens underwent fixation in 4% neutral
formalin (24 h at 4°C), routine dehydration, paraffin-embedding,
preparation into 4-µm sections and staining using hematoxylin-eosin
at room temperature for about 2 h. Immunohistochemical staining was
used for differential diagnoses. Immunohistochemistry was performed
using the indirect immunoperoxidase technique. Bovine serum albumin
(Origene Technologies, Beijing, China) was used for blocking at
room temperature for 1 h. Primary antibodies included pre-diluted
monoclonal antibodies against transcription factor E3 (TFE3;
ZA-0570, Origene Technologies; 1:100), vimentin (ZM-0260; 1:200),
desmin (ZM-0091; 1:200), myogenin (ZM-0402; 1:200), S-100 (ZM-0224;
1:200), cytokeratin (ZM-0069; 1:100), neurone-specific enolase
(ZM-0203; 1:200), smooth muscle actin (ZM-0003; 1:200), epithelial
membrane antigen (ZM-0095; 1:200), synaptophysin (ZM-0246; 1:200),
chromogranin A (ZM-0076; 1:100), which were incubated for 12 h at
4°C. The SuperPicture™ 3rd Gen IHC Detection kit (Invitrogen;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) was used to
evaluate staining, according to the manufacturer's protocol. For
antigen retrieval, slides were boiled in EDTA buffer (pH 8.0;
ZLI-9066; Origene Technologies; Tris 30.27 g, EDTA 1.461 g and
H2O 500 ml) under high pressure. Slides were
counterstained with hematoxylin. Appropriate positive and negative
controls were used. Quantitative evaluation of TFE3 was obtained by
calculating the percentage of TFE3-positive nuclei in 100 tumor
cells from the microscopic field (light microscope; magnification,
×100; Konghai Co., Beijing, China) with the highest density of
labeled nuclei.
Results
Clinical presentation
The clinical features of the eight patients in the
present study are summarized in Table
I. The ages of patients ranged between 15 and 33 years (mean,
25.3 years). The sex ratio was 3:1 male to female. The duration of
symptoms ranged between1 and 22 weeks. Headache and scalp mass were
the most common initial symptoms. The most common site of the
primary tumor was in the extremities (6/8 patients, 75%), with the
lower extremity being involved in 5 of these 6 patients (83.3%) and
the upper extremity in one patient (16.6%). The torso was involved
in two patients (25%): The chest of one patient and the abdominal
region of the other. In addition, 5/8 (62.5%) patients with brain
metastases had concurrent pulmonary metastases. The median
preoperative KPS score was 86.3±5.2 (Table I).
Neuroradiological findings
Preoperative MRI results were available in all 8
patients (Table II). The location of
the tumors were as follows: Left frontal in two patients (cases 1
and 6); right frontal in two patients (cases 2 and 7); left
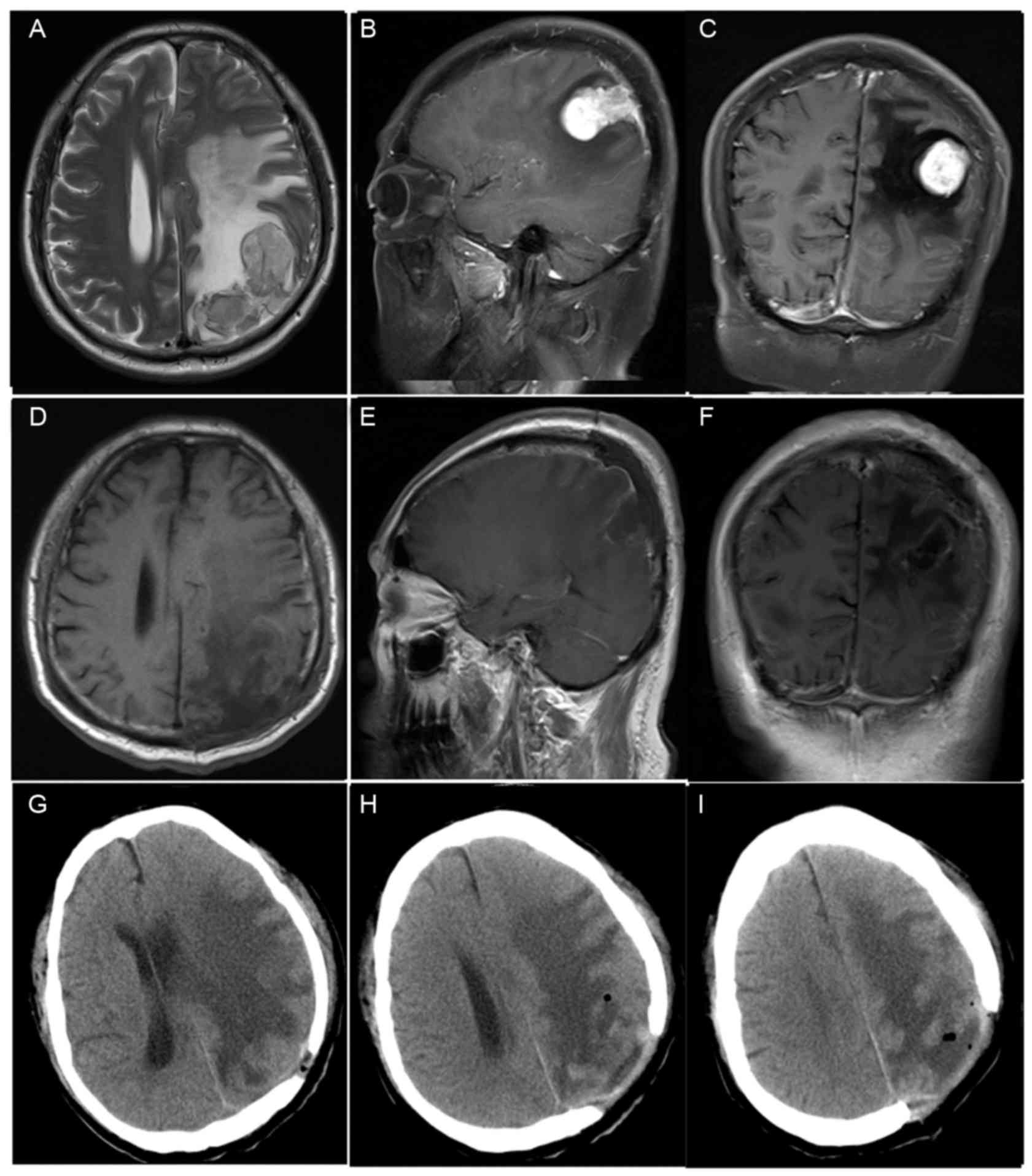
parietal in two patients (cases 4 and 8; Fig. 1); right parietal in one patient (case
5); left anterior cranial fossa in one patient (case 3). Tumor size
(maximum diameter on MRI) ranged between 2.5 and 5.4 cm (median,
3.4 cm). In total, 4 tumors were <3.0 cm in the longest
dimension, 3 were 3.0–5.0 cm and 1 was >5.0 cm. MRI revealed
well-circumscribed lesions and peritumoral edema was observed in 4
patients (cases 1, 2, 4, 6; Fig. 1).
T1-weighted images revealed a hypointense signal in all eight
patients. T2-weighted imaging showed a hyperintense signal in six
patients and an isointense signal in two patients. Moderate
enhancement was observed in three cases (cases 3, 7 and 8), and
bright contrast enhancement was observed in five cases (cases 1, 2,
4, 5 and 6; Fig. 1).
 | Table II.Magnetic resonance imaging features
of eight patients with brain metastatic alveolar soft-part
sarcoma. |
Table II.
Magnetic resonance imaging features
of eight patients with brain metastatic alveolar soft-part
sarcoma.
| Patient number | Location | T1-weighted
imaging | T2-weighted
imaging | Enhancement | Margins | Max diameter,
cm | Edema |
|---|
| 1 | Left frontal | Hypo | Hyper | Marked | Well
demarcated | 2.8 | + |
| 2 | Right frontal | Hypo | Hyper | Marked | Well
demarcated | 4.1 | + |
| 3 | Left anterior
cranial fossa | Hypo | Hyper | Moderate | Well
demarcated | 3 | − |
| 4 | Left parietal | Hypo | Hyper | Marked | Well
demarcated | 2.7 | + |
| 5 | Right parietal | Hypo | Hyper | Marked | Well
demarcated | 4 | − |
| 6 | Left frontal | Hypo | Hyper | Marked | Well
demarcated | 2.5 | + |
| 7 | Right frontal | Hypo | Iso | Moderate | Well
demarcated | 5.4 | − |
| 8 | Left parietal | Hypo | Iso | Moderate | Well
demarcated | 2.7 | − |
Histological findings
A histological examination revealed that a partially
encapsulated tumor was sharply demarcated from gliotic brain
parenchyma in every patient. Adjacent parenchyma exhibited a
perivascular mononuclear inflammatory infiltrate. The tumor cells
were large, round-polygonal with distinct borders, abundant
granular eosinophilic-clear cytoplasm and had large vesicular
nuclei with prominent nucleoli. The most notable
immunohistochemical feature was strong, granular cytoplasmic
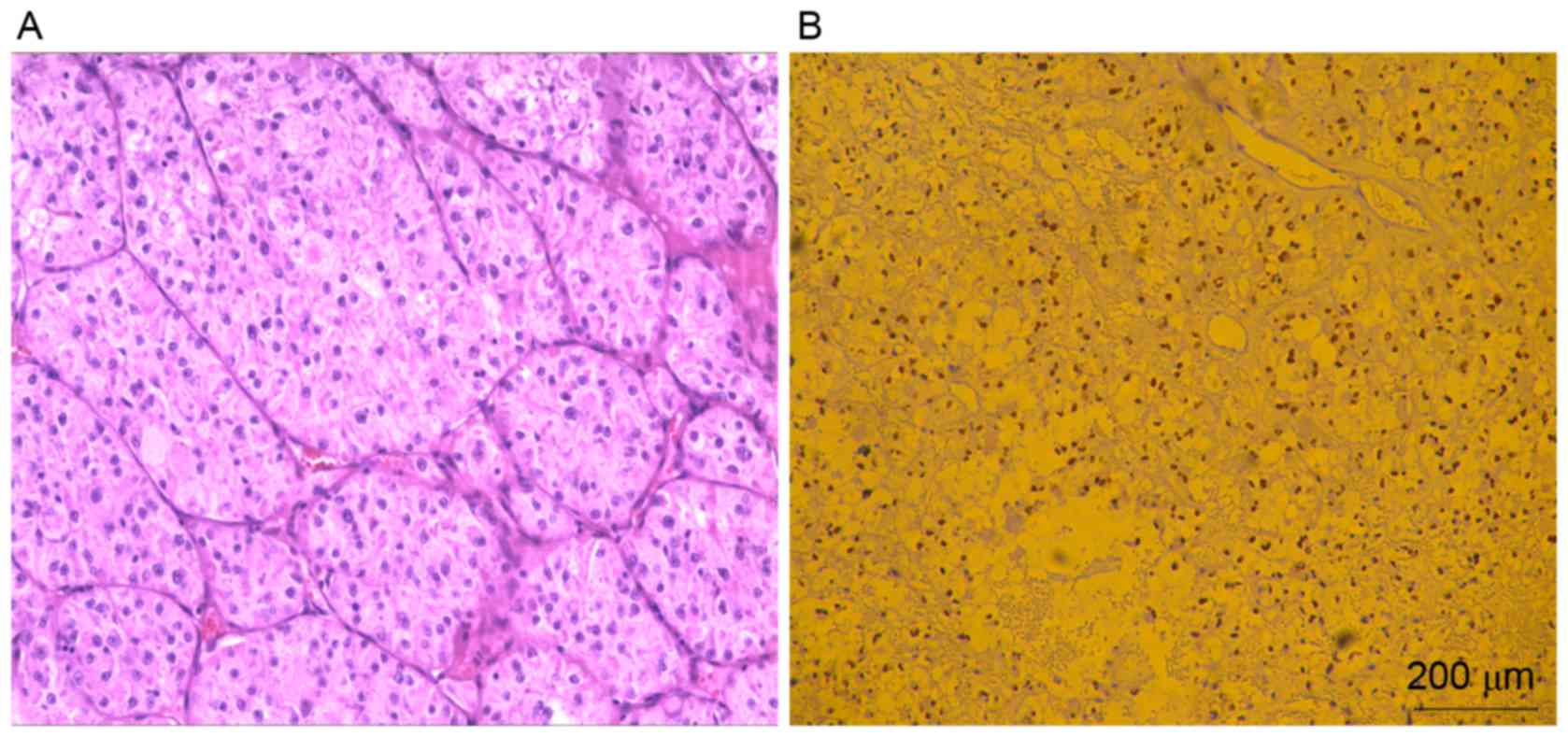
staining for TFE3 (Fig. 2) in 100%
(8/8) cases. Focal but strong cytoplasmic staining for vimentin was
observed in 50% of cases (4/8). The tumor cells lacked staining for
other immunohistochemical markers, including desmin, myogenin and
S-100 (Table III).
 | Table III.Results of immunohistochemistry of
brain metastatic alveolar soft-part sarcoma. |
Table III.
Results of immunohistochemistry of
brain metastatic alveolar soft-part sarcoma.
| IHC staining | Total, n (%) |
|---|
| TFE3 | 8 (100) |
| Vimentin | 4 (50) |
| Desmin | 0 (0) |
| Myogenin | 0 (0) |
| S-100 | 2 (25) |
| CK | 0 (0) |
| NSE | 1 (12.5) |
| SMA | 1 (12.5) |
| EMA | 1 (12.5) |
| SYN | 0 (0) |
| CgA | 2 (25) |
| PAS | 1 (12.5) |
Surgical findings and outcomes
All patients underwent a craniotomy to remove the
tumor. Intraoperatively, the tumors typically appeared as
medium-texture, reddish-gray solid masses, some of which eroded the
skull. Gross total resection was performed in all eight patients,
and seven of the tumors had an abundant blood supply.
Postoperatively, the follow-up data were available for all eight
patients. The mean follow-up time was 29 months (range, 6–69
months). Four patients (cases 1, 2, 5 and 6) experienced tumor
recurrence during follow-up and cases 1 and 6 underwent a second
surgery. One patient (case 5) succumbed to disease after 20 months
of follow-up. Data analysis revealed that the four relapsed
patients had a long history of ASPS, and had experienced multiple
systemic metastases (such as to the liver or kidney); each case
experienced lung metastasis that had not been treated surgically.
The primary disease in these patients had not been well controlled,
as the primary lesions had not been completely resected or multiple
metastases were already established at the time of diagnosis,
eventually leading to intracranial metastasis. In addition,
intracranial metastasis recurred a number of years following total
resection of the primary intracranial tumor. The four non-relapsing
patients (cases 3, 4, 7 and 8) had their primary tumors completely
resected, resulting in satisfactory disease control. Of these
patients, only one experienced lung metastasis, for which resection
was timely performed. None of these four patients relapsed
following total resection of the intracranial tumors. Case 6 had
multiple metastatic intracranial tumors associated with multiple
liver and lung metastases. As of the last follow-up, the patient
had received three craniotomies, with the liver and lung lesions
remaining untreated. Two patients (cases 3 and 4) underwent
adjuvant radiotherapy and one (case 1) received adjuvant
chemotherapy. Case 1 exhibited no response to two cycles of oral
chemotherapy with sorafenib. At follow-up, five patients (cases 2,
3, 4, 7 and 8) had KPS scores that were higher than their
preoperative scores.
Discussion
ASPS is a rare tumor that accounts for 0.5–1% of all
soft-tissue tumors (10).
Christopherson et al (2)
coined the term ASPS when in their study of 12 cases, which first
described its unique histological and cytological features. It is
primarily a tumor that presents in young adults, with a peak age
incidence between 15–35 years and a higher incidence in females
(11). The mean age of presentation
in this study was 25 years and a majority (21–84%) of the patients
presenting with disease aged <30 years. Although other series
have documented a larger proportion of female ASPS patients, the
present study had a male preponderance as 3:1 male to female ratio.
The majority of patients had primary tumors in the lower limbs and
exhibited right-sided laterality, as described by Fassbender
(12). The site of tumor origin
remains controversial, with either myogenic or neurogenic origins
proposed (13–15).
Imaging characteristics of brain metastases of ASPS
have not been well described in the literature. The appearances of
the brain metastases in the present study differ substantially from
that of other brain metastases, such as those originating from lung
and breast carcinomas (16,17). On computed tomography (CT) images, the
appearance of primary and metastatic ASPS reflects a rich
vascularity, with large vessels being a prominent feature of the
tumor (18,19). Tumor invasion of blood vessels and
central non-enhancement, indicating necrosis, are frequent observed
on CT images (18). On MRI, ASPS
usually present as hyperintense T1-weighted and T2-weighted images.
Avid enhancement with contrast is also typical, with or without a
non-enhancing, necrotic core (18,19). When
present, hemosiderin staining on gradient-echo sequences indicates
prior hemorrhage (19). Metastatic
ASPS can be considered in the differential diagnosis for
haemorrhagic intracranial metastases in young patients, along with
other more common diagnoses, such as meningioma, renal cell
carcinoma, granular cell tumor, paraganglioma and
choriocarcinoma.
In the past 10 years, genetic studies have
demonstrated that ASPS is a result of a chromosomal abnormality
associated with an unbalanced translocation between chromosomes X
and 17, der(17)t(X:17)(p11; p25).
This translocation results in a fusion of the ASPL gene on
chromosome 17 and the TFE3 gene on the X chromosome. As a result of
this fusion, the C-terminus of TFE3 is considered to be a specific
highly sensitive marker for ASPS (1,20,21). An antibody directed against the
C-terminus of TFE3 has emerged as a highly sensitive and specific
method of detecting ASPS (22); the
present study also confirmed brain metastatic ASPS using TFE3
immunohistochemical staining. Furthermore, molecular analysis of
fresh tissue may serve a role in the diagnosis of primary and
metastatic ASPS. However, it is not currently possible to assay for
this chromosomal translocation in formalin-fixed paraffin-embedded
tissue. As such, it was not possible to perform this test on any of
the retrospective cases in the present study. In the future,
however, it is expected that molecular methods may assist in the
diagnosis of difficult cases of ASPS (23).
ASPS has the highest incidence of brain metastasis
(19–30%) of all sarcomas (24–26). The
reason for this high incidence of brain metastases is unknown. It
may be because ASPS has a high propensity for haematogenous
metastasis (18). The primary
therapeutic option for ASPS brain metastases is radical surgical
resection. The aggressive removal of all accessible brain lesions
is recommended in patients with ASPS who are not terminally ill,
which can result in a particularly favorable prognosis (27). Radiotherapy is recommended following
surgical excision (28). The use of
chemotherapy is controversial for ASPS, and the majority of authors
consider it to be ineffective (3,5,29). In the present study, two patients
received adjuvant radiotherapy, one patient received adjuvant
chemotherapy and the remaining patients underwent surgery for gross
total resection alone. Follow-up data were available for all eight
patients: Five exhibited an improvement in their symptoms; four
experienced tumor recurrence, one of whom succumbed to the disease
as a result of this recurrence. The role served by radiotherapy was
unclear due to the limited number of patients who underwent
radiotherapy and the short follow-up time. Additional therapy is
largely dependent on clinical circumstances with respect to
recurrence, ability to undergo complete surgical excision and other
clinical factors. The patient series in the present study indicates
that adjuvant therapy may not be necessary if ASPS brain metastases
can be completely resected.
The resistance of ASPS tumors to conventional
chemotherapy and radiotherapy means treatment of this type of tumor
is challenging. However, a number of clinical trials are
investigating novel targeted therapies (29,30). Some
trials seek to focus on the over activity of the MET receptor
tyrosine kinase gene induced by the ASPSCR1-TFE3 fusion protein
(31). In addition, the highly
vascularized nature of this tumor also indicates that there may be
a potential therapeutic role for antiangiogenic agents (32).
Limitations of the present study include the small
sample size and the retrospective nature of the study. The high
incidence of brain metastasis in the current study is likely to be
due to referral bias in a tertiary cancer center. Statistical
analysis was not performed owing to the small number of patients,
but this is unavoidable, considering the rarity of the disease.
ASPS is an uncommon soft tissue tumor that has a
propensity to recur or metastasize late in the follow-up period.
ASPS often metastasizes to the lungs, bones and brain. Brain
metastases should be considered in the differential diagnosis of an
intracranial mass with the radiographic characteristics of a
meningioma, particularly if clinical or radiographic findings are
even marginally unusual. TFE3 immunohistochemical staining and
knowledge of the characteristic microscopic features of ASPS could
facilitate an early diagnosis, with early total resection possibly
the most effective treatment for brain metastatic ASPS. With the
development of radiotherapy, chemotherapy and targeted therapies, a
multidisciplinary treatment is essential to achieve extended
disease-free survival.
Acknowledgements
The present study was supported by a research grant
from the National Natural Science Foundation of China (no.
81471238).
References
|
1
|
Lieberman PH, Brennan MF, Kimmel M,
Erlandson RA, Garin-Chesa P and Flehinger BY: Alveolar soft-part
sarcoma. A clinico-pathologic study of half a century. Cancer.
63:1–13. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Christopherson WM, Foote Fw Jr and Stewart
FW: Alveolar soft-part sarcomas; structurally characteristic tumors
of uncertain histogenesis. Cancer. 5:100–111. 1952. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ahn SH, Lee JY, Wang KC, Park SH, Cheon
JE, Phi JH and Kim SK: Primary alveolar soft part sarcoma arising
from the cerebellopontine angle. Childs Nerv Syst. 30:345–350.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lewis AJ: Sarcoma metastatic to brain.
Cancer. 61:593–601. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cohen DB, Jones DM, Fergus AH, Qian J and
Schwartz MS: Metastatic alveolar soft-part sarcoma of the
intracranial skull base: Case report. Skull Base. 12:33–38. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mandal S, Majumdar K, Saran Kumar R and
Jagetia A: Meningeal alveolar soft part sarcoma masquerading as a
meningioma: A case report. ANZ J Surg. 83:693–694. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Akiyama Y, Baba T, Ibayashi Y, Asai Y and
Houkin K: Alveolar soft part sarcoma in brain with cardiac
metastasis: A case report. Int J Cardiol. 114:e93–e95. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hartmann K and Kuffer M: Karnofsky's score
modified for cats. Eur J Med Res. 3:95–98. 1998.PubMed/NCBI
|
|
9
|
Louis DN, Ohgaki H, Wiestler OD, Cavenee
WK, Burger PC, Jouvet A, Scheithauer BW and Kleihues P: The 2007
WHO classification of tumours of the central nervous system. Acta
Neuropathol. 114:97–109. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Schenning R, Vajtai P, Troxell M, Pollock
J and Hopkins K: Alveolar soft part sarcoma: Unusual etiology of
mediastinal mass in an adolescent. Clin Pract. 3:e262013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Folpe AL and Deyrup AT: Alveolar soft-part
sarcoma: A review and update. J Clin Pathol. 59:1127–1132. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fassbender HG: Alveolar myoblastic sarcoma
of the skeletal musculature. Oncologia. 13:184–191. 1960.(In
German). View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kang WD, Heo SH, Choi YD, Choi HS and Kim
SM: Alveolar soft part sarcoma of the uterine cervix in a woman
presenting with postmenopausal bleeding: A case report and
literature review. Eur J Gynaecol Oncol. 32:359–361.
2011.PubMed/NCBI
|
|
14
|
Yang CQ, Cui Z, Yao JJ and Liu DG:
Meningeal alveolar soft tissue sarcoma misdiagnosed as meningioma:
Report of a case. Zhonghua Bing Li Xue Za Zhi. 40:193–194. 2011.(In
Chinese). PubMed/NCBI
|
|
15
|
Xin FY, Rana N, Ming Z and Lang YB:
Alveolar soft part sarcoma of the retro peritoneum. J Cancer Res
Ther. 6:117–119. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yavuz A, Göya C, Bora A and Beyazal M:
Primary alveolar soft part sarcoma of the scapula. Case Rep Oncol.
6:356–361. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim JM, Im SA, Oh SN and Chung NG:
Alveolar soft part sarcoma arising from the kidney: Imaging and
clinical features. Korean J Radiol. 15:381–385. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sood S, Baheti AD, Shinagare AB,
Jagannathan JP, Hornick JL, Ramaiya NH and Tirumani SH: Imaging
features of primary and metastatic alveolar soft part sarcoma:
Single institute experience in 25 patients. Br J Radiol.
87:201307192014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Suh JS, Cho J, Lee SH, Shin KH, Yang WI,
Lee JH, Cho JH, Suh KJ, Lee YJ and Ryu KN: Alveolar soft part
sarcoma: MR and angiographic findings. Skeletal Radiol. 29:680–689.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kayton ML, Meyers P, Wexler LH, Gerald WL
and LaQuaglia MP: Clinical presentation, treatment, and outcome of
alveolar soft part sarcoma in children, adolescents, and young
adults. J Pediatr Surg. 41:187–193. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ladanyi M, Lui MY, Antonescu CR,
Krause-Boehm A, Meindl A, Argani P, Healey JH, Ueda T, Yoshikawa H,
Meloni-Ehrig A, et al: The der(17)t(X;17)(p11;q25) of human
alveolar soft part sarcoma fuses the TFE3 transcription factor gene
to ASPL, a novel gene at 17q25. Oncogene. 20:48–57. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Argani P, Lal P, Hutchinson B, Lui MY,
Reuter VE and Ladanyi M: Aberrant nuclear immunoreactivity for TFE3
in neoplasms with TFE3 gene fusions: A sensitive and
specificimmunohistochemical assay. Am J Surg Pathol. 27:750–761.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fanburg-Smith JC, Miettinen M, Folpe AL,
Weiss SW and Childers EL: Lingual alveolar soft part sarcoma; 14
cases: Novel clinical and morphological observations.
Histopathology. 45:526–537. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Portera CA Jr, Ho V, Patel SR, Hunt KK,
Feig BW, Respondek PM, Yasko AW, Benjamin RS, Pollock RE and
Pisters PW: Alveolar soft part sarcoma: Clinical course and
patterns of metastasis in 70 patients treated at a single
institution. Cancer. 91:585–591. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Reichardt P, Lindner T, Pink D,
Thuss-Patience PC, Kretzschmar A and Dörken B: Chemotherapy in
alveolar soft part sarcomas. What do we know? Eur J Cancer.
39:1511–1516. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ogose A, Morita T, Hotta T, Kobayashi H,
Otsuka H, Hirata Y and Yoshida S: Brain metastases in
musculoskeletal sarcomas. Jpn J Clin Oncol. 29:245–247. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bindal RK, Sawaya RE, Leavens ME, Taylor
SH and Guinee VF: Sarcoma metastatic to the brain: Results of
surgical treatment. Neurosurgery. 35:185–191. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kumar Sujit GS, Chacko G, Chacko AG and
Rajshekhar V: Alveolar soft-part sarcoma presenting with multiple
intracranial metastases. Neurol India. 52:257–258. 2004.PubMed/NCBI
|
|
29
|
Mitton B and Federman N: Alveolar soft
part sarcomas: Molecular pathogenesis and implications for novel
targeted therapies. Sarcoma. 2012:4287892012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ghose A, Tariq Z and Veltri S: Treatment
of multidrug resistant advanced alveolar soft part sarcoma with
sunitinib. Am J Ther. 19:e56–e58. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Stacchiotti S, Negri T, Zaffaroni N,
Palassini E, Morosi C, Brich S, Conca E, Bozzi F, Cassinelli G,
Gronchi A, et al: Sunitinib in advanced alveolar soft part sarcoma:
Evidence of a direct antitumor effect. Ann Oncol. 22:1682–1690.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Genin O, Rechavi G, Nagler A, Ben-Itzhak
O, Nazemi KJ and Pines M: Myofibroblasts in pulmonary and brain
metastases of alveolar soft-part sarcoma: A novel target for
treatment? Neoplasia. 10:940–948. 2008. View Article : Google Scholar : PubMed/NCBI
|