Introduction
Esophageal cancer is the eighth most common cancer
type and the sixth-leading cause of cancer-associated mortality
globally (1). Esophageal squamous
cell carcinoma (ESCC) is the main subtype of esophageal cancer,
comprising ~70% of esophageal cancer occurrences, with incidence
rates varying widely with geographic location (2–4). The World
Health Organization estimated that ~50% of total ESCC cases
occurred in China, while there were 197,472 occurrences of
ESCC-associated mortality globally in 2012 (5). In China, the majority of patients with
ESCC are diagnosed with advanced-stage disease and the 5-year
survival rate is 4.4% (5). At
present, the treatment of this disease is relatively ineffective,
owing to recurrence, metastasis and the development of resistance
to radiation, and chemotherapy resistance (6,7).
Therefore, the identification of novel therapeutic agents for
cancer with high selectivity and low toxicity may represent a step
forward in treatment.
The phosphatidylinositol 3-kinase (PI3K)/protein
kinase B (AKT)/mechanistic target of rapamycin (mTOR) signaling
pathway is involved in multiple essential processes, including
cellular metabolism, proliferation, cell cycle regulation,
cytoskeletal reorganization and tumor development (8,9). In cancer
cells, this pathway has a marked effect on the regulation of cell
growth and survival, ultimately conferring a competitive growth
advantage, inducing entry into the cell cycle, and enabling
metastatic competence, angiogenesis and resistance to therapy
(10,11). PI3K is activated by the binding of a
growth factor or ligand to its cognate growth factor receptor
tyrosine kinase, which leads to the phosphorylation and activation
of Akt, a serine/threonine kinase. Phosphorylation of AKT
stimulates protein synthesis and cell growth by activating mTOR
(12).
Celastrus orbiculatus (Celastraceae) is a
traditional medicine used to treat numerous symptoms and diseases,
including ache, arthritis and other inflammatory diseases (13). Previous studies indicate that
Celastrus orbiculatus extracts (COE) exhibit multiple
biological properties, including antitumor, anti-inflammatory,
analgesic, antifertility, antibacterial and antiviral properties
(14,15). COE have been identified to exhibit
anticancer effects. Several studies have demonstrated that COE can
interfere with growth of stomach, colon and liver cancer cells
through the inhibition of proliferation, angiogenesis, invasion and
metastasis, and the induction of apoptosis in vitro and
in vivo (16–24). However, the therapeutic effects of COE
on ESCC have not been investigated. The present study aimed to
elucidate the anticancer effects of COE, and whether they were
mediated via growth inhibition, cell cycle arrest, apoptosis and
DNA damage in ESCC; it also aimed to identify the possible
mechanism underlying these effects by inhibiting the PI3K/AKT/mTOR
signaling pathway.
Materials and methods
Preparation of COE
Celastrus orbiculatus plants (production
batch no. 070510) were purchased from Guangzhou Zhixin
Pharmaceutical Co., Ltd. (Guangzhou, China) in 2007 and extracted
at the Department of Chinese Materia Medica Analysis, China
Pharmaceutical University (Nanjing, China) as described previously
(22). The chemical constituents of
the stems of COE were investigated and compounds were isolated as
described previously (24,25). The resultant COE micropowder was
dissolved in DMSO (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany)
and diluted to produce a range of different concentrations prior to
use. The final concentration of DMSO in the cell culture did not
exceed 0.1%.
Cell culture
The human esophageal squamous carcinoma ECA-109 cell
line was obtained from the Cell Bank of Chinese Academy of
Sciences, Shanghai Institute of Cell Biology (Shanghai, China).
Cells were cultured in RPMI-1640 containing 10% fetal bovine serum
(both Gibco; Thermo Fisher Scientific, Waltham, MA, USA) in a 5%
CO2 incubator at 37°C in a humidified atmosphere.
Cell viability assay
A total of 2.0×103 ECA-109 cells/well
were seeded into 96-well plates and incubated at 37°C in a 5%
CO2 incubator for 24 h. Next, cells were treated with
different concentrations of COE (320, 160, 80, 40 or 20 mg/l) for
24, 48 or 72 h. A negative control group consisting of untreated
cells was also included. The plate was subjected to treatment with
5 mg/ml MTT (Sigma-Aldrich, Merck KGaA) dissolved in sterile PBS in
dark for 4 h in a 5% CO2 incubator at 37°C, and the
optical density (OD) value was measured at 490 nm. The tests were
independently performed ≥3 times. The cell viability rate was
calculated as follows: (OD value of each concentration group/OD
value of negative control group) ×100.
Cell cycle analysis
A total of 1×106 ECA-109 cells treated
with 0, 20, 40 or 80 mg/l COE for 24 h were harvested, and then
fixed in 70% ethanol at 4°C for 2 h. After 24 h, the cells were
washed twice with ice-cold PBS, stained with enough neat propidium
iodide (PI)/RNase Staining Solution (Cell Signaling Technology,
Inc., Danvers, MA, USA) at 25°C for 15 min in the dark. The
measurements were performed using a FACSCaliber flow cytometer and
Cell Quest Pro software version 349226 (BD Biosciences, Franklin
Lakes, NJ, USA). The data were analyzed using FlowJo 7.6 software
(Tree Star, Inc., Ashland, OR, USA). The tests were performed ≥3
independent times.
Cell apoptosis assay
A total volume of 1×106 ECA-109 cells
were treated with 0, 20, 40 or 80 mg/l COE for 24 h, and the
harvested cells were washed twice with ice-cold PBS. Apoptotic
cells were identified using the Annexin V-Fluorescein
Isothiocyanate (FITC)/PI Apoptosis Detection kit (Nanjing KeyGen
Biotech Co., Ltd., Nanjing, China). After centrifugation at 100 × g
for 5 min at 4°C, 290 µl of 1X binding buffer, 5 µl of Annexin
V-FITC and 5 µl of PI were added to the pellet, and incubated at
room temperature (25°C) for 15 min in the dark. Next, 200 µl of 1X
binding buffer was added prior to measurement. The data were
measured and analyzed with the same machine and software as the
cell cycle assay. The cell apoptosis assay was performed with 3
independent experiments.
Evaluation of mitochondrial membrane
depolarization
The Mitochondrial Membrane Potential Detection kit
JC-1 (Beyotime Institute of Biotechnology, Hangzhou, China) was
used to measure the changes of the mitochondria membrane potential.
Following treatment with 0, 20, 40 or 80 mg/l COE for 24 h, the
cells were incubated with 1 ml JC-1 working solution for 20 min at
37°C in the dark, and then washed twice with JC-1 buffer. The
results were observed using a fluorescence microscope at
magnification, ×100.
Transmission electron microscopy
ECA-109 cells in the logarithmic growth phase were
incubated with 0, 20, 40 or 80 mg/l COE for 24 h. Cells were
harvested and the supernatant was discarded. Cells were then washed
twice with PBS, and 2.5% glutaraldehyde was added over 2 h at 4°C.
Following a wash with 0.1 mM PBS, the cells were fixed in 1% osmium
teroxide for 2 h at 4°C, and then washed, dehydrated in a graded
alcohol series and acetone. Cells were embedded in different
proportions of Epon 812 resin, sectioned at 50 nm and stained with
uranyl acetate for 30 min and lead citrate for 15 min at room
temperature, the cells were observed under a CM100 transmission
electronic microscope at magnification, ×6,600 (Philips Medical
Systems B.V., Eindhoven, The Netherlands).
Terminal
deoxynucleotidyltransferase-mediated dUTP nick-end labeling
(TUNEL)
DNA fragmentation was detected using the TUNEL
technique with the In Situ Cell Apoptosis Detection kit, POD
(Nanjing KeyGen Biotech Co., Ltd., Nanjing, China) performed
according to the manufacturer's protocol. The cells grew prior on
the glass slide were treated with COE at different concentrations
for 24 h. Next, 4% (w/v) paraformaldehyde in PBS (pH 7.4) was used
for fixation of cells (30 min at room temperature) and rinsed twice
with PBS. The fixed cells were then incubated in permeabilization
solution (0.1% Triton X-100 in 0.1% sodium citrate) for 3 min at
room temperature, and then the cells were incubated with blocking
solution (3% H2O2 in methanol) for 10 min at
25°C and rinsed with PBS. Subsequently, 50 µl of reaction mixture
containing TdT enzyme and nucleotide was added to the cells, and
the cells were all incubated for 1 h at 37°C in the dark. The
slides were washed with PBS and incubated with 50 µl
streptavidin-horseradish peroxidase working solution for 30 min at
37°C in the dark, and rinsed three times with PBS. Finally, the
cells incubated with DAB were analyzed by light microscopy.
Western blot analysis
Expression levels of B-cell lymphoma 2 (BCL2;
Epitomics, Burlingame, California, USA; cat. no. S0820; dilution,
1:1,000), Bcl-2-associated X [Bax; Cell Signaling Technology (CST),
Inc. Danvers, MA, USA; cat. no. 2772; dilution, 1:1,000], PI3K
(CST; cat. no. 4249; dilution, 1:1,000), AKT (CST; cat. no. 4691;
dilution, 1:1,000), phosphorylated (p)-AKT(Ser473) (CST; cat. no.
4058; dilution, 1:1,000), mTOR (CST; cat. no. 2983; dilution,
1:1,000), phosphorylated mTOR (Ser2448; CST; cat. no. 5536;
dilution, 1:1,000) in ECA-109 cells treated with 0, 20, 40 or 80
mg/l COE were detected by western blot analysis. β-actin (CST; cat.
no. 4970; dilution, 1:1,000) was used as a marker for cytosolic
proteins. Lysate proteins were resolved by 10% SDS-PAGE and
transferred onto nitrocellulose membranes (EMD Millipore,
Billerica, MA, USA). Membranes were blocked in blocking buffer (5%
not-fat dry milk and 1% Tween-20 in PBS) for 2 h at room
temperature, and then incubated with appropriate primary antibodies
in blocking buffer at 4°C overnight. Subsequently, the membranes
were washed with TBS containing Tween-20 to remove the residual
primary antibodies mentioned above, and incubated with horseradish
peroxidase conjugated goat anti-rabbit immunoglobulin G secondary
antibodies (Huaan Biotechnology company, Hangzhou, Zhejiang, China;
cat. no. HA-1001-100; dilution, 1:2,000) for 2 h at room
temperature. Enhanced chemiluminescence was used to detect signals,
using the Super Signal West Pico Chemiluminescent Substrate (Thermo
Fisher Scientific, Waltham, MA, USA) on a Molecular Imager Chemi
Doc XRS System (Bio-Rad Laboratories, Inc., Hercules, CA, USA). The
experiments were repeated 3 times. The bands from western blotting
were quantified using Quantity One analysis software version 4.62
(Bio-Rad Laboratories, Inc.).
Inhibitor treatment
To investigate the effect of mTOR inhibition on cell
growth, apoptosis and the cell cycle in ECA-109 cells further,
confluent cell cultures were pretreated with 100 nM rapamycin
(Sigma-Aldrich, Merck KGaA; cat. no. R0395) for 1 h and then
incubated in the presence or absence of COE (80 mg/l) for 24 h. The
cells were then subjected to the cell apoptosis assay, cell cycle
analysis, evaluation of mitochondrial membrane depolarization,
TUNEL assay, transmission electron microscopy and western blotting,
as aforementioned.
Statistical analysis
Each experiment was repeated ≥3 times. The
experimental results were analyzed using SPSS 16.0 software (SPSS,
Inc., Chicago, IL, USA). Data are expressed as the mean ± standard
deviation. Comparison between two groups was performed using the
Student's t-test. Statistical comparisons of more than two groups
were performed by one-way analysis of variance with Bonferroni's
post hoc test. P<0.05 was considered to indicate a statistically
significant difference. The graphs were obtained using GraphPad
Prism 5.0 software (GraphPad, Inc., La Jolla, CA, USA).
Results
COE inhibits the proliferation of
ESCC
To assess the anticancer activity of COE on ESCC,
the effect of COE on the proliferation of the ESCC ECA-109 cell
line was investigated. Morphologically, with increasing
concentrations of COE, cells were increasingly shrunk and detached
from the coverslip (Fig. 1A). As a
result, the viability of cells decreased with COE treatment in a
time- and dose-dependent manner, as assessed by MTT assay (Fig. 1B). The inhibitory effects on
proliferation were not significant at 24 and 48 h in the 20 mg/l
group.
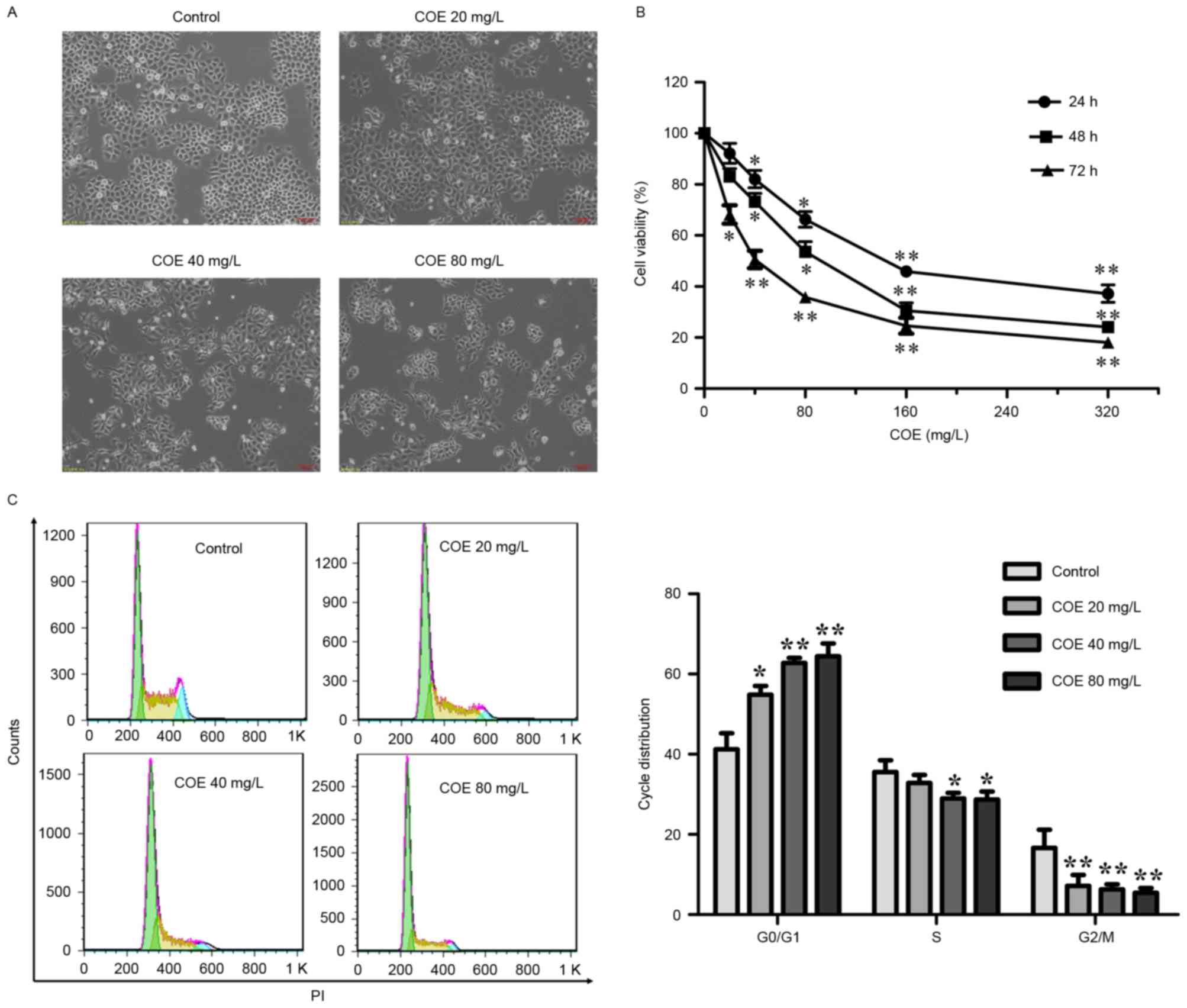 | Figure 1.COE inhibited the proliferation of
ESCC ECA-109 cells and induced cell cycle arrest. (A) ECA-109 cells
were treated with COE at the indicated concentrations for 24, 48 or
72 h, followed by morphological observation. Scale bar, 100 µm. (B)
The effect of COE on cell viability in ECA-109 cells. Cells were
treated with 20, 40, 80, 160 or 320 mg/l COE for 24, 48 or 72 h.
Cell viability was measured by MTT assay. *P<0.05 and
**P<0.01 vs. control. (C) COE triggered
G0/G1 cell cycle arrest. Cells were treated
with COE at different concentrations (20, 40 or 80 mg/l) for 24 h,
followed by PI staining and fluorescence-activated cell sorting
analysis of the cell cycle profile. Representative images (left)
and results of statistical analysis (right) are presented.
*P<0.05 and **P<0.01 vs. control. COE, Celastrus
orbiculatus extracts; ESCC, esophageal squamous carcinoma
cells; PI, propidium iodide. |
COE induces
G0/G1 cell cycle arrest in ESCC
The cell cycle assay demonstrated that COE produced
a significant decrease in the number of cells in the
G2/M phase, and a significant accumulation in the number
of cells in the G0/G1 phase. These findings
clearly demonstrated that COE triggered G0/G1
cell cycle arrest in ECA-109 cells in a dose-dependent manner
(Fig. 1C).
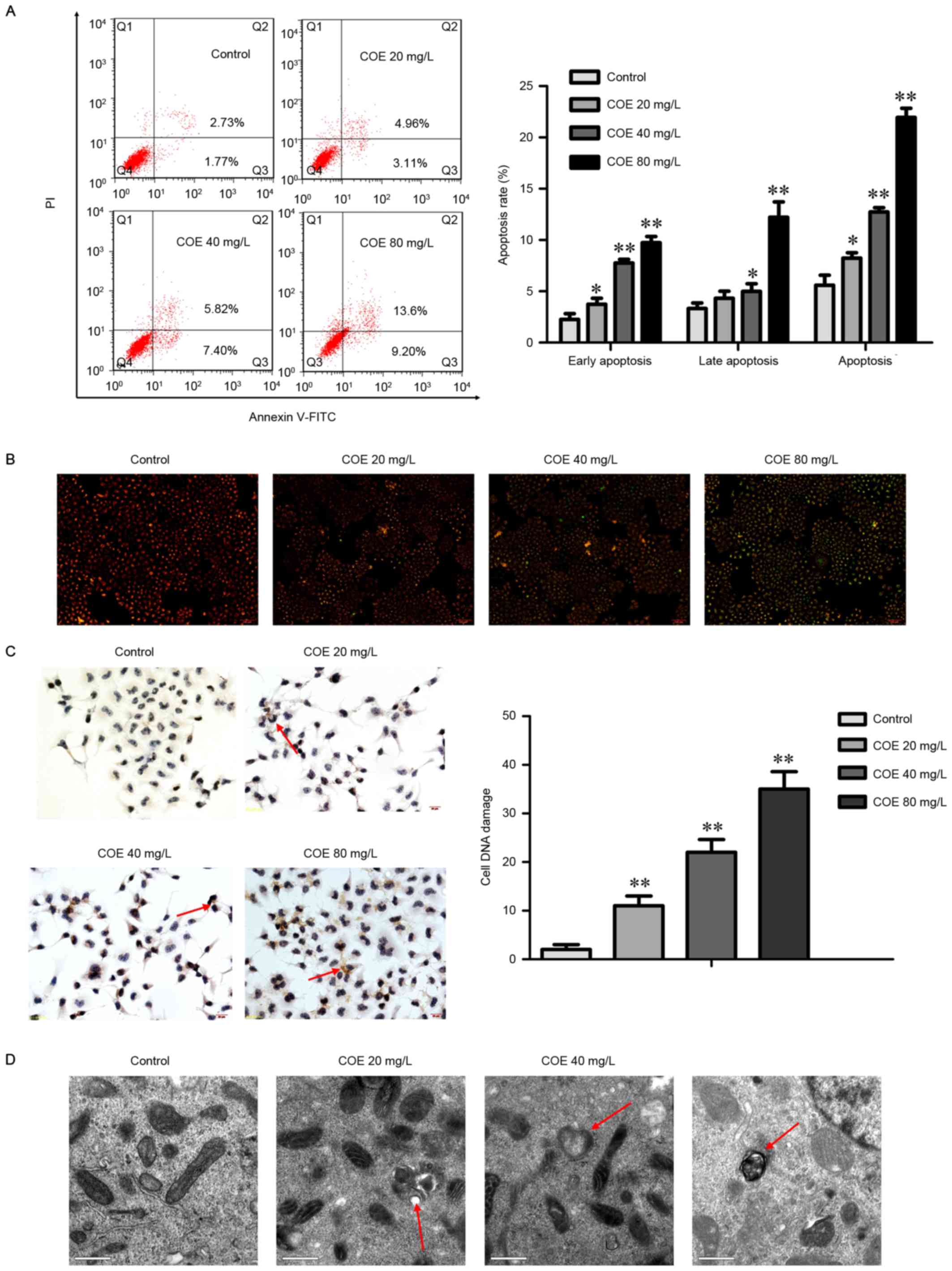
COE induces apoptosis in ESCC
Next, whether apoptosis was responsible for the
anticancer activity of COE was assessed. The results demonstrated
that COE treatment led to the significant accumulation of cells in
early-(Annexin V+/PI−) and late-stage
(Annexin V+/PI+) apoptosis in a
dose-dependent manner (Fig. 2A). The
proportion of cells in early apoptosis significantly increased when
COE was added, whereas the percentage of late apoptosis cells
treated with 20 mg/l COE did not significantly change.
COE induced the loss of mitochondrial membrane
potential (Fig. 2B), a classical
marker of the activation of intrinsic apoptosis, which indicated
that COE triggered mitochondrial apoptosis. This was indicated by
the transition of JC-1 fluorescence from red to green gradually
with the increasing concentration gradient.
COE induces DNA damage and increases
the amount of apoptotic bodies in ESCC
To determine whether COE induces the DNA damage
response, a TUNEL assay was performed to detect apoptotic nuclei in
ECA-109 cells (Fig. 2C). The TUNEL
staining revealed gradual and significant increases in the rate of
apoptosis in the groups treated with 20, 40, and 80 mg/l COE.
To verify apoptosis, transmission electron
microscopy was used to observe organelles in ECA-109 cells treated
with COE (Fig. 2D). The results
revealed that untreated cells possessed normal mitochondria and
cytoplasm, whereas COE-treated cells exhibited the appearance of
apoptotic bodies of various sizes. Swollen and transparent
mitochondria were also observed by electron microscopy, which
occurred in a DOE dose-dependent manner.
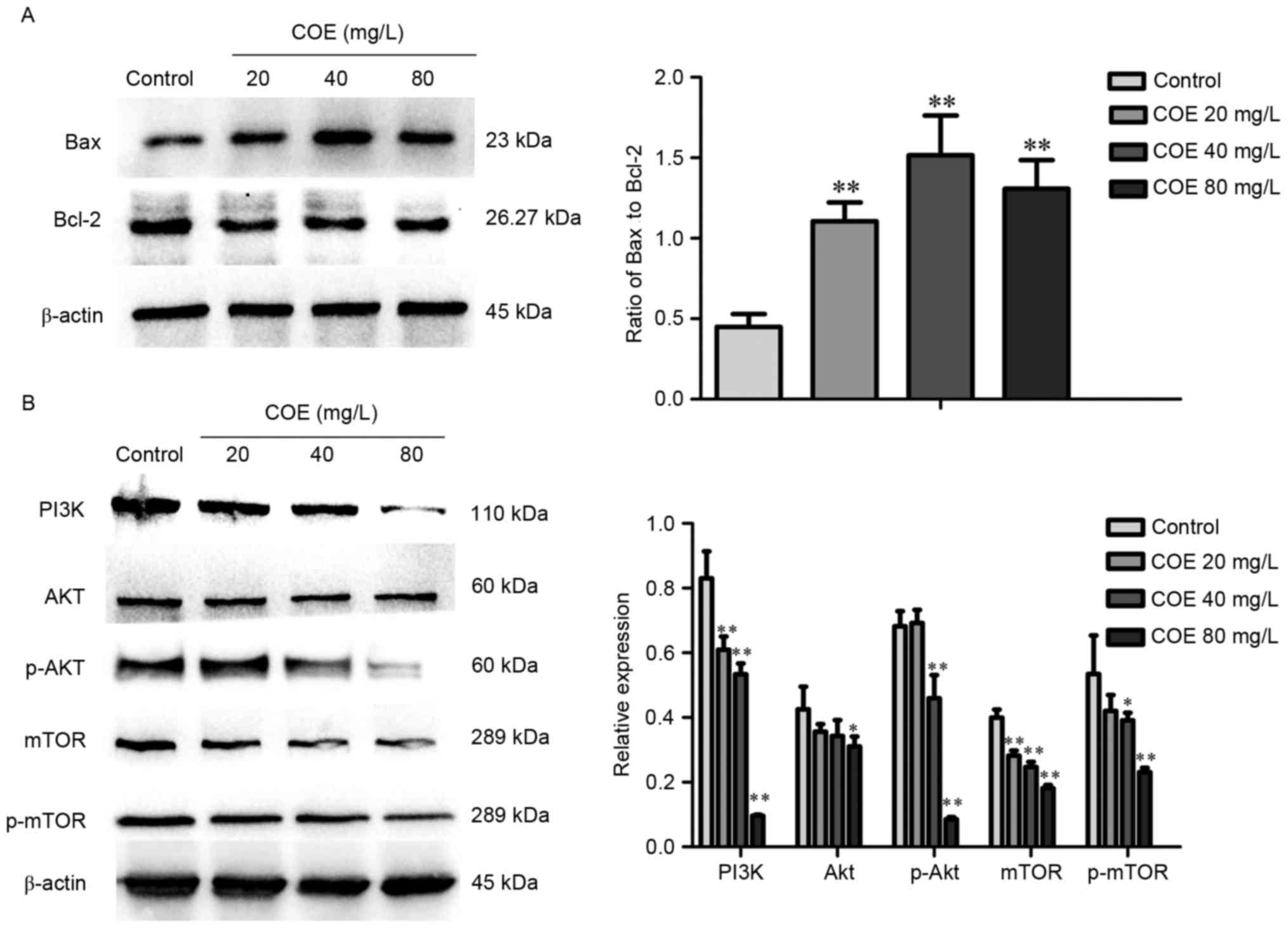
COE inhibits the PI3K/Akt/mTOR
signaling pathway in ESCC
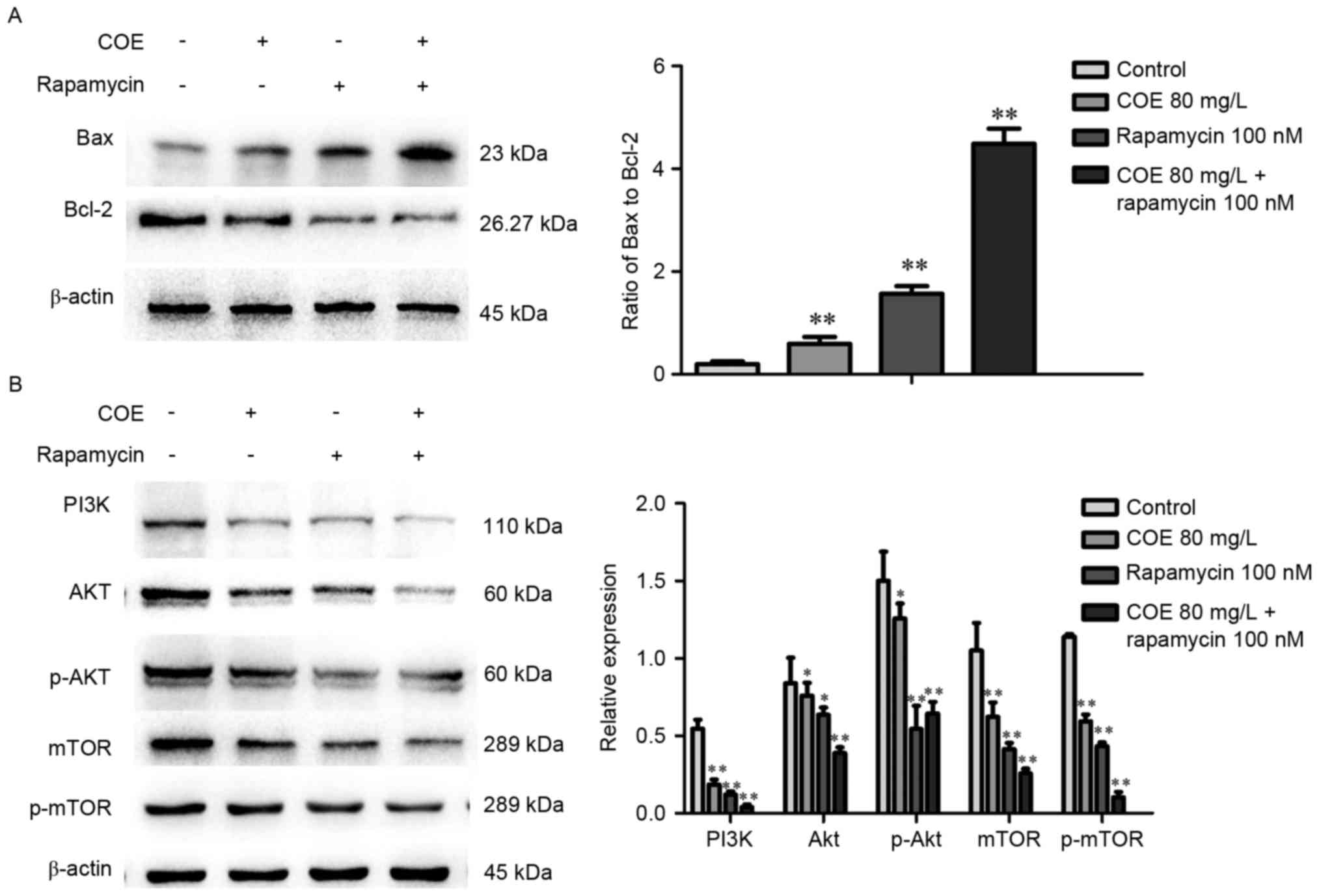
Western blot analysis demonstrated that treatment
with COE significantly increased the ratio of Bax to Bcl-2 protein,
whereby the effect was larger in the 40 mg/l compared with 80 mg/l
treatment group (Fig. 3A). As
presented in Fig. 3B, levels of PI3K,
phosphorylated Akt, mTOR, phosphorylated mTOR were significantly
lower in groups treated with COE compared with the control group,
with no significant changes in Akt expression overall.
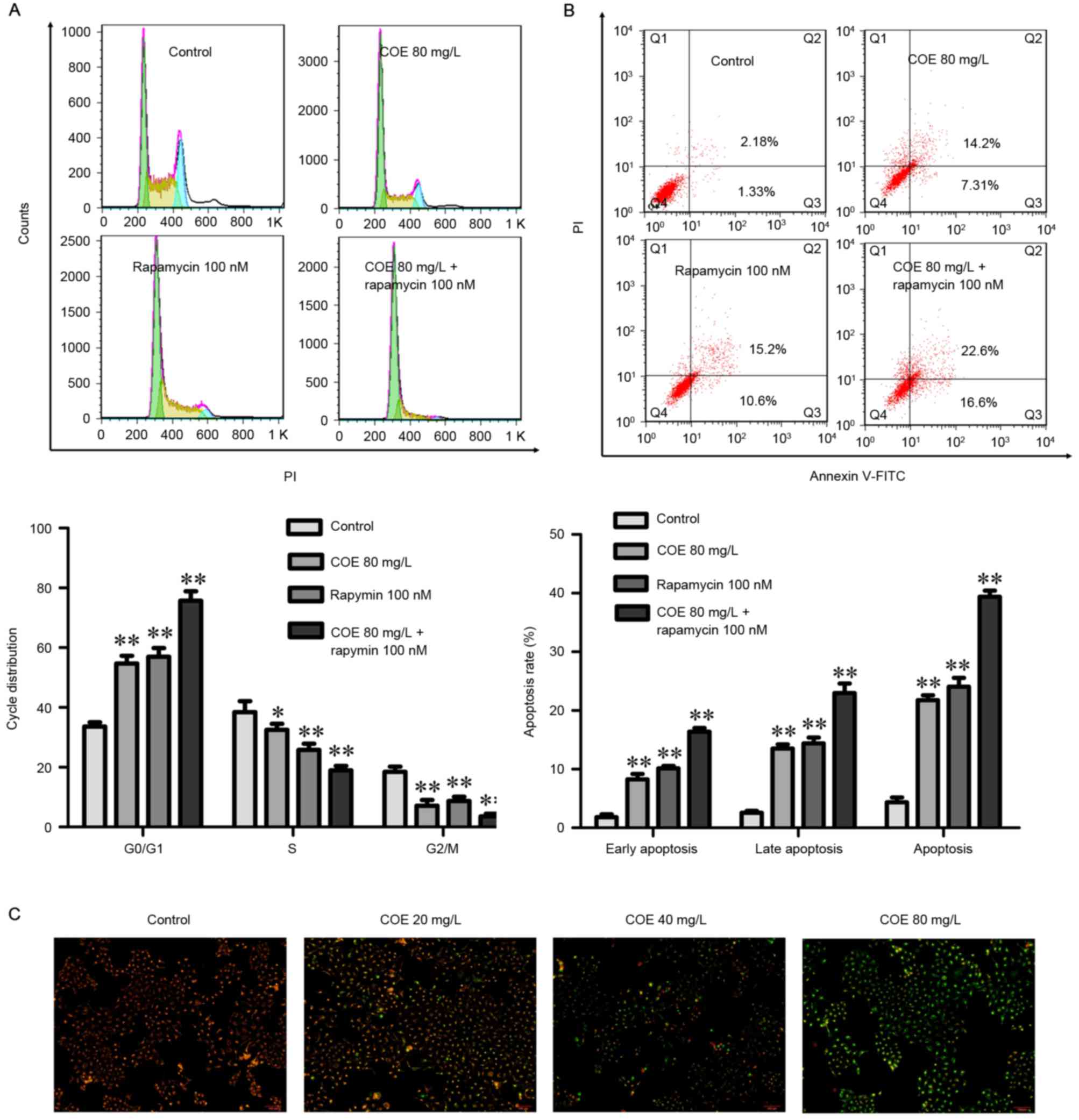
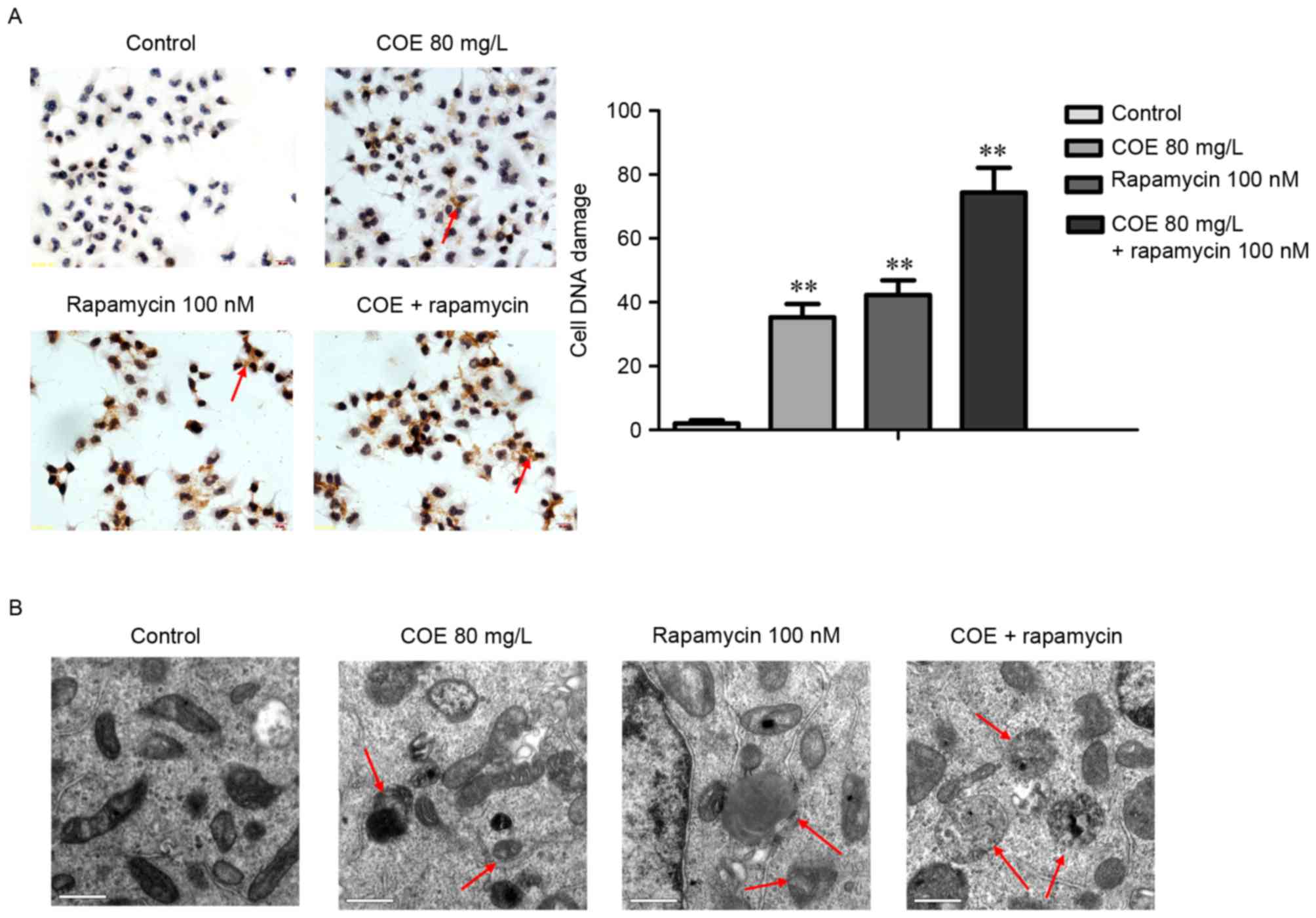
COE inhibits proliferation, and
induces cell cycle arrest and apoptosis through PI3K/Akt/mTOR
signaling pathways
The combined treatment of rapamycin and COE
significantly induced cell cycle arrest (Fig. 4A), and apoptosis (Fig. 4B) compared with treatment with COE
alone. In addition, the effects of combination treatment on the
decrease of mitochondrial membrane potential were significantly
more evident compared with treatment with COE alone (Fig. 5A). Furthermore, apoptotic bodies
induced by combination treatment were markedly more evident
compared with COE alone (Fig. 5B).
Western blot analysis indicated that the combination treatment
significantly enhanced the observed changes that occurred following
treatment with COE or rapamycin alone, including changes to the
Bax/Bcl-2 ratio (Fig. 6A), and PI3K,
Akt, p-Akt, mTOR and p-mTOR protein expression levels (Fig. 6B).
Discussion
Traditional Chinese herbs have been widely used in
the treatment of cancer in Asia (23). The antitumor effects of traditional
Chinese medicine have been further studied, including their
antiproliferative, anti-angiogenic and pro-apoptotic effects
(26,27). COE is extracted from the plant of
Celastrus orbiculatus, which is widely used in traditional
Chinese medicine. Previous studies had reported that treatment with
COE exerts an antitumor effect on several types of tumors in a
cell-type-dependent manner (16–24). COE
exhibits distinct antitumor activities, including the inhibition of
cell proliferation, induction of cell apoptosis and inhibition of
angiogenesis (16–24). Qian et al (16) reported that COE inhibited tumor
angiogenesis by modulating the vascular endothelial growth factor
signaling pathway. Zhu et al (22–24)
reported that COE suppressed tumor growth factor-β1-induced
epithelial-mesenchymal transition by inhibiting HSP27 and the tumor
necrosis factor-α-induced nuclear factor-κB/Snail signaling pathway
in human gastric adenocarcinoma. Therefore, COE may be a novel
therapeutic agent targeted at digestive tract cancer cells.
However, the antitumor effects and the associated mechanisms of COE
treatment for ESC remain undefined.
The present study assessed and validated the
efficacy of treatment with COE alone and in combination with
rapamycin in ESCC in vitro. COE induced cells change from
normal adherence to a shrunk and floated status with increasing
concentrations of COE. An MTT assay revealed that COE decreased the
viability of cells in a time- and dose-dependent manner. To exclude
any cytotoxic effect, a low-toxicity concentration was chosen
(<80 mg/l) of COE for further experiments. The cell cycle assay
indicated that COE may induce cell cycle arrest by triggering
induction of the G0/G1 phase. COE induced
early and late apoptosis in ECA-109 cells at 40, and 80 mg/l. The
mitochondrial membrane potential assay also demonstrated the action
of COE on mitochondria, which are central to the process of
apoptosis. Furthermore, the TUNEL assay revealed that COE caused
DNA damage in a dose-dependent manner. To observe the organelles
within ECA-109 cells treated with COE further, transmission
electron microscopy was used, revealing that mitochondria were
swollen and transparent, and apoptotic bodies appeared in a
dose-dependent manner. To determine the effects of COE at the
molecular level, the protein expression of Bax and Bcl-2 were
detected, whereby a decrease in the ratio of Bcl-2 to Bax
expression is known to be associated with apoptosis (28,29). The
results revealed that COE increased the BAX/Bcl-2 ratio, and the
effect of 40 mg/l was preferable to 80 mg/l. Previous studies have
reported that COE promoted apoptosis in human hepatocellular
carcinoma cells via inhibition of the AKT signaling pathway and
inhibited human colorectal carcinoma cell metastasis via
suppression of the mTOR signaling pathway (16–20). On
the basis of these previous results, the present study assessed the
expression of the PI3K/AKT/mTOR signaling pathway. It was
demonstrated that the levels of PI3K, p-AKT, mTOR and p-mTOR
decreased significantly, while the expression of AKT did not change
following COE treatment. In addition, the combined treatment of
rapamycin and COE further inhibited cellular proliferation, and
induced cell cycle arrest and apoptosis.
In conclusion, the present study revealed that COE
treatment inhibits proliferation and induces apoptosis in ECA-109
cells by triggering G0/G1 cell cycle arrest
and DNA damage. Furthermore, the effects of COE combined with
rapamycin are superior to treatment with either alone. These
results suggest that the possible mechanism of COE on ESCC involves
the PI3K/AKT/mTOR signal pathway. The findings of the present study
extend the understanding of COE and suggest that it may be
considered as a treatment option for ESCC, alone or in combination
with other drugs. However, all of the experiments in the present
study were performed in vitro, and further study will be
performed to evaluate the efficacy of COE on cell growth and
apoptosis of ESCC in vivo.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81773944 and
81573656) and the Natural Science Foundation of Jiangsu Province
(grant no. BK20171290 and BK20170516).
References
|
1
|
Zhang Y: Epidemiology of esophageal
cancer. World J Gastroenterol. 19:5598–5606. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Roshandel G, Majdzadeh R, Keshtkar A,
Aramesh K, Sedaghat SM and Semnani S: Healthcare utilization in
patients with esophageal cancer in a high risk area in northeast of
Iran. Asian Pac J Cancer Prev. 12:2437–2442. 2011.PubMed/NCBI
|
|
3
|
Maleki I, Shekarriz R, Nosrati A and Orang
E: Simultaneous esophageal squamous cell carcinoma and
adenocarcinoma: A case report. Middle East J Dig Dis. 7:257–260.
2015.PubMed/NCBI
|
|
4
|
Roshandel G, Boreiri M, Sadjadi A and
Malekzadeh R: A diversity of cancer incidence and mortality in West
Asian populations. Ann Glob Health. 80:346–357. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lu CL, Lang HC, Luo JC, Liu CC, Lin HC,
Chang FY and Lee SD: Increasing trend of the incidence of
esophageal squamous cell carcinoma, but not adenocarcinoma, in
Taiwan. Cancer Causes Control. 21:269–274. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
He YT, Hou J, Chen ZF, Qiao CY, Song GH,
Meng FS, Jin HX and Chen C: Trends in incidence of esophageal and
gastric cardia cancer in high-risk areas in China. Eur J Cancer
Prev. 17:71–76. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Porta C, Paglino C and Mosca A: Targeting
PI3K/Akt/mTOR Signaling in Cancer. Front Oncol. 14:642014.
|
|
9
|
Lee JJ, Loh K and Yap YS: PI3K/Akt/mTOR
inhibitors in breast cancer. Cancer Biol Med. 12:342–354.
2015.PubMed/NCBI
|
|
10
|
Hu A, Sun M, Yan D and Chen K: Clinical
significance of mTOR and eIF4E expression in invasive
ductalcarcinoma. Tumori. 100:541–546. 2014.PubMed/NCBI
|
|
11
|
Liu NB, Zhang JH, Liu YF, Li J, Zhang ZZ,
Li JW, Liu WY, Huang C, Shen T, Gu CW, et al: High DEPTOR
expression correlates with poor prognosis in patients with
esophageal squamous cell carcinoma. Onco Targets Ther. 8:3449–3455.
2015.PubMed/NCBI
|
|
12
|
Burris HA III: Overcoming acquired
resistance to anticancer therapy: Focus on the PI3K/AKT/mTOR
pathway. Cancer Chemother Pharmacol. 71:829–842. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang M: Research progress of a Chinese
herb Celastrus on antitumor effect. J Chinese Medicine.
25:1055–1057. 2010.(In Chinese).
|
|
14
|
Li JJ, Yang J, Lu F, Qi YT, Liu YQ, Sun Y
and Wang Q: Chemical constituents from the stems of Celastrus
orbiculatus. Chin J Nat Med. 10:279–283. 2012.(In Chinese).
View Article : Google Scholar
|
|
15
|
Zan K, Chen X, Wang Q and Cao L: Chemical
constituents in stem of Celastrus orbiculatus. Chin Tradit Herbal
Drugs. 38:14552007.(In Chinese).
|
|
16
|
Qian YY, Zhang H, Hou Y, Yuan L, Li GQ,
Guo SY, Hisamits T and Liu YQ: Celastrus orbiculatus extract
inhibits tumor angiogenesis by targeting vascular endothelial
growth factor signaling pathway and shows potent antitumor activity
in hepatocarcinomas in vitro and in vivo. Chin J Integr Med.
18:752–760. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang M, Zhang X, Xiong X, Yang Z, Sun Y,
Yang Z, Hoffman RM and Liu Y: Efficacy of the Chinese traditional
medicinal herb Celastrus orbiculatus Thunb on human hepatocellular
carcinoma in an orthothopic fluorescent nude mouse model.
Anticancer Res. 32:1–1220. 2012.PubMed/NCBI
|
|
18
|
Zhang H, Qian Y, Liu Y, Li G, Cui P, Zhu
Y, Ma H, Ji X, Guo S and Tadashi H: Celastrus orbiculatus extract
induces mitochondrial-mediated apoptosis in human hepatocellular
carcinoma cells. J Tradit Chin Med. 32:621–626. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Qian YY and Liu YQ: Inhibition of
Celastrus orbiculatus extracts on VEGF expression in hepatoma cells
of mice. Chinese Herbal Med. 2:72–76. 2010.
|
|
20
|
Hui MA, Yayun Q, Hua Z, Xue J, Yaodong Z,
Pingfang C and Yanqing L: Celastrus orbiculatus extract could
inhibit human colorectal carcinoma HT-29 cells metastasis via
suppression of the mTOR signaling pathway. Life Sci J.
10:1704–1710. 2013.
|
|
21
|
Li G, Liu D, Guo S, Sunagawa M, Hisamitsu
T and Liu Y: Anti-invasive effects of Celastrus orbiculatus extract
on interleukin-1 betaand tumour necrosis factor-alpha
combination-stimulated fibroblast-likesynoviocytes. BMC Complement
Altern Med. 14:622014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhu Y, Liu Y, Qian Y, Dai X, Yang L, Chen
J, Guo S and Hisamitsu T: Antimetastatic effects of Celastrus
orbiculatus on human Gastricadenocarcinoma by inhibiting
epithelial-mesenchymal transition and NF-κB/snail signaling
pathway. Integr Cancer Ther. 14:271–281. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhu YD, Liu YQ, Qian YY, Zhang H, Li GQ
and Yang L: Extracts of Celastrus orbiculatus exhibit
anti-proliferative and anti-invasiveeffects on human gastric
adenocarcinoma cells. Chin J Integr Med. Nov 10–2014.(Epub ahead of
print). View Article : Google Scholar
|
|
24
|
Zhu Y, Liu Y, Qian Y, Yang L, Chen J, Guo
S and Hisamitsu T: Research on the efficacy of Celastrus
orbiculatus in suppressing TGF-β1-induced epithelial-mesenchymal
transition by inhibiting HSP27 and TNF-α-induced NF-κB/Snail
signaling pathway in human gastric adenocarcinoma. BMC Complement
Altern Med. 14:4332014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tao W, Luo X, Cui B, Liang D, Wang C, Duan
Y, Li X, Zhou S, Zhao M and Li Y: Practice of traditional Chinese
medicine for psycho-behavioral intervention improves quality of
life in cancer patients: A systematic review and meta-analysis.
Oncotarget. 6:39725–39739. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xu H, Zhao X, Liu X, Xu P, Zhang K and Lin
X: Antitumor effects of traditional Chinese medicine targeting the
cellularapoptotic pathway. Drug Des Devel Ther. 9:2735–2744.
2015.PubMed/NCBI
|
|
27
|
Zhang YS, Shen Q and Li J: Traditional
Chinese medicine targeting apoptotic mechanisms for esophageal
cancer therapy. Acta Pharmacol Sin. 37:295–302. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu Z, Ding Y, Ye N, Wild C, Chen H and
Zhou J: Direct activation of bax protein for cancer therapy. Med
Res Rev. 36:313–341. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Thomas S, Quinn BA, Das SK, Dash R, Emdad
L, Dasgupta S, Wang XY, Dent P, Reed JC, Pellecchia M, et al:
Targeting the Bcl-2 family for cancer therapy. Expert Opin Ther
Targets. 17:61–75. 2013. View Article : Google Scholar : PubMed/NCBI
|