Introduction
To reproduce the three-dimensional (3D) organization
and the cell-cell and cell-matrix features that are found in normal
or tumor tissues, cells can be cultured as 3D aggregates, also
called spheroids. Over the last decade, spheroids have been
recognized as essential 3D culture models for high-throughput
screening and pharmacological evaluation (1). They are also of utmost interest in the
field of tissue engineering because they represent basic bricks
that can be used to generate original cell assemblages and
organization, and to produce larger tissues (2).
Spheroids derived from primary cells or immortalized
cancer cell lines are often made using the classical hanging drop
or centrifugation methods (3,4). Although these techniques are robust and
their variability is acceptably low, there are potential
reproducibility issues linked to various classical experimental
problems and batch-to-batch variability. Storage in liquid
nitrogen, in the presence of a cryoprotectant, has been used for
brain cell and hepatocyte spheroids with successful preservation of
morphological markers and functionality (5,6). However,
most researchers rely on repeated custom production, depending on
the need. Alternatively, spheroids can be ordered from companies
that deliver standardized and perfectly controlled biological
material, usually shipped in conditions that maintain the
microtissues at 37°C during transit. In such case, temperature
stabilization and shipping efficiency become critical issues.
Several reports have shown that spheroid growth
results in the generation of a hypoxia and nutrient gradients
(7–9)
where cells localized in the inner region exit the cell cycle and
enter a G0 quiescent state. The induced cell quiescence in the
spheroid central region mimics the situation observed in
vivo in microtumor domains and the subsequent resistance to
classical chemotherapeutic agents (10,11).
Moreover, we recently demonstrated that oxygen partial pressure is
a rate-limiting parameter for cell proliferation in 3D spheroids
(12). Thus, we hypothesized that
culturing spheroids in anoxic conditions could be an efficient way
to induce their reversible growth arrest. In order to make the
procedure as simple and inexpensive as possible, we investigated
whether oxygen absorbers could be used to generate an anoxic
environment for microtissues. Oxygen absorbers can efficiently
reduce oxygen concentration to less than 0.0001%. Consequently,
their composition and packaging has been adapted for a very large
range of applications (13).
Currently, oxygen absorbers are used to preserve many food products
(bread, meat, fish and seafood, fruits, nuts, cheese) from food
spoilage due to aerobic microorganism proliferation and to prevent
fat oxidation. They are also used in the pharmaceutical industry to
improve molecule protection and safety. Oxygen absorbers are also
employed to generate oxygen-free environments to control and
eradicate museum insect pest and to preserve art works (14,15).
Here, we report that in oxygen absorber-induced
anoxia, spheroid growth can be reversibly stopped at 4°C for up to
18 days. Moreover, after anoxic storage, they can be successfully
used in pharmacological assays. This oxygen absorber-based method
to reversibly stop cell proliferation could represent a tremendous
advance in the field of 3D microtissue engineering with obvious
immediate applications for spheroid storage and shipment.
Materials and methods
Cell culture
HCT116 colon adenocarcinoma cells (ATCC) were
cultured in DMEM (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) containing 10% foetal calf serum (FCS), 2 mM/l
glutamine and penicillin/streptomycin in a tissue culture incubator
(humidified atmosphere of 5% CO2 at 37°C). Spheroids
were prepared as previously described(9). Briefly, 500
cells/well were distributed in ultra-low attachment 96-round bottom
well plates. After centrifugation at 200 g for 6 min, plates were
placed in the tissue culture incubator. After 3 days, each well
contained a single spheroid. The spheroid maximal area was
determined by automated measurement with the High Content Screening
ArrayScan Cellomics® platform (Thermo Fischer Scientific
Inc.).
Packaging with oxygen absorbers and
oxygen concentration measurement
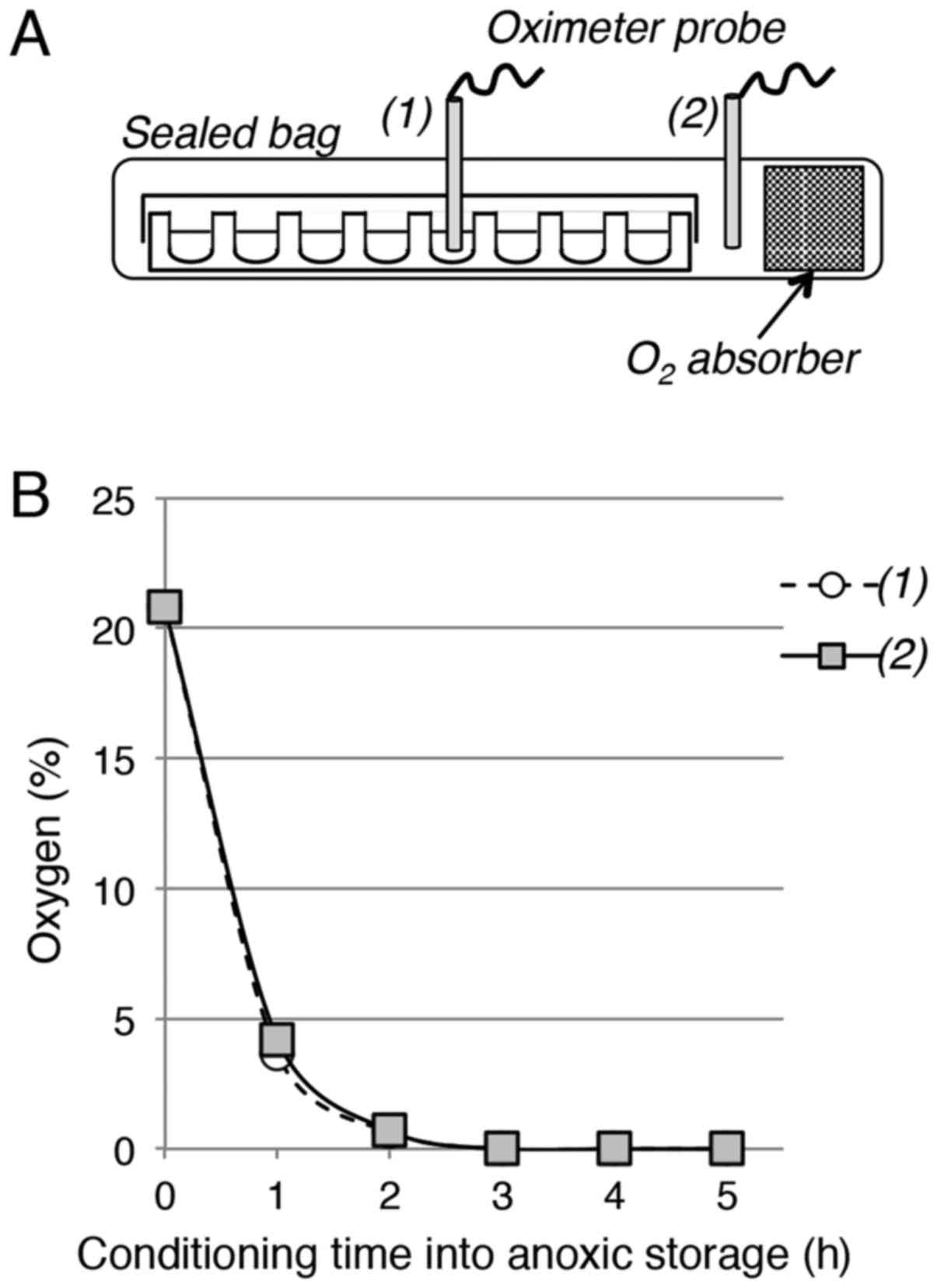
For anoxic storage, each 96-well plate was placed in
an ATCO Biocult P plastic bag with or without (control) an
ATCO® Biosystem 96P oxygen absorber (Fig. 1A). The bag was immediately heat-sealed
and placed in the tissue culture incubator (storage at 37°C) or in
a cold room (storage at 4°C). The ATCO® Biosystem 96P
oxygen absorbers used in this study were specifically designed for
96-well plates. The residual oxygen concentration in the bag was
determined using a Servomex 570A oximeter (accuracy in the range of
± 0.1%; Servomex, Norwood, MA, USA). The oximeter probe was
inserted in the sealed bag in an airtight manner and was positioned
either outside or inside the 96-well plate. The residual oxygen
level was calculated as the mean of at least three measurements at
each time point. At the end of the storage period in anoxic
conditions, bags were opened and plates returned to the tissue
culture incubator.
Pharmacological evaluation
Spheroids were prepared in normoxia and then stored
at 4°C in oxygen absorber-induced hypoxia (0% oxygen) for 4, 7 or
14 days and then transferred to normoxia (21% oxygen at 37°C) for
24 h. This recovery time was chosen in order to use spheroids of
approximately 350–400 µm in diameter to allow comparison with
controls (spheroids of similar size cultured in normoxia). To test
their response to etoposide, 100 µl of culture medium per well was
mixed with 100 µl of culture medium containing etoposide
(Sigma-Aldrich, St. Louis, MO, USA). In each plate, six different
concentrations were obtained by serial dilution (4–6
wells/spheroids for each concentration). Spheroid size was
determined with the High Content Screening ArrayScan
Cellomics® platform after 72 h-incubation with
etoposide. The half maximal inhibitory concentration
(IC50) was calculated using the Prism®
software.
Results and Discussion
Use of an oxygen absorber to generate
an anoxic environment for 96-well plates
Multi-well plates are the most commonly used cell
culture disposable material to produce and transfer 3D spheroids.
To analyse whether oxygen absorbers could be used to generate a
hypoxic environment for spheroids cultured in 96-well plates, the
oxygen concentration was measured with an oximeter needle probe
positioned either on one side or in the middle of the 96-well plate
packaged with an oxygen absorber in a heat-sealed plastic bag
(Fig. 1A). The oxygen concentration
measured inside the bag and in the plate rapidly decreased from
20.8% to ~4% after 1 h, to 0.7% after 2 h and nearly to 0% after 3
h (Fig. 1B). This indicates that this
very simple experimental setting allows the fast and efficient
removal of oxygen to generate an anoxic environment compatible with
cell culture disposable material, such as 96-well plates.
Oxygen absorber-induced anoxia
reversibly arrests spheroid growth at 37°C
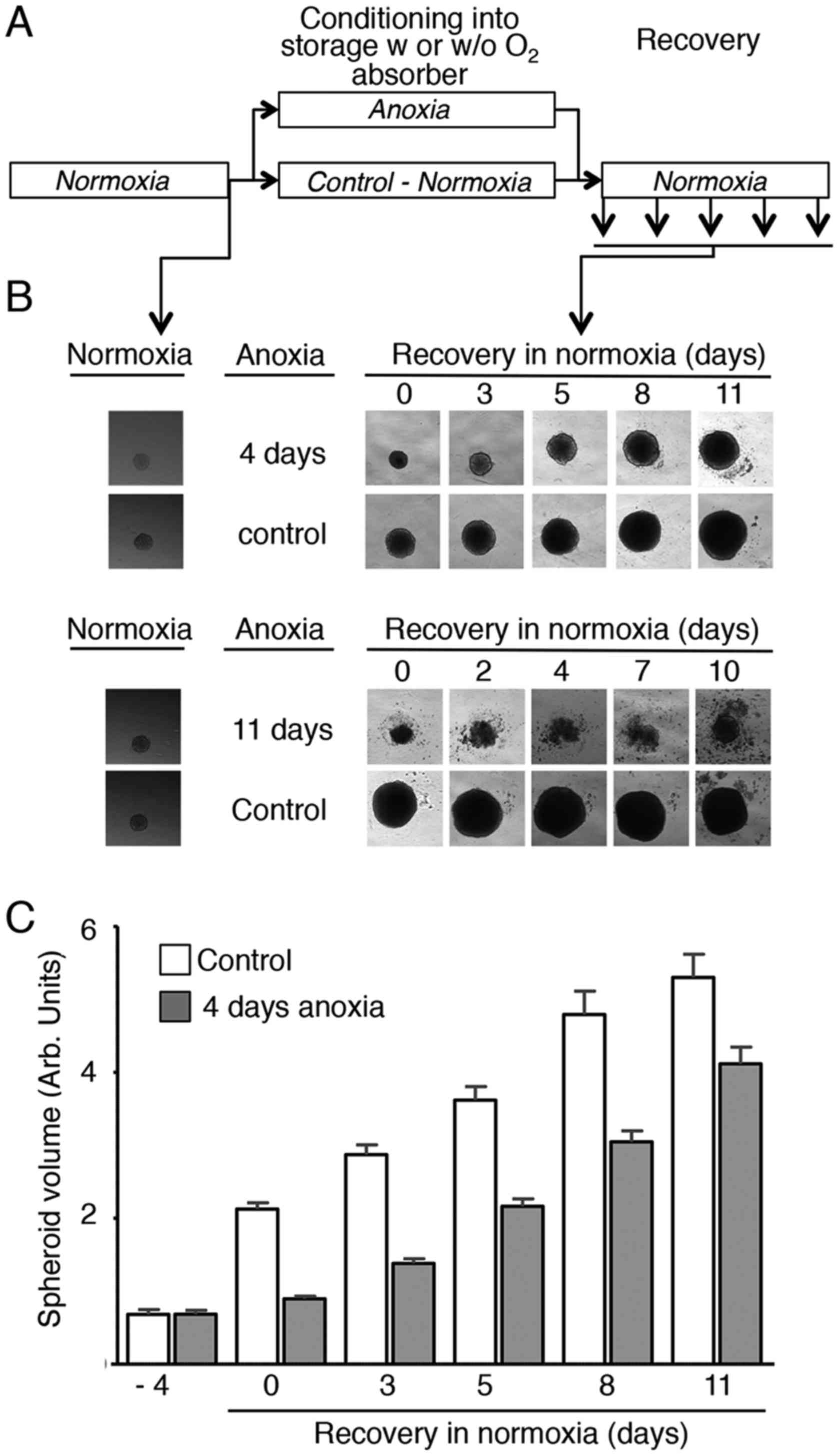
The effect of absorber-induced anoxia on spheroid
proliferation was then analysed with the experimental setting
depicted in Fig. 2A that was used for
all the experiments of this study. After 3 days in normoxia at
37°C, each 96-well plate that contained one HCT116 colon
adenocarcinoma spheroid per well was placed, with or without
(control) one ATCO Biosystem 96P oxygen absorber, in a plastic bag
that was heat-sealed and put back in the tissue culture incubator
for 4 or 11 days. Visual inspection under an inverted microscope of
spheroids after return to normoxic conditions (Fig. 2B, upper and lower panels,
respectively) showed that control spheroids (no oxygen absorber)
kept growing during storage and after removal from the bag.
Conversely, growth of spheroids packed with the oxygen absorber was
completely inhibited by anoxia. Upon return to normoxia, spheroid
growth resumption was observed only after anoxic storage for 4
days, but not for 11 days (Fig. 2B).
Indeed, spheroids kept in anoxic conditions for 11 days, rapidly
dissociated and did not resume growth upon return to normoxia
(Fig. 2B, lower panels).
Quantification of the spheroid volume (60 spheroids/condition for
each time point) confirmed that growth of spheroids stored at 37°C
in anoxia for 4 days was totally inhibited during the storage
period, but proliferation resumed once back to normoxia, with a
slope similar to controls (Fig.
2C).
Together, these observations indicate that when
placed at 37°C in an anoxic environment obtained with an ATCO
Biosystem 96P oxygen absorber, spheroid proliferation is arrested.
Back to normoxia, proliferation resumes in the same way as for
control spheroids.
Spheroid growth is reversibly arrested
by storage in oxygen absorber-induced anoxia at 4°C up to 18
days
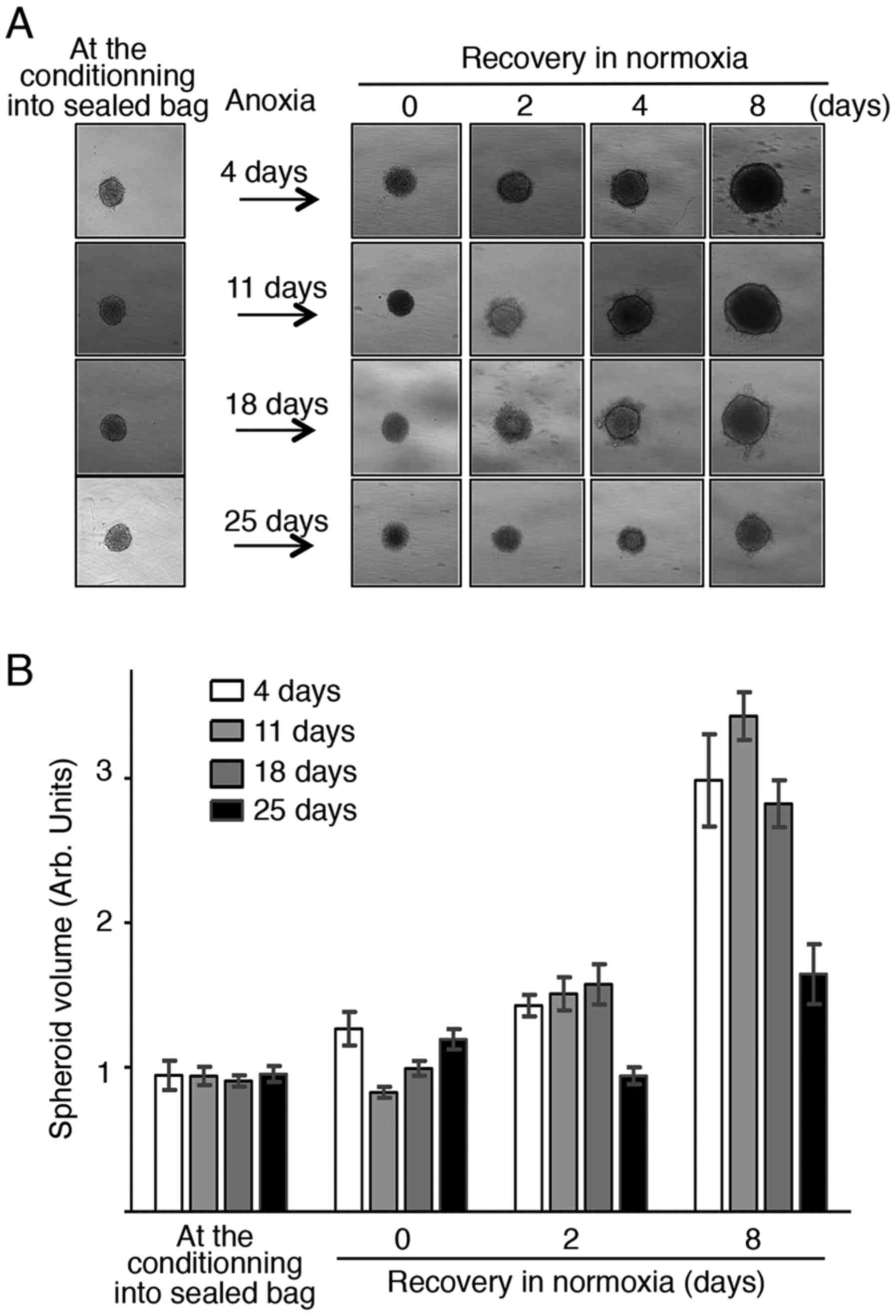
Although it may be of interest to slow down or stop
microtissue proliferation at 37°C for a short period, the storage
and transport of such biological material would be more easily
performed in standardized refrigerated conditions. Therefore, the
ability of spheroids to resume proliferation after storage in
oxygen absorber-induced anoxia at 4°C was assessed using the same
experimental set-up (Fig. 2A). Plates
were kept in anoxia, or not (controls), at 4°C for 4, 11, 18 and 25
days, before returning to normoxia at 37°C for recovery. Similarly
to the results obtained at 37°C, oxygen absorber-induced anoxia led
to growth arrest without loss of structure integrity or structure
changes (Fig. 3A). Growth was resumed
after return to normoxia, although it was less fast in spheroids
stored in anoxia at 4°C for 18 and 25 days compared with up to 11
days. On the other hand, spheroids stored at 4°C without oxygen
absorber could not resume growth after return to normal culture
conditions (data not shown). Quantification of the spheroid volume
(50–60 spheroids/condition per time point) at different time points
after return to normoxia (Fig. 3B)
confirmed growth resumption after storage at 4°C for up to 18
days.
These results indicate that storage of spheroids at
4°C in an anoxic environment obtained with an ATCO Biosystem 96P
oxygen absorber leads to fully reversible growth arrest (up to 18
days of anoxia), which is technically very easy to achieve.
Spheroid storage in oxygen
absorber-induced anoxia does not modify the response to
etoposide
To definitively confirm that oxygen absorber-induced
anoxia may represent a major advance for spheroid storage, spheroid
preservation was evaluated by assessing their response to
etoposide, a DNA polymerase inhibitor currently used in the clinic
for cancer treatment. To this aim, spheroids stored with the oxygen
absorber at 4°C for 4, 7 or 14 days were allowed to recover in
normoxia for 24 h before incubation with increasing concentrations
of etoposide for 72 h. Growth inhibition caused by etoposide
cytotoxic effect was comparable in stored spheroids and in controls
(spheroids of similar diameter at the time of treatment grown in
normoxia), with similar IC50 values (Table I). Thus, storage of spheroids in
anoxia at 4°C for 4, 7 or 14 days does not modify their response to
a reference genotoxic agent.
 | Table I.Determination of the IC50
for etoposide in spheroids stored in anoxic conditions. |
Table I.
Determination of the IC50
for etoposide in spheroids stored in anoxic conditions.
|
| IC50
(µM) |
|---|
|
|
|
|---|
| Anoxic storage
duration | Control | Anoxia at 4°C |
|---|
| 4
days | 1.4 | 2.3 |
| 7
days | 2.1 | 2.4 |
| 14 days | 1.6 | 1.7 |
In this study, we investigate whether anoxia
generated by using ATCO Biosystem 96 P oxygen absorbers represents
a valid method for the storage and shipment of 3D tumor spheroids.
We found that by simply packing a 96-well plate in a heat-sealed
plastic bag that contains an oxygen absorber, total anoxia can be
generated in about 2 h. Moreover, we show that in conditions of
total anoxia, spheroid growth is fully stopped and that
proliferation can be resumed after up to 4 days of storage in
anoxia at 37°C.
Mammalian cells are in principle unable to survive
in hypoxic conditions because the unbalance between the decreasing
ATP supply and the demand to ensure homeostasis progressively leads
to mitochondria dysfunction and cell death. However, adaptive
molecular responses allow a hypometabolic response that transiently
prevents cell death (16). Recent
work has shown that in acidic conditions, hypoxia can promote tumor
cell survival by preserving the ATP level (17). Furthermore, a study on pancreatic
islet conservation prior to transplantation demonstrated that
storage at low temperatures prevent cell damage associated with
hypoxia and may improve transplantation efficiency (18). In line with these reports, here we
found that lowering the temperature to 4°C offers the possibility
to fully resume spheroid growth after up to 18 days of anoxia.
Although further work is needed to validate this storage method in
other cancer cell lines our results already open a real and major
opportunity for spheroid storage, functional preservation and
shipment.
Acknowledgements
The support of the TRI-Genotoul and ITAV imaging
facility is gratefully acknowledged. The authors would like to
thank Elisabetta Andermarcher for expert manuscript editing. The
work in the laboratory of B.D. and V.L. is supported by the
University of Toulouse, the CNRS, Agence Nationale de la Recherche
ANR and la Ligue Contre le Cancer.
References
|
1
|
Hirschhaeuser F, Menne H, Dittfeld C, West
J, Mueller-Klieser W and Kunz-Schughart LA: Multicellular tumor
spheroids: An underestimated tool is catching up again. J
Biotechnol. 148:3–15. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fennema E, Rivron N, Rouwkema J, van
Blitterswijk C and de Boer J: Spheroid culture as a tool for
creating 3D complex tissues. Trends Biotechnol. 31:108–115. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ivascu A and Kubbies M: Rapid generation
of single-tumor spheroids for high-throughput cell function and
toxicity analysis. J Biomol Screen. 11:922–932. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Del Duca D, Werbowetski T and Del Maestro
RF: Spheroid preparation from hanging drops: Characterization of a
model of brain tumor invasion. J Neurooncol. 67:295–303. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Purcell WM, Atterwill CK and Xu J:
Cryopreservation of organotypic brain spheroid cultures. Altern Lab
Anim. 31:563–573. 2003.PubMed/NCBI
|
|
6
|
Magalhaes R, Wang XW, Gouk SS, Lee KH, Ten
CM, Yu H and Kuleshova LL: Vitrification successfully preserves
hepatocyte spheroids. Cell Transplant. 17:813–828. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sutherland RM, Sordat B, Bamat J, Gabbert
H, Bourrat B and Mueller-Klieser W: Oxygenation and differentiation
in multicellular spheroids of human colon carcinoma. Cancer Res.
46:5320–5329. 1986.PubMed/NCBI
|
|
8
|
Kunz-Schughart LA, Freyer JP, Hofstaedter
F and Ebner R: The use of 3-D cultures for high-throughput
screening: The multicellular spheroid model. J Biomol Screen.
9:273–285. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Laurent J, Frongia C, Cazales M, Mondesert
O, Ducommun B and Lobjois V: Multicellular tumor spheroid models to
explore cell cycle checkpoints in 3D. BMC Cancer. 13:732013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sutherland RM: Cell and environment
interactions in tumor microregions: The multicell spheroid model.
Science. 240:177–184. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Karlsson H, Fryknäs M, Larsson R and
Nygren P: Loss of cancer drug activity in colon cancer HCT-116
cells during spheroid formation in a new 3-D spheroid cell culture
system. Exp Cell Res. 318:1577–1585. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gomes A, Guillaume L, Grimes DR,
Fehrenbach J, Lobjois V and Ducommun B: Oxygen partial pressure is
a rate-limiting parameter for cell proliferation in 3D spheroids
grown in physioxic culture condition. PLoS One. 11:e01612392016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Brody A, Strupinsky E and LR K: Active
packaging for food applications. CRC Press; 2001, View Article : Google Scholar
|
|
14
|
Standa Industrie, . Application de
l'absorbeur d'oxygène ATCO dans les musées, bibliothèques et
archives. La lettre de l'OCIM. 60:29–32. 1998.(In French).
|
|
15
|
Maekawa S and Elert K: The use of
oxygen-free environments in the control of museum insect pests. The
Gerry Conservation Institute; Los Angeles: 2003
|
|
16
|
Boutilier RG: Mechanisms of cell survival
in hypoxia and hypothermia. J Exp Biol. 204:3171–3181.
2001.PubMed/NCBI
|
|
17
|
Parks SK, Mazure NM, Counillon L and
Pouysségur J: Hypoxia promotes tumor cell survival in acidic
conditions by preserving ATP levels. J Cell Physiol. 228:1854–1862.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Itoh T, Sugimoto K, Takita M, Shimoda M,
Chujo D, SoRelle JA, Naziruddin B, Levy MF and Matsumoto S: Low
temperature condition prevents hypoxia-induced islet cell damage
and HMGB1 release in a mouse model. Cell Transplant. 21:1361–1370.
2012. View Article : Google Scholar : PubMed/NCBI
|