Introduction
Pancreatic cancer is a result of malignant tumor
growth in the digestive system (1).
Relative to other types of cancer, pancreatic cancer involves late
identification of lesions, a high degree of malignance, fast
progression and poorer prognosis (1).
Previous research has demonstrated that pancreatic cancer primarily
presents in the head of pancreas (2).
The morbidity of patients with pancreatic ductal adenocarcinoma is
the highest amongst pancreatic cancer and accounts for 95% of
pancreatic cancer-associated mortality (2). The 5-year survival rate of pancreatic
cancer is <5% (3), and patients
with pancreatic cancer are expected to survive for ~4–6 months
after diagnosis prior to succumbing to the disease (4). Patients who undergo surgery are expected
to live for ~13–20 months before succumbing to pancreatic cancer
(2). The annual morbidity rate of
patients with pancreatic cancer is ~9/10,000, with the morbidity
rate in China being ~5/10,000 (3).
Annually, ~40,000 people succumb to pancreatic cancer (3). Previous research has revealed that the
morbidity rate of pancreatic cancer in China is increasing
(4).
Inhibition of apoptosis serves a role in the
progression of pancreatic cancer. Proteins in the B-cell lymphoma 2
(Bcl-2) family serve an important role in the regulation of
apoptosis (5). The Bcl-2 family
includes the apoptotic inhibitors Bcl-2, B-cell lymphoma
extra-large (Bcl-xL), Bcl-2-like protein 2 (Bcl-w), Bcl-2-related
protein A1 (Bfl-1), brefeldin A-resistant Arf-guanine nucleotide
exchange factor 1 (Brag-1) and induced myeloid leukemia cell
differentiation protein (Mcl-1), and the apoptotic regulators
Bcl-2-associated X protein (Bax), Bcl-2 homologous
antagonist/killer (Bak), B-cell lymphoma extra-small (Bcl-xS),
Bcl-2-associated death promoter (Bad), BH3 interacting-domain death
agonist (Bid), Bcl-2-interacting killer (Bik) and activator of
apoptosis hara-kiri (Hrk) (6). The
ratio of apoptotic inhibitors to apoptotic inducers determines the
response of cells to apoptosis-triggering signals. To some extent,
induction and inhibition of apoptosis may be regulated by
controlling the expression of various Bcl-2 family members
(7). p53 is the primary regulator of
the Bcl-2 family. The biological behavior of pancreatic cancer
tumor cells is thus affected by the regulatory properties exhibited
by p53 over the various inhibitors and inducers of apoptosis within
the Bcl-2 family (8).
Poly(ADP-ribose) polymerase (PARP) requires an
oxidized nicotinamide-adenine dinucleotide substrate, utilizing
adenosine triphosphate in order to form a poly(ADP-ribose) chain
(PAR chain) at the site of single strand breaks in DNA (9). The formation of a PAR chain signals
other DNA damage repair proteins to the site of the break. PARP has
exhibited involvement in a number of metabolic processes in cells
including genome stabilization, cell cycle progression, gene
transcription, chromosome function and cell death. The PARP family
has a number of distinct members including; PARP-1, PARP-2, PARP-3,
Vault-PARP and tankyrases. PARP-1 was the first member of the PARP
family to be identified, with a molecular mass of 116Kd (9). It comprises three regions: A DNA-binding
domain, an automodification domain and a catalytic domain (10). The primary structure of PARP-1 is
highly conserved in eukaryotes; distinct catalytic regions exhibit
a high degree of homology (10).
Increased expression levels of PARP-1 have been identified in
multiple types of tumor including breast, prostate and pancreatic
cancer, indicating that PARP-1 participates in tumorigenesis and
tumor progression (11). In order to
develop effective novel therapeutic approaches to treat pancreatic
cancer, the underlying molecular mechanisms of the involvement of
PARP-1 in tumor progression require investigation.
Materials and methods
Cell lines and cell culture
Human pancreatic cancer cells (PanC-1) were provided
by the Institute of Biochemistry and Cell Biology (Chinese Academy
of Science Shanghai, China). PanC-1 cells were cultured in
Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal
bovine serum (FBS; Gibco, Thermo Fisher Scientific, Inc.),
penicillin (50 U/ml) and streptomycin (50 µg/ml) at 37°C in a
humidified atmosphere containing 5% CO2.
Cell proliferation assay
PanC-1 cells (1×103 in a 96-well plate)
were cultured in the presence of dimethylsulfoxide (DMSO; Amresco,
LLC, Solon, OH, USA; untreated) or olaparib (1, 5 and 25 µM;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) for 1, 2, 3 and 4
days at 37°C. A second set of experiments was performed, with
PanC-1 cells (1×103 in a 96-well plate) cultured in the
presence of DMSO (untreated), olaparib (5 µM) or olaparib (5 µM) +
KU55933 (5 µM) for 4 days at 37°C. Subsequently, 20 µl/well MTT
solution (5 mg/ml; Amresco, LLC) was added to each well prior to
further incubation at 37°C for 4 h. Subsequently, DMSO solution was
added to each well for solubilization of the formazan product at
37°C for 30 min with gentle mixing. Absorbance was determined at
560 nm using an M200 plate reader (Tecan Group, Ltd., Mannedorf,
Switzerland).
Analysis of apoptosis using flow
cytometry (FCM)
PanC-1 cells (1×106 in a 6-well plate)
were cultured in the presence of DMSO (untreated) or olaparib (1, 5
and 25 µM) for 4 days at 37°C. A second set of experiments was
performed, with PanC-1 cells (1×106 in a 6-well plate)
cultured in the presence of DMSO (untreated), olaparib (5 µM) or
olaparib (5 µM) + KU55933 (5 µM) for 4 days at 37°C. Subsequently
PanC-1 cells were washed twice with PBS and resuspended in 500 µl
binding buffer (BD Biosciences, Franklin Lakes, NJ, USA) and 5 µl
annexin V-fluorescein isothiocyanate (FITC) (BD Biosciences) and
subsequently in 5 µl propidium iodide (PI) (BD Biosciences) in the
dark. The rate of apoptosis was analyzed using FCM (BD
Biosciences).
Caspase-3 activity determination using
a caspase-3 activity kit
The caspase-3 activity kit it was obtained from
Beyotime Institute of Biotechnology (Haimen, China; C1115). PanC-1
cells (1×106 in a 6-well plate) were cultured in the
presence of DMSO (untreated) or olaparib (1, 5 and 25 µM) for 4
days at 37°C. A second set of experiments was performed, with
PanC-1 cells (1×106 in a 6-well plate) cultured in the
presence of DMSO (untreated), olaparib (5 µM) or olaparib (5 µM) +
KU55933 (5 µM) for 4 days at 37°C. Subsequently PanC-1 cells were
washed twice with PBS and incubated with
N-acetyl-Asp-Glu-Val-Asp-p-nitroanilide (C1115; Beyotime Institute
of Biotechnology) at 37°C for 30 min. Absorbance was determined at
405 nm using an M200 plate reader.
Western blot analysis
PanC-1 cells (1×106 in 6-well plate) were
cultured in the presence of DMSO (untreated) or olaparib (1, 5 and
25 µM) for 1, 2, 3 and 4 days at 37°C. A second set of experiments
was performed, with PanC-1 cells (1×106 in a 6-well
plate) cultured in the presence of DMSO (untreated), olaparib (5
µM) or olaparib (5 µM) + KU55933 (5 µM) for 4 days at 37°C.
Subsequently, PanC-1 cells were lysed in radioimmunoprecipitation
assay lysis buffer (Beyotime Institute of Biotechnology) with
phenylmethanesulfonyl fluoride protease and phosphatase inhibitor
(Beyotime Institute of Biotechnology). Total cell protein was
extracted and subsequently quantified using a BCA protein
quantitative assay kit (Beyotime Institute of Biotechnology). An
equivalent amount of protein (50 µg) was resolved using SDS-PAGE
(8–12% gel) and transferred on to a nitrocellulose membrane (Merck
KGaA). Membranes were blocked with skimmed milk (5%) in TBST (with
0.1% Tween-20) for 1 h at 37°C and then incubated with
anti-pro-caspase-3 (sc-98785; 1:500; Santa Cruz Biotechnology,
Inc., Dallas, TX, USA), anti-Bcl-2 (sc-783; 1:300; Santa Cruz
Biotechnology, Inc.), anti-p53 (sc-6243; 1:500; Santa Cruz
Biotechnology, Inc.) and anti-GAPDH (sc-25778, 1:500; Santa Cruz
Biotechnology, Inc.) at 4°C overnight. Horseradish
peroxidase-labeled goat anti-rabbit secondary antibodies (sc-2004;
1:5,000, Sangon Biotech Co., Ltd., Shanghai, China) were added for
1 h at 37°C and detected using enhanced chemiluminescence reagent
(Bio-Rad Laboratories, Inc., Hercules, CA, USA). The protein
expression was measured using ImageJ 1.37 software (National
Institutes of Health, Bethesda, MD, USA).
Statistical analysis
All data are presented as the mean ± standard
deviation. Analysis of variance was used for multiple comparisons
and Student's t-test was used to compare between pairs. P<0.05
was considered to indicate a statistically significant
difference.
Results
Down-expression of PARP-1 suppresses
the proliferation of PanC-1 cells
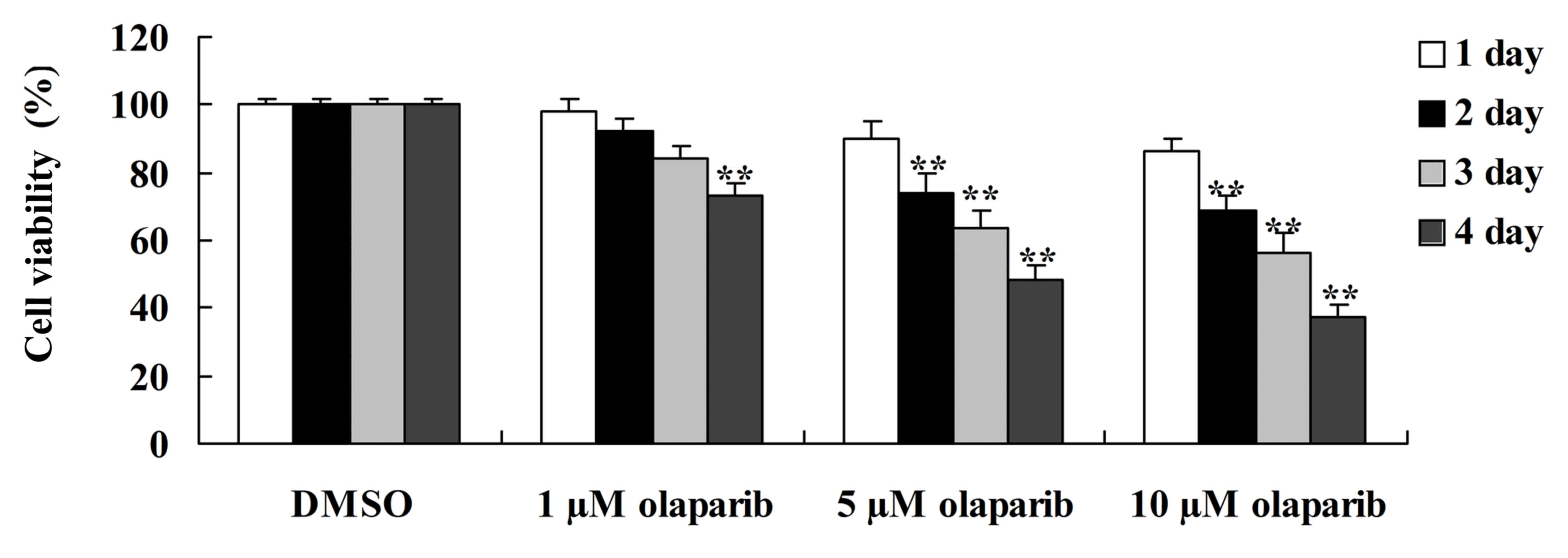
To investigate whether the down-expression of PARP-1
has an effect on human pancreatic cancer, an MTT assay was used to
measure the proliferation of PanC-1 cells following treatment with
olaparib. It was demonstrated that olaparib, a PARP-1 inhibitor,
significantly suppressed the proliferation of PanC-1 cells in a
time- and dose-dependent manner when compared with the DMSO control
group (Fig. 1).
Down-expression of PARP-1 induces
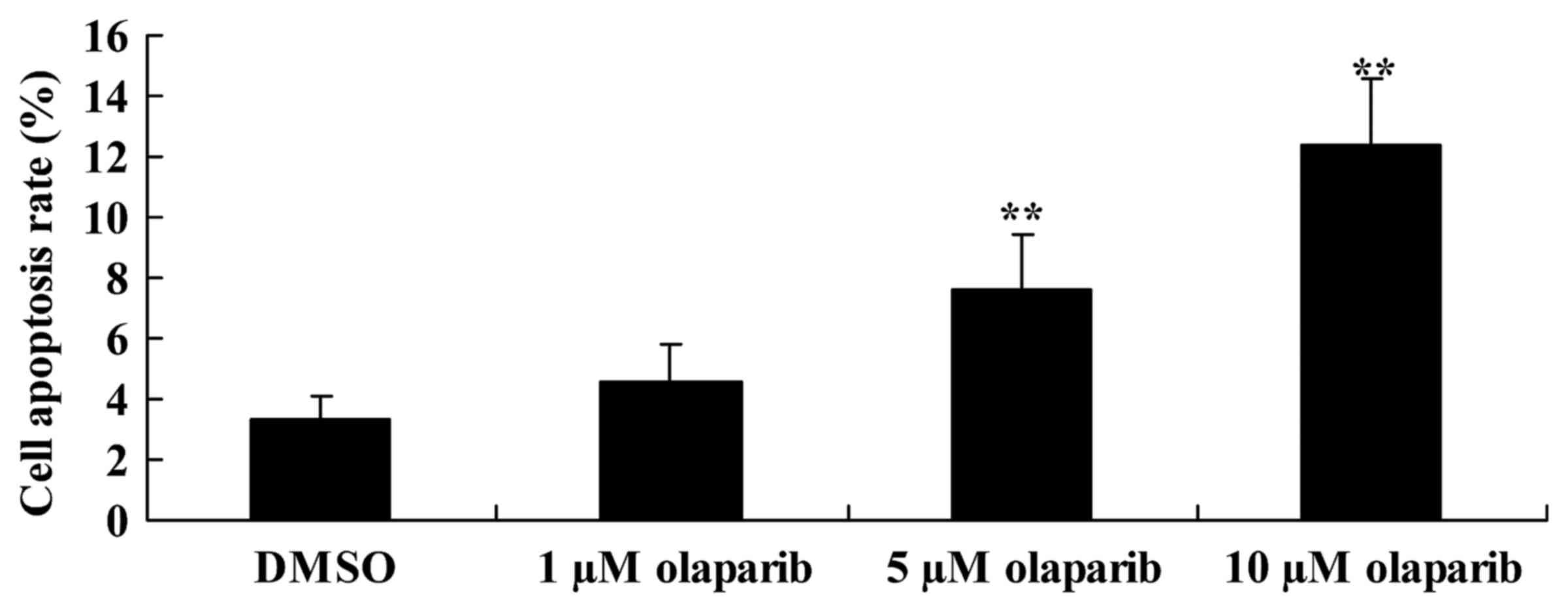
apoptosis of PanC-1 cells
To investigate whether the down-expression of PARP-1
induces apoptosis of PanC-1 cells following olaparib treatment, FCM
was performed. Cells treated with olaparib (5 and 10 µM) exhibited
a significant increase in the rate of apoptosis in a dose-dependent
manner when compared with the DMSO control group (Fig. 2).
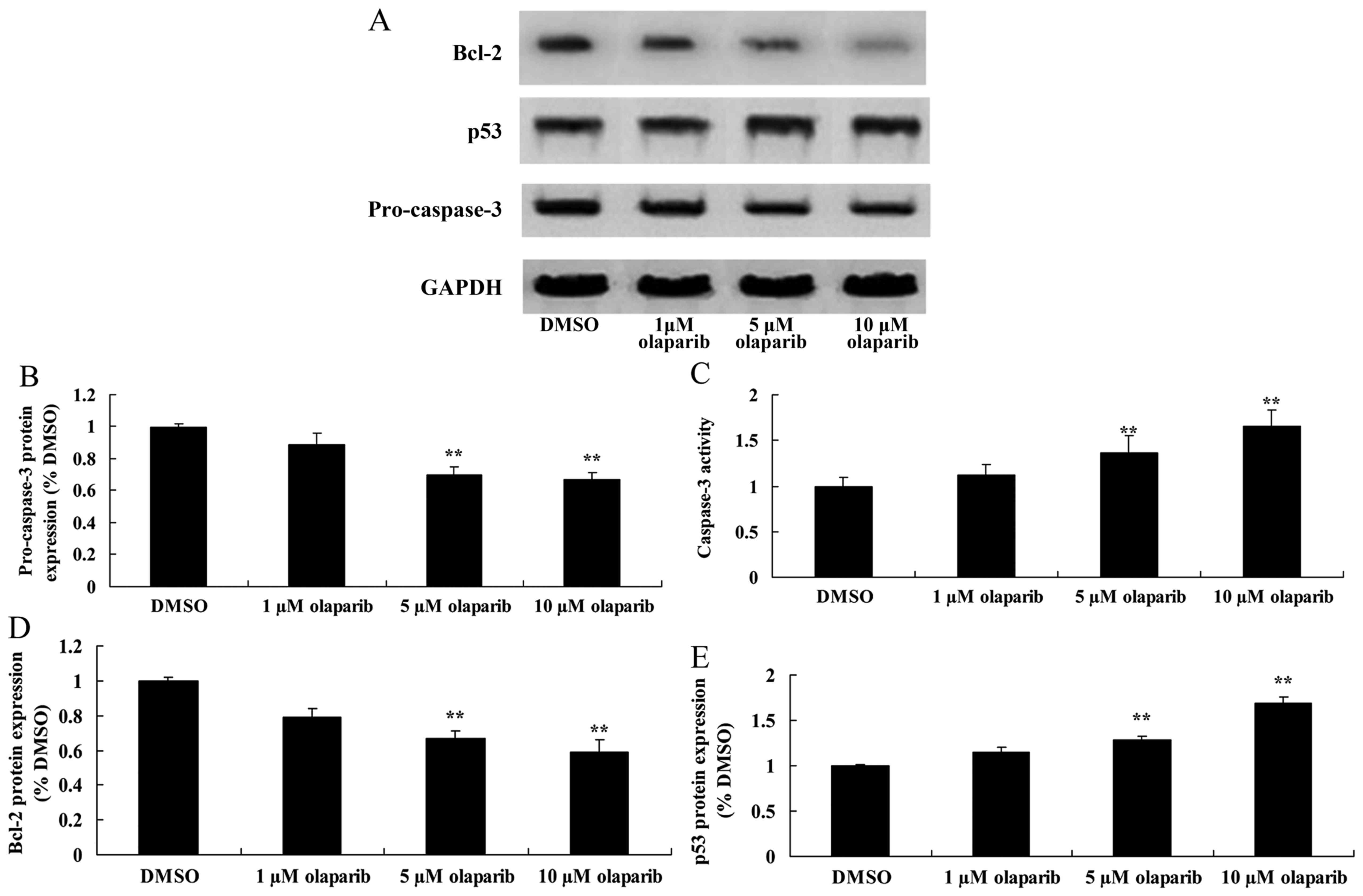
Down-expression of PARP-1 decreases
pro-caspase-3 expression levels and increases caspase-3 activity in
PanC-1 cells
Pro-caspase-3 was analyzed using western blotting
and a caspase-3 activity kit. Pro-caspase-3 expression levels were
significantly suppressed following down-expression of PARP-1,
compared with the DMSO control group. However, caspase-3 activity
was significantly increased following down-expression of PARP-1
when compared with the DMSO control group (Fig. 3).
Down-expression of PARP-1 suppresses
Bcl-2 protein expression of PanC-1 cells
To quantify cell death, Bcl-2 protein expression
levels in PanC-1 cells were measured using western blot analysis.
The results demonstrated that olaparib significantly inhibited the
expression of Bcl-2 protein in a dose-dependent manner when
compared with the DMSO control group (Fig. 3).
Down-expression of PARP-1 induces p53
protein expression in PanC-1 cells
To further investigate the effect of down-expression
of PARP-1 on cell death, the expression levels of p53 protein in
PanC-1 cells was measured using western blot analysis. It was
identified that increasing concentrations of olaparib resulted in
increased expression levels of p53 protein (Fig. 3).
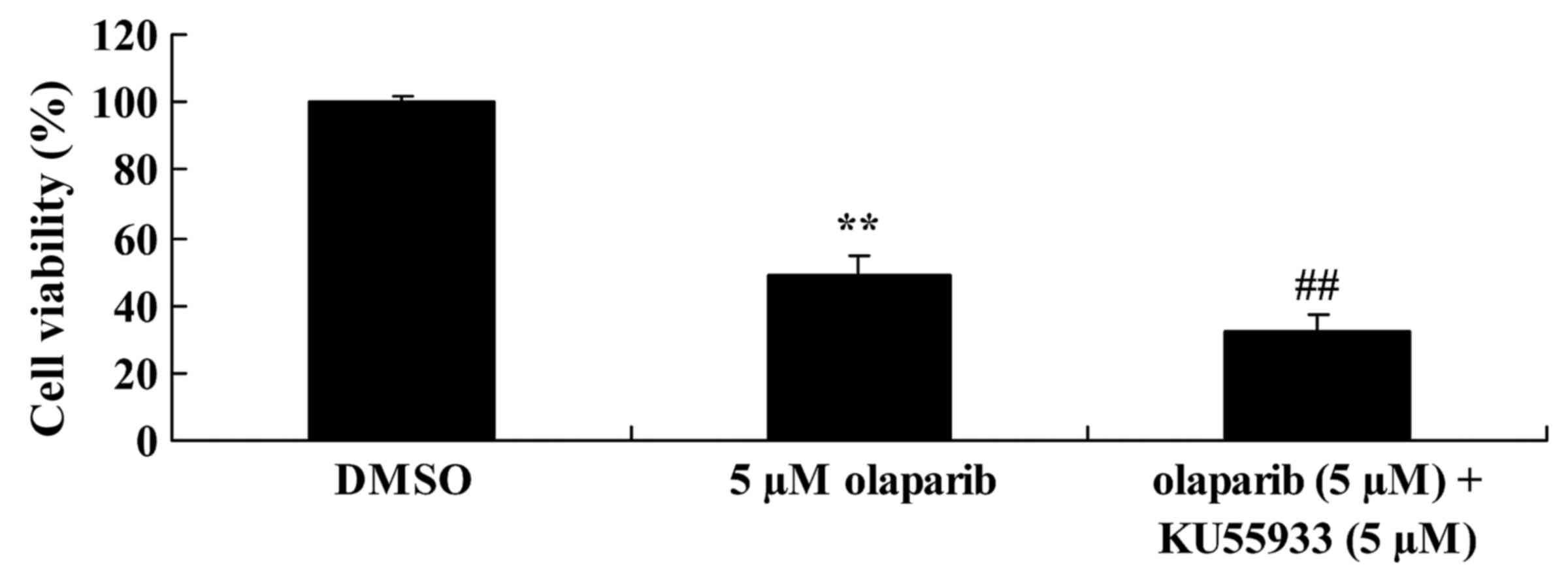
Inhibition of ataxia telangiectasia
mutated (ATM) activity alongside down-expression of PARP-1 further
suppresses the proliferation of PanC-1 cells
Functional ATM caused anchorage-independent
proliferation in pancreatic cancer. KU55933 (5 µM) was used to
inhibit ATM activity. As presented in Fig. 4, following treatment with KU55933 (5
µM) and olaparib (5 µM) cell proliferation was decreased when
compared with the olaparib (5 µM)-treated group.
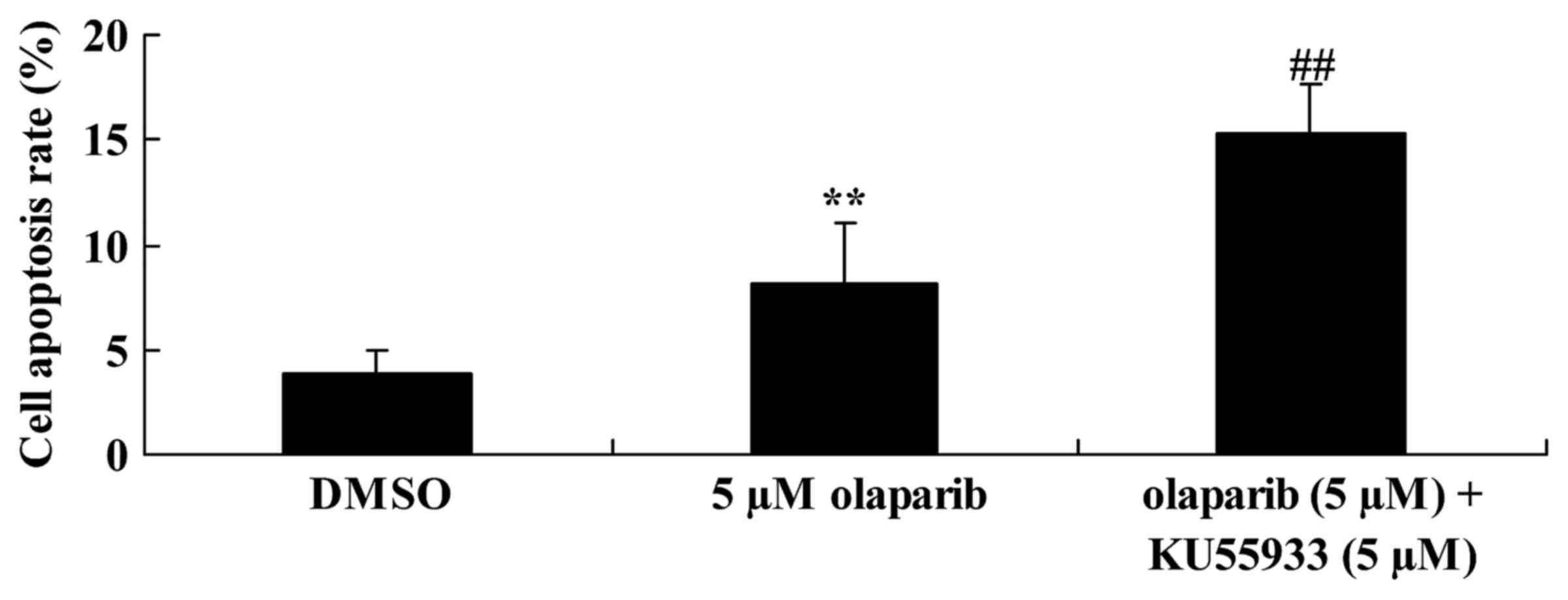
Inhibition of ATM activity alongside
down-expression of PARP-1 further increases the rate of apoptosis
of PanC-1 cells
To investigate ATM activity in PanC-1 cells
following olaparib treatment, the rate of apoptosis of PanC-1 cells
was analyzed using FCM. It was identified that the down-expression
of PARP-1 alongside inhibition of ATM activity (inhibited using
KU55933; 5 µM) significantly increased the rate of apoptosis of
PanC-1 cells when compared with the olaparib (5 µM)-treated group
(Fig. 5).
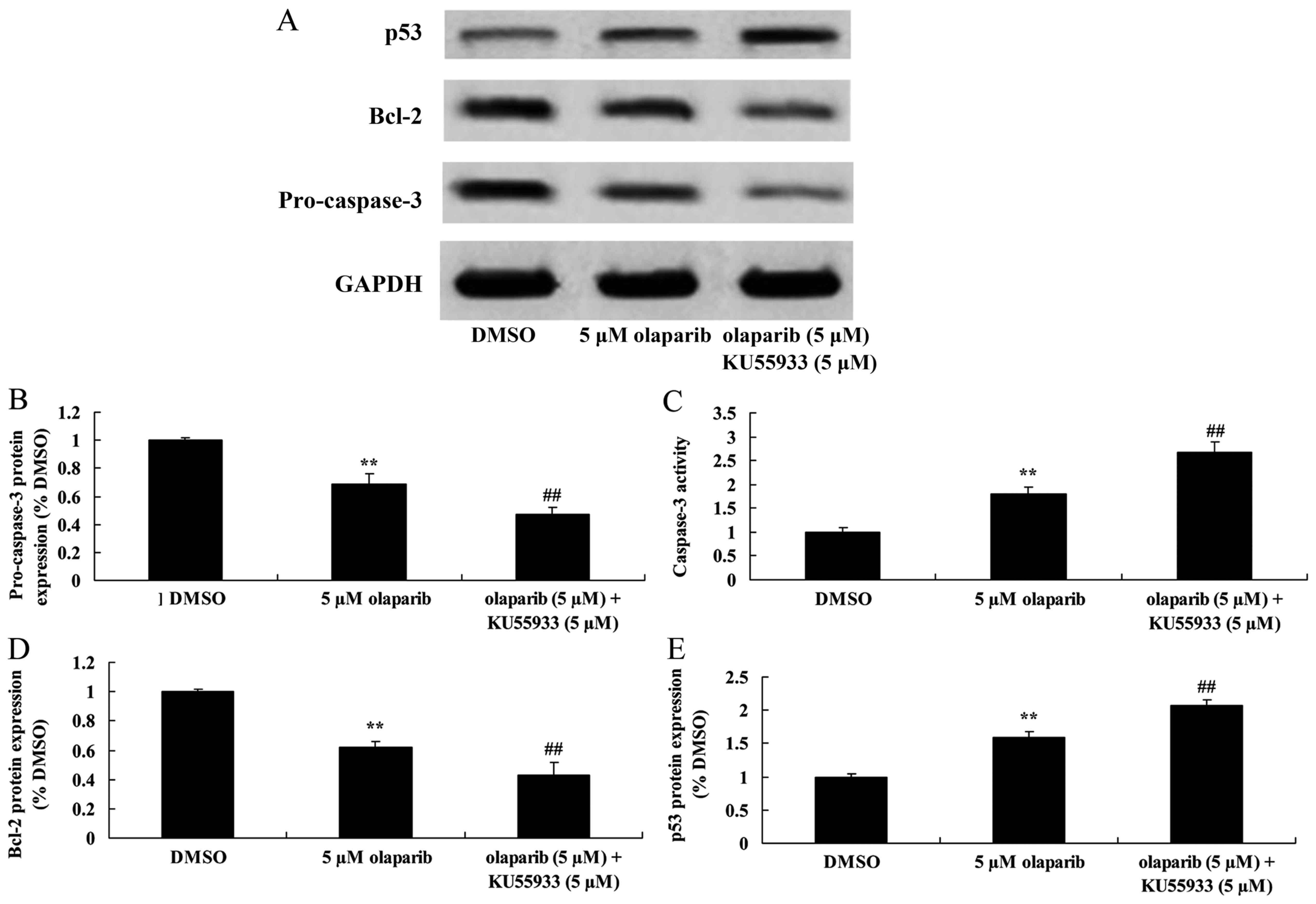
Inhibition of ATM activity in
conjunction with down-expression of PARP-1 decreases pro-caspase-3
expression levels and increases caspase-3 activity in PanC-1
cells
Following treatment with KU55933 (5 µM) and olaparib
(5 µM), pro-caspase-3 expression was significantly decreased and
caspase-3 activity was significantly increased when compared with
the olaparib (5 µM)-treated group (Fig.
6).
Inhibition of ATM activity alongside
down-expression of PARP-1 suppresses Bcl-2 protein expression in
PanC-1 cells
The expression levels of Bcl-2 protein in PanC-1
cells were investigated using western blot analysis following
inhibition of ATM activity using KU55933 (5 µM). It was identified
that down-expression of PARP-1 in conjunction with ATM inhibition
significantly decreased Bcl-2 expression levels when compared with
the olaparib (5 µM)-treated group (Fig.
6).
Inhibition of ATM activity in
conjunction with down-expression of PARP-1 increases p53 protein
expression levels of PanC-1 cells
Expression levels of p53 were measured using western
blot analysis. Treatment with olaparib (5 µM) and KU55933 (5 µM)
resulted in significantly increased p53 expression levels when
compared with the olaparib (5 µM)-treated group (Fig. 6).
Discussion
Pancreatic cancer presents with a malignant tumor of
the digestive tract and is a serious threat to human health. Owing
to a lack of typical clinical symptoms and the need for sensitive
diagnostic tests, by the time the majority of patients receive a
diagnosis, their symptoms are too severe to be treated with
surgical intervention. The 5-year survival rate of pancreatic
cancer is <5% (2). An increased
understanding of the etiology of pancreatic cancer may result in
the development of improved diagnostic tests. It is hypothesized
that pancreatic cancer is the result of multiple factors and genes.
Inactivation of p53 and p16, abnormal expression of the apoptosis
inhibitor gene Bcl-2, inactivation of DNA damage repair genes,
overexpression of growth factors and receptors, and an increase in
telomerase activity are all hypothesized to serve a function in the
development of pancreatic cancer (2).
PARP-1 is present in the majority of eukaryotic cells and serves an
important role in DNA damage repair, genetic transcription, cell
cycle progression, genome stabilization and cell death. In the
present study, it was identified that PARP-1 inhibition
significantly suppressed cell proliferation, suppressed
pro-caspase-3 protein expression and activated caspase-3 activity
in PanC-1 cells.
Apoptosis is a type of programmed cell death
regulated by genes in an active and orderly manner. Effective
apoptosis is achieved via p53 regulation (8). Apoptosis regulation via p53 may be
realized by upregulating Bax and downregulating Bcl-2 or Bcl-xL
(12). Through their interaction, the
permeability of cell mitochondria is regulated, affecting the
function of downstream apoptosis genes. It has been demonstrated
that Bcl-2 in pancreatic cancer tissue with increased expression of
p53 is overexpressed, whereas pancreatic cancer tissue with
decreased expression of p53 exhibits decreased expression levels of
Bcl-2, indicating that Bcl-2 expression may be at least partially
dependent on p53 expression levels (13,14). In
the present study, it was identified that PARP-1 inhibition
significantly increased p53 expression levels in PanC-1 cells.
Deben et al (15) reported
that PARP inhibition induces apoptosis in non-small cell lung
cancer cell lines via the p53 signaling pathway.
Bcl-2 family members exhibit the ability to form
dimers or polymers by themselves or with other family members. For
example, a Bax-Bax dimer may induce apoptosis, whereas a Bcl-2-Bax
dimer is more stable when compared with the former (16). As a consequence of increased Bcl-2
expression, the proportion of Bcl-2-Bax dimers will be increased
relative to Bax-Bax dimers, resulting in a decrease in
pro-apoptotic Bax-Bax activity (17).
In the presence of Bcl-xS, formation of Bcl-xS-Bcl-2 dimer results
in a decrease in Bcl-2-Bax dimer levels, which subsequently
increases Bax-Bax levels, so as to induce apoptosis (18). Therefore, the interaction between
Bcl-2 family proteins regulates survival and apoptosis of cells.
The results of the present study demonstrated that PARP inhibition
significantly inhibited the expression of Bcl-2 protein in PanC-1
cells. Hao et al (19)
provided evidence that Tubeimoside-1 inhibits lung cancer cell
proliferation and induces cell apoptosis through activation of PARP
and the Bcl-2/Bax signaling pathway.
The mitochondrial apoptosis pathway is triggered by
the activation and release of various apoptotic factors which
regulate mitochondria by releasing apoptin which targets
mitochondria (20). Although
regulation of apoptosis may include the Bcl-2 family, the final
execution of apoptosis remains dependent on activation of the
caspase cascade reaction (21). The
caspase family, particularly caspase-3, serves a key role in
apoptosis (21). Various apoptotic
pathways lead to apoptosis by triggering the caspase cascade
reaction. Caspase-3 is the effector molecule in the apoptotic
cascade signaling pathway and is the executive protein of apoptosis
(22). As a DNA damage repair
molecule, PARP is degraded by caspase-3 and other cysteine
proteinases during apoptosis, and its degradation is regarded as an
early molecular sign of apoptosis (23). In the present study, it was also
identified that down-expression of PARP-1 (via olaparib)
significantly decreased the cellular viability of PanC-1 cells,
increased p53 protein expression, decreased expression of
pro-caspase-3, increased caspase-3 activity and suppressed Bcl-2
protein expression following the inhibition of ATM activity. Liu
et al (24) reported that PARP
had an effect on sulfur mustard-induced cutaneous injuries in
vitro and in vivo via the caspase-3 signaling
pathway.
The results of the present study revealed that
PARP-1 inhibition significantly suppressed cell proliferation,
suppressed pro-caspase-3 protein expression and activated caspase-3
activity of PanC-1 cells through p53/Bcl-2 signaling. Subsequently,
inhibition of ATM activity alongside downregulation of PARP-1,
further decreased cellular viability of PanC-1 cells, induced p53
protein expression, decreased expression of pro-caspase-3,
increased caspase-3 activity, and suppressed Bcl-2 protein
expression in PanC-1 cells. It is proposed that down-expression of
PARP-1 suppresses proliferation and induces apoptosis of pancreatic
cancer cells via the ATM-deficient p53 signaling pathway.
References
|
1
|
Suzuki N, Hazama S, Ueno T, Matsui H,
Shindo Y, Iida M, Yoshimura K, Yoshino S, Takeda K and Oka M: A
phase I clinical trial of vaccination with KIF20A-derived peptide
in combination with gemcitabine for patients with advanced
pancreatic cancer. J Immunother. 37:36–42. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ciuleanu TE, Pavlovsky AV, Bodoky G, Garin
AM, Langmuir VK, Kroll S and Tidmarsh GT: A randomised Phase III
trial of glufosfamide compared with best supportive care in
metastatic pancreatic adenocarcinoma previously treated with
gemcitabine. Eur J Cancer. 45:1589–1596. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhao H, Yang G, Wang D, Yu X, Zhang Y, Zhu
J, Ji Y, Zhong B, Zhao W, Yang Z and Aziz F: Concurrent gemcitabine
and high-intensity focused ultrasound therapy in patients with
locally advanced pancreatic cancer. Anticancer Drugs. 21:447–452.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Qing SW, Ju XP, Cao YS and Zhang HJ: Dose
escalation of Stereotactic Body Radiotherapy (SBRT) for locally
advanced unresectable pancreatic cancer patients with CyberKnife:
protocol of a phase I study. Radiat Oncol. 12:62017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pelzer U, Schwaner I, Stieler J, Adler M,
Seraphin J, Dörken B, Riess H and Oettle H: Best supportive care
(BSC) versus oxaliplatin, folinic acid and 5-fluorouracil (OFF)
plus BSC in patients for second-line advanced pancreatic cancer: A
phase III-study from the German CONKO-study group. Eur J Cancer.
47:1676–1681. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li Y, Wang Y, Li L, Kong R1, Pan S1, Ji L,
Liu H, Chen H and Sun B: Hyperoside induces apoptosis and inhibits
growth in pancreatic cancer via Bcl-2 family and NF-κB signaling
pathway both in vitro and in vivo. Tumour Biol. 37:7345–7355. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lindqvist LM and Vaux DL: BCL2 and related
prosurvival proteins require BAK1 and BAX to affect autophagy.
Autophagy. 10:1474–1475. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Carter BZ, Mak PY, Mak DH, Ruvolo VR,
Schober W, McQueen T, Cortes J, Kantarjian HM, Champlin RE,
Konopleva M and Andreeff M: Synergistic effects of p53 activation
via MDM2 inhibition in combination with inhibition of Bcl-2 or
Bcr-Abl in CD34+ proliferating and quiescent chronic myeloid
leukemia blast crisis cells. Oncotarget. 6:30487–30499. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Martinez-Bosch N, Fernandez-Zapico ME,
Navarro P and Yelamos J: Poly(ADP-Ribose) polymerases: New players
in the pathogenesis of exocrine pancreatic diseases. Am J Pathol.
186:234–241. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chakrabarti G, Moore ZR, Luo X, Ilcheva M,
Ali A, Padanad M, Zhou Y, Xie Y, Burma S, Scaglioni PP, et al:
Targeting glutamine metabolism sensitizes pancreatic cancer to
PARP-driven metabolic catastrophe induced by ss-lapachone. Cancer
Metab. 3:122015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lohse I, Kumareswaran R, Cao P, Pitcher B,
Gallinger S, Bristow RG and Hedley DW: Effects of combined
treatment with ionizing radiation and the PARP inhibitor olaparib
in BRCA mutant and wild type patient-derived pancreatic cancer
xenografts. PLoS One. 11:e01672722016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Temiz P, Akkaş G, Neşe N, Uğur Duman F,
Karakaş C and Erhan Y: Determination-of apoptosis and cell cycle
modulators (p16, p21, p27, p53, BCL-2, Bax, BCL-xL and cyclin D1)
in thyroid follicular carcinoma, follicular adenoma and adenomatous
nodules via a tissue microarray method. Turk J Med Sci. 45:865–871.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Castrogiovanni C, Vandaudenard M,
Waterschoot B, De Backer O and Dumont P: Decrease of mitochondrial
p53 during late apoptosis is linked to its dephosphorylation on
serine 20. Cancer Biol Ther. 16:1296–1307. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hill R, Rabb M, Madureira PA, Clements D,
Gujar SA, Waisman DM, Giacomantonio CA and Lee PW:
Gemcitabine-mediated tumour regression and p53-dependent gene
expression: Implications for colon and pancreatic cancer therapy.
Cell Death Dis. 4:e7912013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Deben C, Lardon F, Wouters A, Op de Beeck
K, Van Den Bossche J, Jacobs J, van der Steen N, Peeters M, Rolfo
C, Deschoolmeester V and Pauwels P: APR-246 (PRIMA-1 (MET))
strongly synergizes with AZD2281 (olaparib) induced PARP inhibition
to induce apoptosis in non-small cell lung cancer cell lines.
Cancer Lett. 375:313–322. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xu C, Wu A, Zhu H, Fang H, Xu L, Ye J and
Shen J: Melatonin is involved in the apoptosis and necrosis of
pancreatic cancer cell line SW-1990 via modulating of Bcl-2/Bax
balance. Biomed Pharmacother. 67:133–139. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu Z, Wild C, Ding Y, Ye N, Chen H, Wold
EA and Zhou J: BH4 domain of Bcl-2 as a novel target for cancer
therapy. Drug Discov Today. 21:989–996. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Laulier C and Lopez BS: The secret life of
Bcl-2: Apoptosis-independent inhibition of DNA repair by Bcl-2
family members. Mutat Res. 751:247–257. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hao W, Wang S and Zhou Z: Tubeimoside-1
(TBMS1) inhibits lung cancer cell growth and induces cells
apoptosis through activation of MAPK-JNK pathway. Int J Clin Exp
Pathol. 8:12075–12083. 2015.PubMed/NCBI
|
|
20
|
Kvansakul M and Hinds MG: The Bcl-2
family: Structures, interactions and targets for drug discovery.
Apoptosis. 20:136–150. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen H, Yang X, Feng Z, Tang R, Ren F, Wei
K and Chen G: Prognostic value of Caspase-3 expression in cancers
of digestive tract: A meta-analysis and systematic review. Int J
Clin Exp Med. 8:10225–10234. 2015.PubMed/NCBI
|
|
22
|
Liu SY, Liang QJ, Lin TX, Fan XL, Liang Y,
Heemann U and Li Y: Lipopolysaccharide-enhanced early proliferation
of insulin secreting NIT-1 cell is associated with nuclear
factor-kappaB-mediated inhibition of caspase 3 cleavage. Chin Med J
(Engl). 124:3652–3656. 2011.PubMed/NCBI
|
|
23
|
Tang D, Gao J, Wang S, Yuan Z, Ye N, Chong
Y, Xu C, Jiang X, Li B, Yin W, et al: Apoptosis and anergy of T
cell induced by pancreatic stellate cells-derived galectin-1 in
pancreatic cancer. Tumour Biol. 36:5617–5626. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu F, Jiang N, Xiao ZY, Cheng JP, Mei YZ,
Zheng P, Wang L, Zhang XR, Zhou XB, Zhou WX and Zhang YX: Effects
of poly(ADP-ribose) polymerase-1 (PARP-1) inhibition on sulfur
mustard-induced cutaneous injuries in vitro and in vivo. PeerJ.
4:e18902016. View Article : Google Scholar : PubMed/NCBI
|