Introduction
Carcinogenesis is a complex process forming a cancer
and is determined by cellular, genetic and epigenetic changes. In
recent years, various factors have been investigated to reveal the
mechanism of carcinogenesis and especially tumor microenvironment
has been recognized as an important factor in carcinogenesis. One
of the main components of tumor microenvironment is the
extracellular matrix (ECM) remodeling (1,2). The ECM
is defined as a complex structure build by interacting
extracellular molecules including proteoglycans, polysaccharides,
fibronectin, laminin and fibers such as collagen and elastin. The
ECM provides structural and mechanical support to cells and tissues
(1). During carcinogenesis, the
composition and the overall content of the ECM change and the ECM
is progressively stiffened (3,4). ECM
stiffness has been implicated to promote tumor progression
(5) and is partially associated with
lysyl oxidase (LOX)-mediated collagen cross-linking (2,5).
LOX is a copper-containing amine oxidase that
catalyzes lysine-derived cross-links in collagen and elastin and
stabilizes the ECM (6). For many
years, LOX has been demonstrated that it has diverse functions
including the ability regulating gene transcription, cell growth
control, cell motility and migration and cell adhesions (7). LOX is encoded by the LOX gene
located on chromosome 5 (5q23.3–31.2) and belongs to a copper
dependent amine-oxidase family currently consisting of five members
(LOX, LOX-like protein (LOXL) 1, LOXL2, LOXL3 and LOXL4) (6,8–11). Recent studies have shown that LOX
family is related to tumor fibrosis, invasion and metastasis
(10–13).
ECM stiffness is caused by collagen deposition and
linearization and bundling of interstitial collagen (4) and LOX has a crucial role in
stiffness-associated tumor progression (5,10,14). ECM remodeling including stiffness
contributes to fibrotic changes in tumor (15), which is defined as fibrotic focus
(FF). Since Hasebe et al (16)
have proposed FF as an indicator of tumor aggressiveness in
invasive breast cancer, many studies have shown the relationship
between FF and the prognosis of breast cancer (17).
Inflammation has been also recognized as an
important component of tumorigenesis, and it usually precedes
fibrosis (18). Recently, it has been
described that inflammation plays an important role in fibrosis as
an inducer of epithelial-mesenchymal transition (EMT) in cancer
(19). Also, it has been suggested
that invasion and aggression of breast cancer correlates with ECM
stiffening and immune cell infiltration (4).
Nevertheless, the mechanisms of LOX-mediated tumor
progression in association with fibrosis and inflammation are not
fully understood. In this study, we analyzed the association
between the expression of the members of the LOX family and FF in
relation with inflammation, and investigated the prognostic
significance of the members of the LOX family and FF in human
breast cancer.
Materials and methods
Patients and materials
Patients with primary breast cancer who underwent
surgery between January 2003 and December 2010 at the Daegu
Catholic University Hospital (Daegu, Korea) were enrolled.
Formalin-fixed and paraffin-embedded specimens from the patients
were stained with hematoxylin and eosin (H&E) and
histologically examined and reviewed by an experienced pathologist.
The clinicopathologic characteristics were evaluated based on the
pathologic reports and the medical records. Disease stage was
assessed according to the seventh edition of the American Joint
Committee on Cancer (AJCC) staging manual for breast cancer.
Molecular subtype of the breast cancer was classified according to
the immunohistochemical findings for estrogen receptor (ER),
progesterone receptor (PR), human epidermal growth factor receptor
2 (HER2) and Ki-67 labeling index. The informed written consent was
obtained from all patients for the use of their data. The ethics
review of the study was waived from the Institutional Review Board
at the Daegu Catholic University Hospital according to the
deliberation criteria.
Tissue microarray and
immunohistochemistry
Representative paraffin blocks of invasive breast
carcinomas were selected to prepare for tissue microarray (TMA) and
TMA was constructed following the methods described in our previous
study (20). TMA blocks were cut into
5 µm-thick sections and immunohistochemical staining was performed
on the TMA sections using the Bond Polymer Intense Detection System
(Leica Microsystems, Victoria, Australia) according to the
manufacturer's instruction with minor modifications.
Immunohistochemical staining for the members of the
LOX family were conducted using commercially available primary
antibodies, LOX (1:200, ab31238; Abcam, Cambridge, MA, USA), LOXL1
(1:100, NBP1-82827; Novus Biologicals, Littleton, CO, USA), LOXL2
(1:100, NBP1-32954; Novus Biologicals) and LOXL3 (1:100,
NBP1-85908; Novus Biologicals). The TMA sections were also
immunostained for CD4 (RTU, clone 4B12; Leica Biosystems, Wetzlar,
Germany), CD8 (1:200, clone C8/144B; Dako, Glostrup, Denmark), CD68
(1;200, clone PG-M1; Dako), ER (1:100, clone 6F11; Novocastra,
Newcastle, UK), PR (1:100, clone 16; Novocastra), HER2 (1:250,
A0485; Dako), Ki-67 (1:200, MM1-L; Novocastra), Bcl-2 (1:4, clone
124; Dako), p53 (1:200, BP53.12; Zymed, Carlsbad, CA, USA) and
epidermal growth factor receptor (EGFR) (1:100, clone EGFR.25;
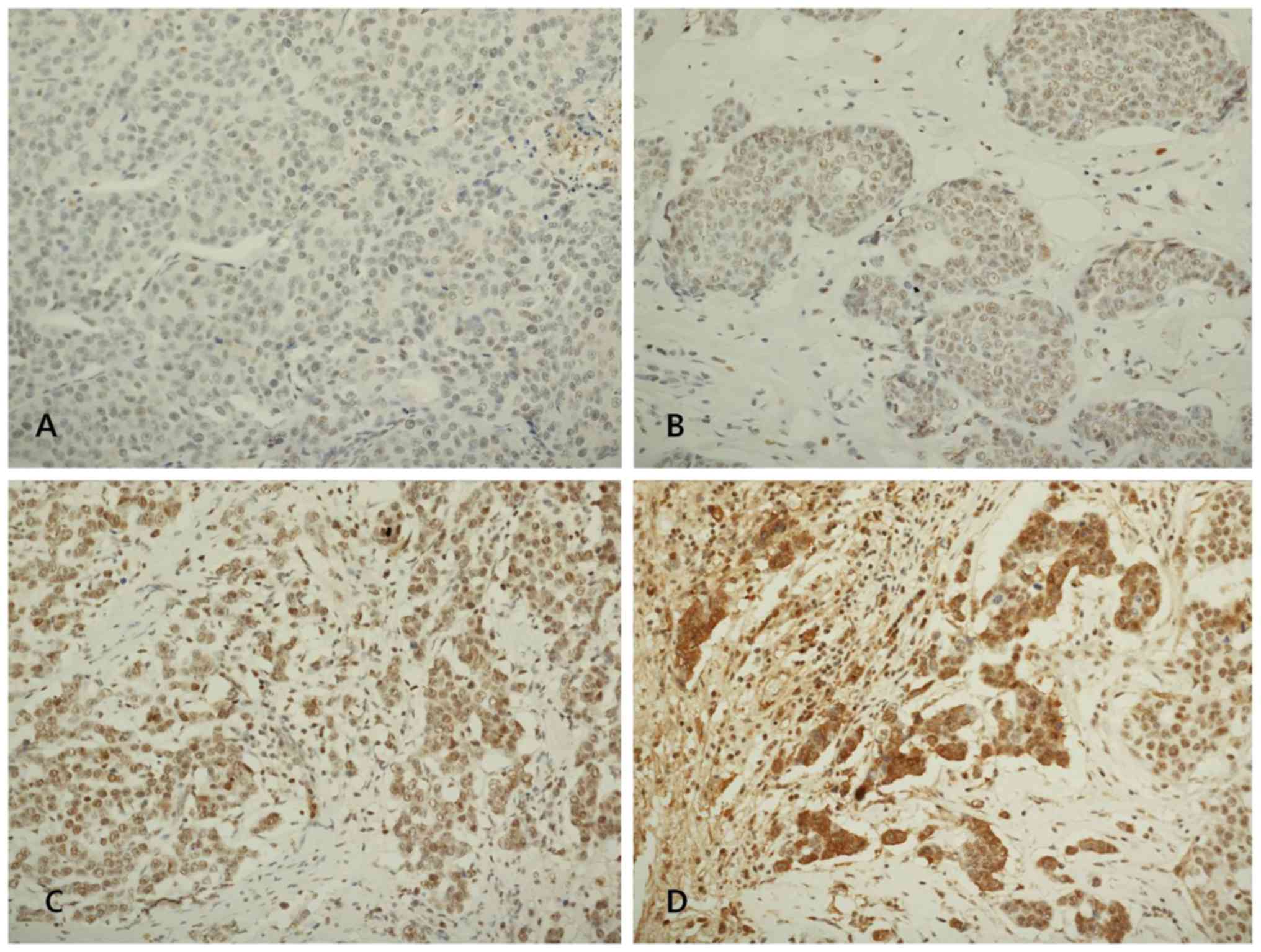
Novocastra). The levels of LOX, LOXL1, LOXL2 and LOXL3 assessed
semiquantitatively as: 0 (no staining); 1+ (minimal intensity,
<10% of cells); 2+ (moderate, 10–49%); and 3+ (marked, ≥50%).
The expression level 0 and 1 were designated as negative and 2 and
3 as positive. Fig. 1 shows
representative microphotographs of immunohistochemical expression
of LOX, LOXL1, LOXL2 and LOXL3. The number of cluster of
differentiation (CD)4+ T cells, CD8+ T cells
and CD68+ macrophages was evaluated under a microscopic
and counted both in the stroma and the cancer cell nest.
Intratumoral (within the tumor boundary) and peritumoral (at the
edge of tumor boundary) lymphocyte infiltration was also assessed
semiquantitatively as follows: 0 (no or scant lymphocytes); 1 (a
few scattered lymphocytic infiltration); 2 (scattered lymphocytic
aggregation); and 3 (diffuse and dense aggregation of lymphocytes),
where 1, 2 and 3 are designated as positive and 0 is as
negative.
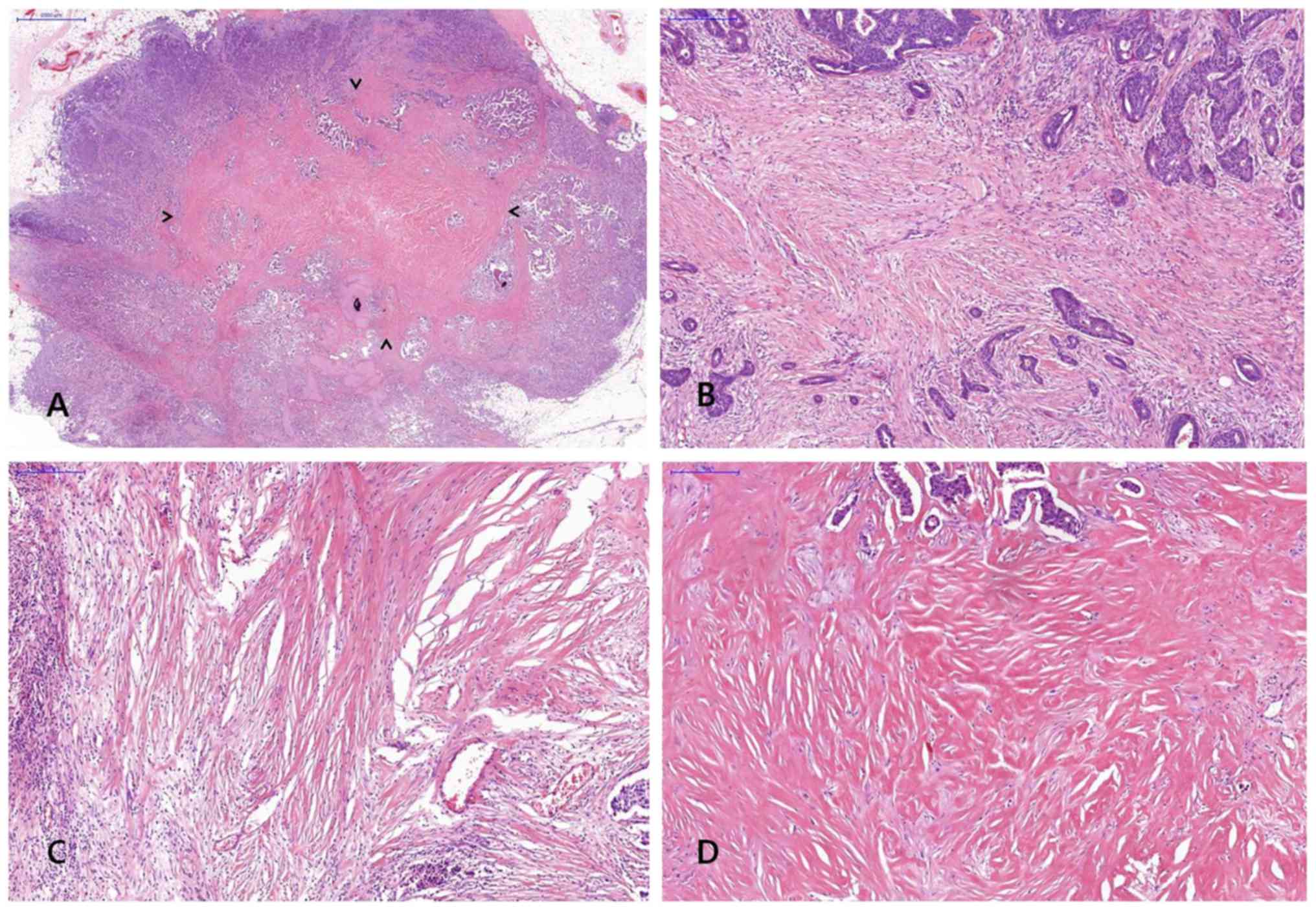
FF was diagnosed when there was a scar-like area or
radially expanding fibrous bands consist of the fibroblasts and
collagen fibers within the tumor, and surrounded by a highly
cellular zone of infiltrating carcinoma cells (17). Fig. 2
shows representative example of FF in H&E stained breast cancer
specimen. The status of FF within the tumor was assessed including
size and grade and a minimal dimension of 1 mm of a fibrosclerotic
core was referred to as FF.
RT-PCR
The levels of cytokines including tumor necrosis
factor (TNF)-α, interleukin (IL)-1, IL-2, IL-4, IL-6, transforming
growth factor (TGF)-β, interferon (IFN)-γ and nuclear factor
(NF)-κB p50 were assessed by the levels of RNA transcripts in
frozen tissue using RT-PCR. Total RNA was isolated from frozen
breast cancer tissues using TRIzol reagent (Invitrogen; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) according to the
manufacturer's protocol. Reverse transcription of total RNA was
performed using a commercial kit (Superscript II RNase H-reverse
transcriptase; Invitrogen; Thermo Fisher Scientific, Inc.). PCR
amplification was performed with specific primers and the sequences
of primers are presented in Table I.
PCR products were analyzed by agarose gel electrophoresis and
visualized by ethidium bromide staining.
 | Table I.Sequences of primers used in PCR. |
Table I.
Sequences of primers used in PCR.
| Primer name | Primer sequence
(5′→3′) | Orientation |
|---|
| TNF-α |
CCCTCAACCTCTTCTGGCTC | Forward |
| TNF-α |
AGGCAGCTCCTACATTGGGT | Reverse |
| IL-1 |
AAATACCTGTGGCCTTGGGC | Forward |
| IL-1 |
TTTGGGATCTACACTCTCCAGCT | Reverse |
| IL-2 |
GCAACTCCTGTCTTGCATTG | Forward |
| IL-2 |
TGCTTTGACAAAAGGTAATCCA | Reverse |
| IL-4 |
ATGGGTCTCACCTCCCAACTGC | Forward |
| IL-4 |
TTCCTGTCGAGCCGTTTCAG | Reverse |
| IL-6 |
TACCCCCAGGAGAAGATTCC | Forward |
| IL-6 |
AAAGCTGCGCAGAATGAGAT | Reverse |
| TGF-β |
CCCAGCATCTGCAAAGCTC | Forward |
| TGF-β |
GTCAATGTACAGCTGCCGCA | Reverse |
| IFN-γ |
AGTTATATCTTGGCTTTTCA | Forward |
| IFN-γ |
ACCGAATAATTAGTCAGCTT | Reverse |
| NF-κB p50 |
CACCTAGCTGCCAAAGAAGG | Forward |
| NF-κB p50 |
TCAGCCAGCTGTTTCATGTC | Reverse |
Statistical analysis
Statistical analyses were performed using SPSS
software version 19.0 (SPSS, Inc., Chicago, IL, USA). Association
between FF and LOX, LOXL1, LOXL2 and LOXL3 was analyzed using the
Chi-square test. Association of FF with number of inflammatory
cells was assessed using two sample t-test. Association of FF with
the clinicopathologic characteristics was analyzed using two sample
t-test and the Chi-square test. Binary logistic regression analysis
was performed to assess the odds ratios (ORs) of the statistically
significant factors in univariate analysis. Association of the
members of the LOX family with number of inflammatory cells was
assessed using two sample t-test. Association between the members
of the LOX family with the clinicopathologic characteristics was
analyzed using two sample t-test and the Chi-square test. Survival
data were analyzed using Kaplan-Meier survival analysis. P<0.05
was considered to indicate a statistically significant
difference.
Results
Clinicopathologic characteristics of
the patients
A total of 291 patients with invasive breast cancer
were included in this study. The mean age of the patients was
52.63±11.01 years (range, 24–90 years). Clinicopathologic
characteristics of the patients are shown in Table II. Among the patients, the most
common histologic type of invasive cancer was invasive ductal
carcinoma not otherwise specified (89.7%), and the remaining were
infiltrating lobular carcinoma (n=7), papillary carcinoma (n=4),
micropapillary carcinoma (n=2), mucinous carcinoma (n=4), tubular
carcinoma (n=4), metaplastic carcinoma (n=3), medullary carcinoma
(n=2), sarcoma (n=1), inflammatory carcinoma (n=1) and mixed type
(n=2).
 | Table II.Patient characteristics. |
Table II.
Patient characteristics.
| Clinicopathologic
variables | Value |
|---|
| Age (years), mean
(range) | 51.81±11.29
(24–90) |
| Menopausal status,
n (%) |
|
|
Premenopausal | 143 (49.14) |
|
Postmenopausal | 148 (50.86) |
| Tumor
size (cm), mean (range) | 2.09±1.80
(0.1–23.0) |
| Histologic grade, n
(%) |
|
| I | 64 (22.15) |
| II | 110 (38.06) |
|
III | 115 (39.79) |
| Lymph node
metastasis, n (%) |
|
|
Negative | 165 (62.26) |
|
Positive | 100 (37.74) |
| Stage, n (%) |
|
| I | 126 (43.9) |
| II | 117 (40.77) |
|
III | 36 (12.54) |
| IV | 8 (2.79) |
| Lymphovascular
invasion, n (%) |
|
|
Negative | 197 (68.4) |
|
Positive | 91 (31.6) |
| ER, n (%) |
|
|
Negative | 102 (35.17) |
|
Positive | 188 (64.83) |
| PR, n (%) |
|
|
Negative | 81 (27.93) |
|
Positive | 209 (72.07) |
| HER2
overexpression, n (%) |
|
|
Negative | 117 (50.87) |
|
Positive | 114 (49.13) |
| Ki-67, n (%) |
|
|
<14% | 129 (58.64) |
|
≥14% | 91 (41.36) |
| Molecular subtype,
n (%) |
|
| Luminal
A | 113 (39.65) |
| Luminal
B | 110 (38.6) |
|
HER2 | 26 (9.12) |
|
Basal-like | 36 (12.63) |
| Fibrotic focus, n
(%) |
|
|
Negative | 178 (62.46) |
|
Positive | 107 (37.54) |
| LOX, n (%) |
|
|
Negative | 148 (50.86) |
|
Positive | 143 (49.14) |
| LOXL1, n (%) |
|
|
Negative | 200 (68.73) |
|
Positive | 91 (31.27) |
| LOXL2, n (%) |
|
|
Negative | 176 (60.48) |
|
Positive | 115 (39.52) |
| LOXL3, n (%) |
|
|
Negative | 252 (86.6) |
|
Positive | 39 (13.4) |
| Intratumoral
inflammation, n (%) |
|
|
Negative | 51 (17.53) |
|
Positive | 240 (82.47) |
| Peritumoral
inflammation, n (%) |
|
|
Negative | 31 (10.65) |
|
Positive | 260 (89.35) |
Association between FF and LOX
families
The percentage of positive FF was 37.5% and positive
rate of LOX expression was 49.1% in primary breast cancer tissues.
The expression of LOX, LOXL1, LOXL2 and LOXL3 was not associated
with presence of FF (Table
III).
 | Table III.Association between the fibrotic
focus and LOX families. |
Table III.
Association between the fibrotic
focus and LOX families.
|
| Fibrotic focus |
|
|---|
|
|
|
|
|---|
| Variables | Negative | Positive | P-value |
|---|
| LOX, n (%) |
|
|
|
|
Negative | 92 (51.69) | 52 (48.6) | 0.614 |
|
Positive | 86 (48.31) | 55 (51.4) |
|
| LOXL1, n (%) |
|
|
|
|
Negative | 124 (69.66) | 71 (66.36) | 0.561 |
|
Positive | 54 (30.34) | 36 (33.64) |
|
| LOXL2, n (%) |
|
|
|
|
Negative | 112 (62.92) | 59 (55.14) | 0.194 |
|
Positive | 66 (37.08) | 48 (44.86) |
|
| LOXL3, n (%) |
|
|
|
|
Negative | 153 (85.96) | 94 (87.85) | 0.649 |
|
Positive | 25 (14.04) | 13 (12.15) |
|
Association of FF with
clinicopathologic features
By univariate analysis, FF was statistically
significantly associated with stage II tumors (P<0.001), larger
tumor size (P<0.001), lymph node metastasis (P<0.001), high
histologic grade (P=0.001), p53 (P=0.018), intratumoral and
peritumoral inflammation (P<0.001 and P<0.001, respectively)
(Table IV). Multivariate analysis
using covariate with P<0.2 in univariate analysis was performed
to assess the independent association of FF with clinicopathologic
features. We found a significant association between FF and tumor
size, histologic grade, lymph node metastasis and p53 (Table V).
 | Table IV.Association of fibrotic focus with
clinicopathologic characteristics. |
Table IV.
Association of fibrotic focus with
clinicopathologic characteristics.
|
| Fibrotic focus |
|
|---|
|
|
|
|
|---|
| Clinicopathologic
variables | Negative | Positive | P-value |
|---|
| Tumor size, n
(%) |
|
|
|
| <2
cm | 120 (67.42) | 38 (35.51) |
<0.001a |
| ≥2
cm | 58 (32.58) | 69 (65.49) |
|
| Histologic grade, n
(%) |
|
|
|
| I | 52 (29.38) | 10 (9.34) |
<0.001a |
| II | 64 (36.16) | 45 (42.06) |
|
|
III | 61 (34.46) | 52 (48.60) |
|
| Lymph node
metastasis, n (%) |
|
|
|
|
Negative | 126 (71.19) | 43 (40.19) |
<0.001a |
|
Positive | 51 (28.81) | 64 (59.81) |
|
| Stage, n (%) |
|
|
|
| I | 95 (53.67) | 28 (26.67) |
<0.001a |
| II | 61 (34.46) | 55 (52.38) |
|
|
III | 16 (9.04) | 20 (19.05) |
|
| IV | 5 (2.83) | 2 (1.90) |
|
| Lymphovascular
invasion, n (%) |
|
|
|
|
Negative | 136 (76.84) | 57 (53.27) |
<0.001a |
|
Positive | 41 (23.16) | 50 (46.73) |
|
| ER, n (%) |
|
|
|
|
Negative | 60 (33.90) | 39 (36.45) | 0.662 |
|
Positive | 117 (66.10) | 68 (63.55) |
|
| PR, n (%) |
|
|
|
|
Negative | 47 (26.55) | 31 (28.97) | 0.658 |
|
Positive | 130 (73.45) | 76 (71.03) |
|
| HER2
overexpression, n (%) |
|
|
|
|
Negative | 67 (46.21) | 46 (57.50) | 0.105 |
|
Positive | 78 (53.79) | 34 (42.50) |
|
| Ki-67, n (%) |
|
|
|
|
<14% | 126 (70.79) | 70 (65.42) | 0.344 |
|
≥14% | 52 (29.21) | 37 (34.58) |
|
| p53, n (%) |
|
|
|
|
Negative | 49 (27.53) | 44 (41.12) | 0.018a |
|
Positive | 129 (72.47) | 63 (58.88) |
|
| Molecular subtype,
n (%) |
|
|
|
| Luminal
A | 68 (38.64) | 43 (41.75) | 0.830 |
| Luminal
B | 71 (40.34) | 38 (36.89) |
|
|
HER2 | 17 (9.66) | 8 (7.77) |
|
|
Basal-like | 20 (11.36) | 14 (13.59) |
|
| Intratumoral
inflammation, n (%) |
|
|
|
|
Negative | 39 (21.91) | 7 (6.54) | 0.001a |
|
Positive | 139 (78.09) | 100 (93.46) |
|
| Peritumoral
inflammation, n (%) |
|
|
|
|
Negative | 24 (13.48) | 2 (1.87) | 0.001a |
|
Positive | 154 (86.52) | 105 (98.13) |
|
 | Table V.Multivariate analysis of association
between fibrotic focus and clinicopathologic characteristics. |
Table V.
Multivariate analysis of association
between fibrotic focus and clinicopathologic characteristics.
| Clinicopathologic
variables | OR | 95% CI for OR | P-value |
|---|
| Tumor size, n
(%) |
|
|
|
| <2
cm | 1 | – | – |
| ≥2
cm | 4.409 | 1.726, 11.264 | 0.002a |
| Histologic grade, n
(%) |
|
|
|
| I | 1 | – | – |
| II | 4.465 | 1.246, 16.002 | 0.022a |
|
III | 5.072 | 1.147, 22.422 | 0.032a |
| Lymph node
metastasis, n (%) |
|
|
|
|
Negative | 1 | – | – |
|
Positive | 4.862 | 1.727, 13.687 | 0.003a |
| Stage, n (%) |
|
|
|
| I | 1 | – | – |
| II | .416 | 0.115, 1.502 | 0.180 |
|
III | .306 | 0.057, 1.636 | 0.166 |
| IV | .129 | 0.012, 1.395 | 0.092 |
| HER2
overexpression, n (%) |
|
|
|
|
Negative | 1 | – | – |
|
Positive | .556 | 0.27, 1.147 | 0.112 |
| p53, n (%) |
|
|
|
|
Negative | 1 | – | – |
|
Positive | .456 | 0.225, 0.926 | 0.030a |
| Intratumoral
inflammation, n (%) |
|
|
|
|
Negative | 1 | – | – |
|
Positive | 2.468 | 0.829, 7.343 | 0.104 |
| Peritumoral
inflammation, n (%) |
|
|
|
|
Negative | 1 | – | – |
|
Positive | 1.356 | 0.353, 5.215 | 0.658 |
Association of LOX families with
clinicopathologic features
By univariate analysis, LOX was statistically
significantly associated with intratumoral and peritumoral
inflammation (P<0.001 and P<0.001, respectively) (Table VI). LOXL1 was statistically
significantly associated with intratumoral inflammation (P=0.021).
LOXL2 was also statistically significantly associated with
intratumoral and peritumoral inflammation (P=0.004 and P=0.041,
respectively). LOXL3 was significantly associated with positive
expression of ER and PR (P<0.001 and P<0.001, respectively)
and molecular subtype (P<0.001).
 | Table VI.Association of LOX families with
clinicopathologic characteristics. |
Table VI.
Association of LOX families with
clinicopathologic characteristics.
|
| LOX | LOXL1 | LOXL2 | LOXL3 |
|---|
|
|
|
|
|
|
|---|
| Clinicopathologic
variables | Positive
expression, n (%) | P-value | Positive
expression, n (%) | P-value | Positive
expression, n (%) | P-value | Positive
expression, n (%) | P-value |
|---|
| Tumor size, n
(%) |
| 0.496 |
| 0.674 |
| 0.740 |
| 0.236 |
| <2
cm | 82 (50.9) |
| 52 (32.3) |
| 65 (40.4) |
| 25 (15.5) |
|
| ≥2
cm | 61 (46.9) |
| 39 (30.0) |
| 50 (38.5) |
| 14 (10.8) |
|
| Histologic grade, n
(%) |
| 0.702 |
| 0.549 |
| 0.558 |
| 0.263 |
| I | 30 (46.9) |
| 18 (27.7) |
| 22 (34.4) |
| 5 (7.8) |
|
| II | 52 (47.3) |
| 32 (29.1) |
| 44 (40.0) |
| 15 (13.6) |
|
|
III | 60 (52.2) |
| 40 (34.8) |
| 49 (42.6) |
| 19 (16.5) |
|
| LN metastasis, n
(%) |
| 0.596 |
| 0.826 |
| 0.367 |
| 0.805 |
|
Negative | 82 (47.7) |
| 54 (31.4) |
| 65 (37.8) |
| 22 (12.8) |
|
|
Positive | 59 (50.9) |
| 35 (30.2) |
| 50 (43.1) |
| 16 (13.8) |
|
| Stage, n (%) |
| 0.916 |
| 0.930 |
| 0.469 |
| 0.752 |
| I | 65 (51.6) |
| 41 (32.5) |
| 50 (39.7) |
| 16 (12.7) |
|
| II | 55 (47.0) |
| 37 (31.6) |
| 47 (40.2) |
| 15 (12.8) |
|
|
III | 18 (50.0) |
| 10 (27.8) |
| 15 (41.7) |
| 7 (19.4) |
|
| IV | 4 (50.0) |
| 2 (25.0) |
| 1 (12.5) |
| 1 (12.5) |
|
| LVI, n (%) |
| 0.636 |
| 0.505 |
| 0.262 |
| 0.905 |
|
Negative | 99 (50.3) |
| 64 (32.5) |
| 83 (42.1) |
| 27 (13.7) |
|
|
Positive | 43 (47.3) |
| 26 (28.6) |
| 32 (35.2) |
| 12 (13.2) |
|
| ER, n (%) |
| 0.469 |
| 0.374 |
| 0.465 |
| 0.001a |
|
Negative | 47 (46.1) |
| 35 (34.7) |
| 43 (42.2) |
| 23 (22.5) |
|
|
Positive | 95 (50.5) |
| 55 (29.3) |
| 71 (37.8) |
| 16 (8.5) |
|
| PR, n (%) |
| 0.663 |
| 0.969 |
| 0.622 |
| <0.001a |
|
Negative | 38 (46.9) |
| 25 (30.9) |
| 30 (37.0) |
| 20 (24.7) |
|
|
Positive | 104 (49.8) |
| 65 (31.1) |
| 84 (40.2) |
| 19 (9.1) |
|
| HER2
overexpression, n (%) |
| 0.954 |
| 0.323 |
| 0.216 |
| 0.101 |
|
Negative | 56 (47.9) |
| 32 (27.4) |
| 42 (35.9) |
| 11 (9.4) |
|
|
Positive | 55 (48.2) |
| 38 (33.3) |
| 50 (43.9) |
| 19 (16.7) |
|
| Ki-67, n (%) |
| 0.182 |
| 0.334 |
| 0.992 |
| 0.503 |
|
<14% | 93 (46.5) |
| 59 (29.5) |
| 79 (39.5) |
| 25 (12.5) |
|
|
≥14% | 50 (54.9) |
| 32 (35.2) |
| 36 (39.6) |
| 14 (15.4) |
|
| Molecular subtype,
n (%) |
| 0.680 |
| 0.467 |
| 0.671 |
|
<0.001a |
| Luminal
A | 56 (49.6) |
| 30 (26.5) |
| 44 (38.9) |
| 13 (11.5) |
|
| Luminal
B | 56 (50.9) |
| 38 (34.5) |
| 46 (41.8) |
| 8 (7.3) |
|
|
HER2 | 10 (38.5) |
| 10 (38.5) |
| 11 (42.3) |
| 11 (42.3) |
|
|
Basal-like | 19 (52.8) |
| 10 (27.8) |
| 11 (30.6) |
| 7 (19.4) |
|
| IT, n (%) |
|
<0.001a |
| 0.021a |
| 0.004a |
| 0.199 |
|
Negative | 11 (21.5) |
| 9 (17.6) |
| 11 (21.6) |
| 4 (7.8) |
|
|
Positive | 132 (55.0) |
| 82 (34.2) |
| 104 (43.3) |
| 35 (14.6) |
|
| PT, n (%) |
|
<0.001a |
| 0.130 |
| 0.041a |
| 0.229 |
|
Negative | 6 (19.4) |
| 6 (19.4) |
| 7 (22.6) |
| 2 (6.5) |
|
|
Positive | 137 (52.7) |
| 85 (32.7) |
| 108 (41.5) |
| 37 (14.2) |
|
Association of FF and LOX families
with inflammatory markers
Immunohistochemical staining for CD4+ T
cells, CD8+ T cells and CD68+ macrophages
were performed in 77 patients who had undergone surgery from
January 2008 to December 2010. The levels of RNA transcripts for
TNF-α, IL-4 and NF-κB p50 were analyzed in frozen tumor tissues
although the expression levels of RNA transcripts for IL-1, IL-2,
IL-6, TGF-β and IFN-γ were too low to be analyzed. In subgroup
analysis, LOX and CD8+ T cell showed significant
correlation (P=0.030) and LOXL1 and IL-4 showed significant
association (P=0.019) (Table
VII).
 | Table VII.Association of fibrotic focus and LOX
families with inflammatory markers. |
Table VII.
Association of fibrotic focus and LOX
families with inflammatory markers.
|
| Fibrotic focus | LOX | LOXL1 | LOXL2 | LOXL3 |
|---|
|
|
|
|
|
|
|
|---|
| Inflammatory
markers | Negative | Positive | P-value | Negative | Positive | P-value | Negative | Positive | P-value | Negative | Positive | P-value | Negative | Positive | P-value |
|---|
| TNF-α, n (%) |
|
| 0.458 |
|
| 0.712 |
|
| 0.703 |
|
| 0.817 |
|
| 0.627 |
|
Negative | 5
(27.8) | 6 (40.0) |
| 4 (26.7) | 6
(35.3) |
| 6
(28.6) | 4 (36.4) |
| 7
(29.2) | 3 (33.3) |
| 8
(28.6) | 2 (40.0) |
|
|
Positive | 13 (72.2) | 9 (60.0) |
| 11 (73.3) | 11 (64.7) |
| 15 (71.4) | 7 (63.6) |
| 17 (70.8) | 6 (66.7) |
| 20 (71.4) | 3 (60.0) |
|
| IL-4, n (%) |
|
| 0.898 |
|
| 0.982 |
|
| 0.019a |
|
| 0.259 |
|
| 0.175 |
|
Negative | 10 (55.6) | 8 (53.3) |
| 7 (46.7) | 8 (47.1) |
| 13 (61.9) | 2 (18.2) |
| 10 (41.7) | 6 (66.7) |
| 12 (42.9) | 4 (80.0) |
|
|
Positive | 8
(44.4) | 7 (46.7) |
| 8 (53.3) | 9 (52.9) |
| 8
(38.1) | 9 (81.8) |
| 14 (58.3) | 3 (33.3) |
| 16 (57.1) | 1 (20.0) |
|
| NF-κB p50, n
(%) |
|
| 0.354 |
|
| 0.469 |
|
| 0.344 |
|
| 0.273 |
|
| 0.152 |
|
Negative | 1 (5.6) | 0 (0.0) |
| 1 (6.7) | 0 (0.0) |
| 0 (0.0) | 1 (9.1) |
| 0 (0.0) | 1 (11.1) |
| 0 (0.0) | 1 (20.0) |
|
|
Positive | 17 (94.4) | 15 (100.0) |
| 14 (93.3) | 17 (100.0) |
| 21 (100.0) | 10 (90.9) |
| 24 (100.0) | 8 (88.9) |
| 28 (100.0) | 4 (80.0) |
|
| CD4+ T
cell, mean (n) | 34.7 | 24.6 | 0.408 | 24.3 |
36.8 | 0.248 | 28.3 | 34.0 | 0.627 |
31.5 | 32.9 | 0.907 | 28.3 |
34.0 | 0.627 |
| CD8+ T
cell, mean (n) | 105.3 | 103.4 | 0.944 | 79.0 | 132.8 |
0.030c | 95.0 | 123.2 | 0.299 | 102.7 | 106.4 | 0.891 | 95.0 | 120.2 | 0.299 |
| CD68+ cell | 31.9 | 30.0 | 0.801 |
29.14 |
33.8 | 0.515 | 31.7 | 30.4 | 0.868 |
31.9 | 30.1 | 0.814 | 31.4 |
24.7 | 0.868 |
| (Macrophage), mean
(n) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Association of FF and LOX family with
patient outcomes
The median follow-up period was 72 months (range
1–147 months). Among 291 patients, 35 patients (12.0%) showed tumor
recurrence and 26 patients died from breast cancer. The 5-year
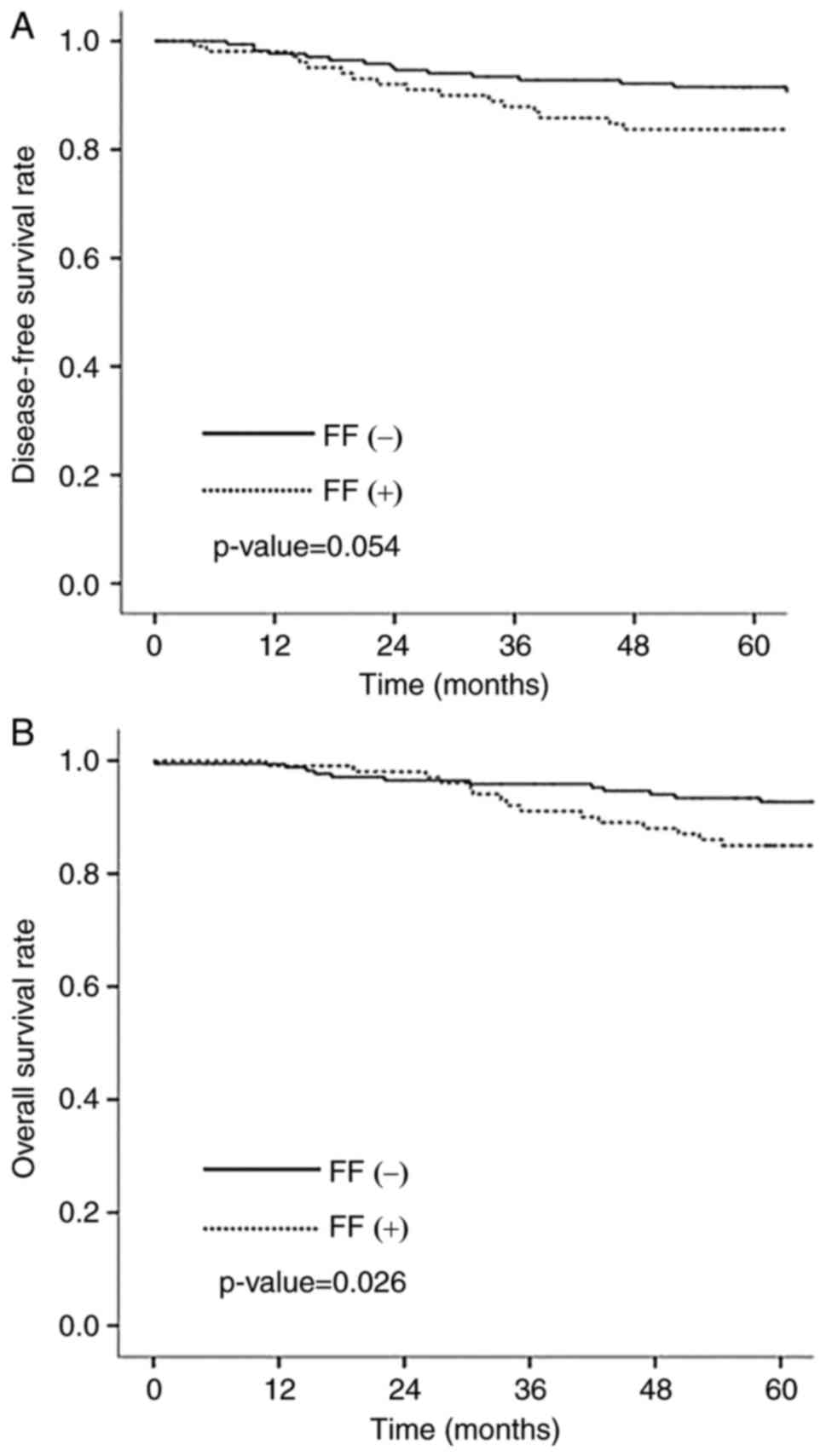
survival rate was 90.0%. Overall survival (OS) was statistically
significantly longer in negative FF group than in positive FF group
(P=0.026) (Fig. 3A). Disease free
survival (DFS) was longer in negative FF group although there was
no statistical significance (P=0.054) (Fig. 3B). There was no significant
association between LOX family and patients outcomes.
Discussion
Tumor fibrosis and inflammation have been
increasingly recognized as important factors which influence tumor
progression and metastasis. Recent studies have demonstrated that
LOX is involved in creating fibrotic microenvironment and
fibrosis-enhanced cancer metastasis (5,21,22). Nevertheless, the relationship between
LOX, FF, inflammation and tumor progression in human breast cancer
have yet to be fully elucidated. FF is distinguished from organ
fibrosis by the characteristics such as irregular or storiform
patterned arrangement of fibroblast and collagen fibers, which
shows radially expanding fibrous bands surrounded by a highly
cellular zone of infiltrating carcinoma cells, and the location in
the center of the tumor (17). In
this study, we aimed to investigate whether LOX contribute to the
formation of FF, especially in association with inflammation. There
was no direct correlation between the members of the LOX family and
FF in this study. However, FF, LOX, LOXL1 and LOXL2 showed
significant correlation with intratumoral inflammation and FF, LOX
and LOXL2 also related to peritumoral inflammation. These results
suggest that the members of the LOX family and FF interact in
relation with inflammation in breast cancer. Although the
mechanisms by which LOX mediates FF formation in tumor are not
clarified, functions of the LOX family in association with fibrosis
in the cardiovascular system are relatively clear (23). LOX has been shown to be up-regulated
by cytokines including TGF-β, TNF-α and IL-6 (23). Also, it has been implicated that
LOX-mediated collagen cross-linking is critical in ECM stiffness
(2,5).
Acerbi et al (4) have shown
that both ECM stiffness and cellular TGF-β signaling correlated
positively with immune cell infiltration in human breast cancer.
Leight et al. have demonstrated that increased matrix
stiffness regulates TGF-β signaling, a potent inducer of EMT in
tumor cells (24). In recent years,
it has been suggested that EMT contributes to increase the collagen
producing fibroblasts (21) and
fibroblasts form FF in fibrotic tissues (17). Taken together these results also
support our hypothesis. As far as I know, this is the first study
to investigate the relationship between the members of the LOX
family and FF.
It is well documented that organ fibrosis is
associated with chronic inflammatory diseases (18,19). A
number of studies have suggested the role of inflammatory cells
such as macrophage and T-lymphocytes in fibrotic tissue (18,25,26). Also,
various inflammatory mediators including TGF-β, TNF-α, IL-4 and
NF-κB have been demonstrated to regulate the inflammatory responses
as well as the fibrotic signaling cascade (18,19). TNF-α
is crucial for the induction of NF-κB (27), the major factor in the inflammatory
response and activated NF-κB is known to contribute to organ
fibrosis and cancer development (19,28,29).
Furthermore, hypoxia in fibrosis and tumor microenvironment
cooperates with inflammatory response in the induction of EMT
process (19,30,31).
Interestingly, hypoxia induces LOX expression in tumor cells
(32) and contributes to promote
fibrogenesis and tumor progression through the reinforcement of the
EMT process (19,33). In this study, we analyzed the
association between FF, the members of the LOX family and
inflammatory response. Although the results did not show any
association between FF and inflammatory markers, there was
significant correlation between LOX and CD8+ T cell.
CD8+ T cells are important for the adaptive immune
responses and have antitumor effects through various mechanisms.
One of the mechanisms, CD8+ T cells secret cytokines
such as TNF-α and IFN-γ, and LOX is known to be up-regulated by
cytokines including TNF-α (23). In
this regards, CD8+ T cells may affect the expression of
LOX. Also, our results showed significant association between LOXL1
and IL-4. IL-4 is a T-lymphocyte-associated cytokine that is
involved in humoral and adaptive immunity (34). IL-4 has many biological effects and
affects various cell types (34).
Interestingly, IL-4 stimulates fibroblasts proliferation34 and
LOXL1 is secreted by fibrogenic cells including fibroblasts
(9). Although the mechanism of
regulation of LOXL1 has not been fully elucidated, our results
suggest that IL-4 may affect the expression of LOXL1.
In previous studies, FF has been reported to be
associated with poor prognosis in breast cancer (17). Hasebe et al (35) have demonstrated that the presence of a
FF was associated with larger tumor size, high histologic grade,
lymph and blood vessel invasion, presence of lymph node metastases,
pTNM stage and a shorter DFS and OS in survival analysis. In
consistent with the results of previous studies, our results showed
that the presence of a FF is associated with more aggressive
characteristics of breast cancer. FF was statistically
significantly associated with tumor stage, larger tumor size, lymph
node metastasis, high histologic grade and p53. Also, OS and DFS
were statistically significantly longer in negative FF group than
in positive FF group in this study.
In recent years, diverse functions of LOX have been
revealed, and LOX has emerged as therapeutic target in cancer
therapy. Many pre-clinical studies have described that LOX promotes
tumor progression and metastasis (5,13,36,37). In
clinical studies, most of studies have also shown that LOX and LOXL
expression correlated with a poor prognosis in cancer (13,14,38–40),
although study methods were somewhat different from each other. In
our study, however, LOX as well as LOXL1 and LOXL2 did not show
clinical relevance except LOXL3. Nevertheless, our study describes
for the first time that LOXL3 was significantly associated with
positive expression of ER and PR and molecular subtype in breast
cancer.
Limitations of the present study include the use of
the paraffin-embedded tumor tissues in detecting FF and the members
of the LOX family, but the fresh frozen tissue in analyzing
inflammatory mediators. Tissue processing can cause genetic
alterations and molecular changes in tissues (41), and the levels of mRNA transcripts or
protein in the paraffin-embedded tissues can be different from
those in fresh frozen tissue. Also, we employed immunohistochemical
staining to assess the levels of the members of the LOX family, but
this method is semiquantitative and less accurate than other
objective methods such as quantitative fluorescence analysis or
real-time quantitative PCR. Furthermore, we did not include control
group such as normal or benign breast tissue. A comparative study
between tumor tissue and control group may be necessary to clarify
the role of LOX and FF in breast cancer.
In conclusion, our study showed that FF and LOX
family are associated with inflammation in breast cancer, although
there is no direct correlation between FF and the members of the
LOX family. In consistent with the previous studies, our results
showed that FF is associated with poor prognosis. And for the first
time, we found that LOXL3 was significantly associated with
positive expression of ER and PR and molecular subtype in breast
cancer. Further studies are required to clarify the mechanisms
relating to the LOX family, FF and inflammation in breast
cancer.
Acknowledgements
This study was supported by the grant of Research
Institute of Medical Science, Catholic University of Daegu
(2013).
References
|
1
|
Pupa SM, Ménard S, Forti S and Tagliabue
E: New insights into the role of extracellular matrix during tumor
onset and progression. J Cell Physiol. 192:259–267. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Levental KR, Yu H, Kass L, Lakins JN,
Egeblad M, Erler JT, Fong SFT, Csiszar K, Giaccia A, Weninger W, et
al: Matrix crosslinking forces tumor progression by enhancing
integrin signaling. Cell. 139:891–906. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gilkes DM, Semenza GL and Wirtz D: Hypoxia
and the extracellular matrix: Drivers of tumour metastasis. Nat Rev
Cancer. 14:430–439. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Acerbi I, Cassereau L, Dean I, Shi Q, Au
A, Park C, Chen YY, Liphardt J, Hwang ES and Weaver VM: Human
breast cancer invasion and aggression correlates with ECM
stiffening and immune cell infiltration. Integr Biol (Camb).
7:1120–1134. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cox TR, Bird D, Baker AM, Barker HE, Ho
MW, Lang G and Erler JT: LOX-mediated collagen crosslinking is
responsible for fibrosis-enhanced metastasis. Cancer Res.
73:1721–1732. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Csiszar K: Lysyl oxidase: A novel
multifunctional amine oxidase family. Prog Nucleic Acid Res Mol
Biol. 70:1–32. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Payne SL, Hendrix MJC and Kirschmann DA:
Paradoxical roles for lysyl oxidases in cancer-a prospect. J Cell
Biochem. 101:1338–1354. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Smith-Mungo LI and Kagan HM: Lysyl
oxidase: Properties, regulation and multiple functions in biology.
Matrix Biol. 16:387–398. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kagan HM and Li W: Lysyl oxidase:
Properties, specificity, and biological roles inside and outside of
the cell. J Cell Biochem. 88:660–672. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Molnar J, Fong KSK, He QP, Hayashi K, Kim
Y, Fong SF, Fogelgren B, Szauter KM, Mink M and Csiszar K:
Structural and functional diversity of lysyl oxidase and the
LOX-like proteins. Biochim Biophys Acta. 1647:220–224. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lucero HA and Kagan HM: Lysyl oxidase: An
oxidative enzyme and effector of cell function. Cell Mol Life Sci.
63:2304–2316. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Baker AM, Cox TR, Bird D, Lang G, Murray
GI, Sun XF, Southall SM, Wilson JR and Erler JT: The role of lysyl
oxidase in SRC-dependent proliferation and metastasis of colorectal
cancer. J Natl Cancer Inst. 103:407–424. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Erler JT, Bennewith KL, Nicolau M,
Dornhöfer N, Kong C, Le QT, Chi JT, Jeffrey SS and Giaccia AJ:
Lysyl oxidase is essential for hypoxia-induced metastasis. Nature.
440:1222–1226. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Barker HE, Chang J, Cox TR, Lang G, Bird
D, Nicolau M, Evans HR, Gartland A and Erler JT: LOXL2-mediated
matrix remodeling in metastasis and mammary gland involution.
Cancer Res. 71:1561–1572. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cox TR and Erler JT: Remodeling and
homeostasis of the extracellular matrix: Implications for fibrotic
diseases and cancer. Dis Model Mech. 4:165–178. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hasebe T, Tsuda H, Hirohashi S, Shimosato
Y, Iwai M, Imoto S and Mukai K: Fibrotic focus in invasive ductal
carcinoma: An indicator of high tumor aggressiveness. Jpn J Cancer
Res. 87:385–394. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Van den Eynden GG, Colpaert CG, Couvelard
A, Pezzella F, Dirix LY, Vermeulen PB, Van Marck EA and Hasbe T: A
fibrotic focus is a prognostic factor and a surrogate marker for
hypoxia and (lymph)angiogenesis in breast cancer: Review of the
literature and proposal on the criteria of evaluation.
Histopathology. 51:440–451. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ueha S, Shand FH and Matsushima K:
Cellular and molecular mechanisms of chronic
inflammation-associated organ fibrosis. Front Immunol. 3:712012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
López-Novoa JM and Nieto MA: Inflammation
and EMT: An alliance towards organ fibrosis and cancer progression.
EMBO Mol Med. 1:303–314. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jeong YJ, Bong JG, Park SH, Choi JI and Oh
HK: Expression of leptin, leptin receptor, adiponectin, and
adiponectin receptor in ductal carcinoma in situ and invasive
breast cancer. J Breast Cancer. 14:96–103. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Radisky DC, Kenny PA and Bissell MJ:
Fibrosis and cancer: Do myofibroblasts come also from epithelial
cells via EMT? J Cell Biochem. 101:830–839. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ertz N: Cancer: Opening LOX to metastasis.
Nature. 522:41–42. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nishioka T, Eustace A and West C: Lysyl
oxidase: From basic science to future cancer treatment. Cell Struct
Funct. 37:75–80. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Leight JL, Wozniak MA, Chen S, Lynch ML
and Chen CS: Matrix rigidity regulates a switch between TGF-β
1-induced apoptosis and epithelial-mesenchymal transition. Mol Biol
Cell. 23:781–791. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wynn TA and Barron L: Macrophages: Master
regulators of inflammation and fibrosis. Semin Liver Dis.
30:245–257. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Luzina IG, Todd NW, Iacono AT and Atamas
SP: Roles of T lymphocytes in pulmonary fibrosis. J Leuko Biol.
83:237–244. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Meldrum KK, Metcalfe P, Leslie JA, Misseri
R, Hile KL and Meldrum DR: TNF-alpha neutralization decreases
nuclear factor-kappaB activation and apoptosis during renal
obstruction. J Surg Res. 131:181–188. 2006. View Article : Google Scholar
|
|
28
|
Tashiro K, Tamada S, Kuwabara N, Komiya T,
Takekida K, Asai T, Iwao H, Sugimura K, Matsumura Y, Takaoka M, et
al: Attenuation of renal fibrosis by proteasome inhibition in rat
obstructive nephropathy: Possible role of nuclear factor kappaB.
Int J Mol Med. 12:587–592. 2003.PubMed/NCBI
|
|
29
|
Karin M and Greten FR: NF-kappaB. Linking
inflammation and immunity to cancer development and progression.
Nat Rev Immunol. 5:749–759. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Julien S, Puig I, Caretti E, Bonaventure
J, Nelles L, van Roy F, Dargemont C, de Herreros AG, Bellacosa A
and Larue L: Activation of NF-kappaB by Akt upregulates Snail
expression and induces epithelium mesenchyme transition. Oncogene.
26:7445–7456. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kim HJ, Litzenburger BC, Cui X, Delgado
DA, Grabiner BC, Lin X, Lewis MT, Gottardis MM, Wong TW, Attar RM,
et al: Constitutively active type I insulin-like growth factor
receptor causes transformation and xenograft growth of immortalized
mammary epithelial cells and is accompanied by an
epithelial-to-mesenchymal transition mediated by NF-kappaB and
snail. Mol Cell Biol. 27:3165–3175. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Denko NC, Fontana LA, Hudson KM, Sutphin
PD, Raychaudhuri S, Altman R and Giaccia AJ: Investigating hypoxic
tumor physiology through gene expression patterns. Oncogene.
22:5907–5914. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Higgins DF, Kimura K, Bernhardt WM,
Shrimanker N, Akai Y, Hohenstein B, Saito Y, Johnson RS, Kretzler
M, Cohen CD, et al: Hypoxia promotes fibrogenesis in vivo via HIF-1
stimulation of epithelial-to-mesenchymal transition. J Clin Invest.
117:3810–3820. 2007.PubMed/NCBI
|
|
34
|
Nagai S and Toi M: Interleukin-4 and
breast cancer. Breast Cancer. 7:181–186. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hasebe T, Sasaki S, Imoto S, Muki K,
Yokose T and Ochiai A: Prognostic significance of fibrotic focus in
invasive ductal carcinoma of the breast: A prospective
observational study. Mod Pathol. 15:502–516. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hollosi P, Yakushiji JK, Fong KS, Csiszar
K and Fong SF: Lysyl oxidase-like 2 promotes migration in
noninvasive breast cancer cells no in normal breast epithelial
cells. Int J Cancer. 125:318–327. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kirschmann DA, Seftor EA, Fong SF, Nieva
DR, Sullivan CM, Edwards EM, Sommer P, Csiszar K and Hendrix MJ: A
molecular role for lysyl oxidase in breast cancer invasion. Cancer
Res. 62:4478–4483. 2002.PubMed/NCBI
|
|
38
|
Hellman J, Jansen MP, Ruigrok-Ritstier K,
van Staveren IL, Look MP, Meijer-van Gelder ME, Sieuwerts AM, Klijn
JG, SleiJfer S, Foekens JA and Berns EM: Association of an
extracellular matrix gene cluster with breast cancer prognosis and
endocrine therapy response. Clin Cancer Res. 14:5555–5564. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Patani N, Jiang W, Newbold R and Mokbel K:
Prognostic implications of carboxyl-terminus of Hsc70 interacting
protein and lysyl-oxidase expression in human breast cancer. J
Carcinog. 9:92010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Peinado H, Moreno-Bueno G, Hardisson D,
Pérez-Gómez E, Santos V, Mendiola M, de Diego JI, Nistal M,
Quintanilla M, Portillo F and Cano A: Lysyl oxidase-like 2 as a new
poor prognosis marker of squamous cell carcinomas. Cancer Res.
68:4541–4550. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
McSherry EA, McGoldrick A, Kay EW, Hopkins
AM, Gallagher WM and Dervan PA: Formalin-fixed paraffin-embedded
clinical tissues show spurious copy number changes in array-CGH
profiles. Clin Genet. 72:441–447. 2007. View Article : Google Scholar : PubMed/NCBI
|