Introduction
Liver cancer is a common type of cancer with a high
mortality rate worldwide. Hepatocellular carcinoma (HCC) is the
most common type of liver cancer and its incidence rate is
currently on the rise (1).
Hepatectomy and liver transplantation remain the curative
treatments for this fatal disease; however, only 30–40% of patients
with HCC are eligible. For late-stage patients, non-curative
strategies, including transcatheter arterial chemoembolization and
radiofrequency ablation, are available; however, the survival time
is prolonged by only a few months (2). Furthermore, there are no effective
chemotherapeutic drugs available due to the overexpression of
multidrug-resistance genes in HCC (3). Currently, a multi-targeted tyrosine
kinase inhibitor, sorafenib, only slightly improves the survival of
certain patients with HCC (4).
Therefore, the development of novel chemotherapeutic agents and
more effective therapies for the treatment of HCC are required.
Triterpenoids are compounds that are presented
extensively in numerous plants and have been used in traditional
medicine. Olive oil is an important source of pentacyclic
triterpenoids, including oleanolic acid. Oleanolic acid exhibits
potential antitumor activity against numerous types of tumors,
including hepatoma cells (5),
pancreatic cancer cells (6) and
bladder cancer cells (7). In
addition, oleanolic acid has been revealed to arrest cell cycle
progression, and to induce apoptosis via reactive oxygen
species-mediated mitochondrial depolarization and lysosomal
membrane permeabilization in human pancreatic cancer cells
(6). Furthermore, an oleanolic acid
derivative has been demonstrated to induce human hepatoma cell
apoptosis via a reactive oxygen species/mitogen-activated protein
kinase-dependent mitochondrial signaling pathway (5). Additionally, ursolic acid has been
revealed to induce an endoplasmic reticulum stress response to
activate apoptosis signal-regulating kinase 1-c-Jun N-terminal
kinase signaling and to induce apoptosis in T24 human bladder
cancer cells (7). These findings
suggest that oleanolic acid and ursolic acid may be promising
agents for the treatment of HCC. However, the molecular
pharmacology of oleanolic acid and ursolic acid remains
unknown.
The present study investigated the molecular
pharmacology of an oleanolic acid derivative, a newly synthesized
compound. In addition, the potential effects of this oleanolic acid
derivative on the viability of SMMC-7721 human cells were evaluated
and the underlying mechanism involved in oleanolic acid
derivative-mediated cell death was explored.
Materials and methods
Chemicals and reagents
The oleanolic acid derivative was provided by Dr Lei
of Beijing University of Chinese Medicine (Beijing, China) and
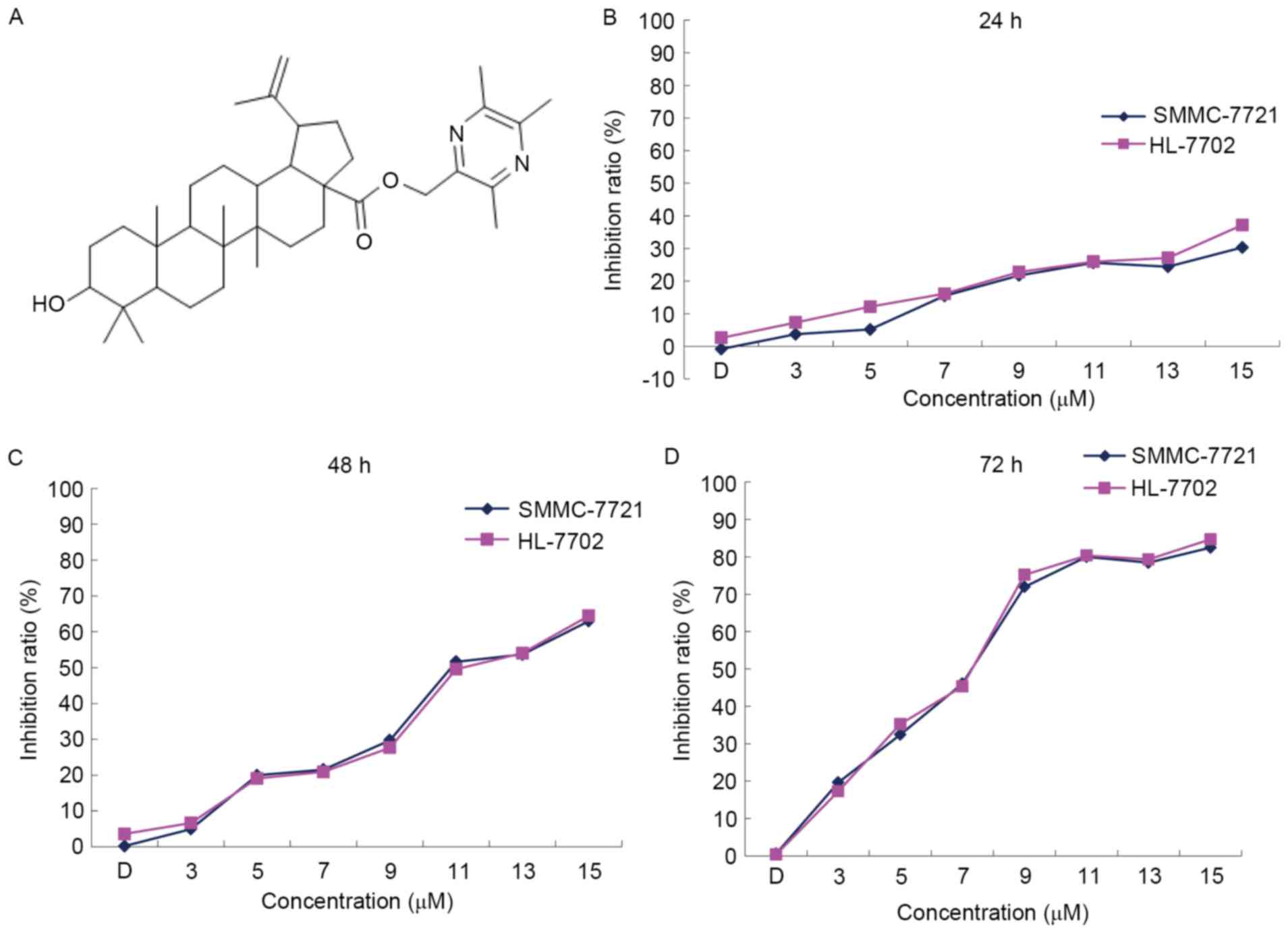
dissolved in DMSO. The chemical structure of the oleanolic acid
derivative is presented in Fig. 1A.
RPMI-1640, fetal bovine serum, penicillin, streptomycin and trypsin
were purchased from Gibco (Thermo Fisher Scientific, Inc., Waltham,
MA, USA). SDS, ponceaus, dithiothreitol,
phenylmethylsulfonylfluoride, bovine serum albumin, MTT, the
Annexin V-fluorescein isthocyanate (FITC)/propidium iodide (PI)
Apoptosis Detection kit and JC-1 were purchased from Sigma-Aldrich
(Merck KGaA, Darmstadt, Germany). Anti-caspase-9 (catalog no.,
9502), anti-caspase-3 (catalog no., 9662) were obtained from Cell
Signaling Technology, Inc. (Danvers, MA, USA). Bcl-2 (catalog no.,
BS1031) and Bax (catalog no., BS1030) were obtained from Biogot
Technology Co., Ltd. (Nanjing, China). Horseradish
peroxidase-conjugated goat anti-rabbit antibodies (catalog no.,
BA1054) were obtained from Boster Biological Technology Co. Ltd
(Wuhan, China). Polyvinylidene difluoride (PVDF) microporous
membranes and the enhanced chemiluminescence detection system were
purchased from Takara Biotechnology Co., Ltd. (Dalian, China). RIPA
cell lysis buffer for western blot analysis was purchased from
Beyotime Institute of Biotechnology (Haimen, China). The kit for
detection of adenosine triphosphate (ATP) was purchased from
Nanjing Jiancheng Bioengineering Institute (Nanjing, China). The
Human cytochrome c (Cyt-C) ELISA kit was purchased from Shanghai
Ximei Chemical (Shanghai, China). The Cytoplasmic and Mitochondrial
Protein Extraction kit was purchased from Sangon Biotech Co., Ltd.
(Shanghai, China).
Cell culture and treatment
SMMC-7721 human HCC cells were obtained from the
American Type Culture Collection (Manassas, VA, USA). HL-7702
normal human liver cells were obtained from Obio Technology
(Shanghai, China). The cells were maintained in RPMI-1640 medium
supplemented with 10% heat-inactivated fetal bovine serum and 1%
penicillin-streptomycin at 37°C in a 5% CO2 incubator.
Cells were treated with vehicle (0.15% DMSO) alone or with 3, 7, 9,
11, 13 or 15 µM oleanolic acid derivative for 24, 48 and 72 h.
Cell viability assay
Cell viability was determined using the MTT assay.
SMMC-7721 and HL-7702 cells were seeded at a density of
1×105 cells/well in 96-well plates. Following incubation
for ~24 h at 37°C in a 5% CO2 incubator, the cells were
treated with various concentrations of oleanolic acid derivative
(0, 3, 5, 7, 9, 11, 13 and 15 µM). The MTT assay was performed
after 24, 48 and 72 h of treatment. The culture medium was
discarded, then 30 µl 0.5% (w/v) MTT dissolved in 1X
phosphate-buffered saline was added to each well, and the plate was
incubated for 3 h at 37°C. Following incubation for 3 h, the
culture medium was discarded and 120 µl DMSO was added into each
well. Following incubation for 30 min and gentle agitation for 15
min at 37°C, the absorbance at 490 nm was evaluated using a
microplate reader. The experiment was repeated in triplicate. Cell
viability was expressed as a percentage of proliferation against
the controls (untreated cells), which was set at 100%.
Detection of cell membrane
integrity
The cell membrane integrity of SMMC-7721 cells was
assessed using annexin V-FITC and PI double staining. Briefly,
~1×105 SMMC-7721 cells were seeded/well in 6-well
plates. Following incubation for 24 h, the cells were treated with
various concentrations of oleanolic acid derivative (7, 9 or 11 µM)
or vehicle (0.11% DMSO) alone for 12, 24 or 36 h. Subsequently, the
drug-containing medium was removed at each time point, and the
SMMC-7721 cells were harvested, washed and stained with annexin
V-FITC/PI, according to the manufacturer's protocol. Samples were
then diluted with binding buffer and were analyzed using a
fluorescence microscope (×200) within 1 h.
Western blot analysis
Following treatment with the oleanolic acid
derivative (7, 9 or 11 µM) for 48 h at room temperature, the
SMMC-7721 cells were harvested. Total protein was extracted using
RIPA cell lysis buffer on ice. The protein concentration was
determined using a BCA protein assay. Proteins were then mixed with
loading buffer (Beijing ComWin Biotech Co., Ltd., Beijing, China)
and boiled at 95°C for 10 min. Equal amounts of proteins (60 µg)
were separated by SDS-PAGE (12% gradient gel), transferred to PVDF
membranes and detected using the specific antibodies. The PVDF
membranes were then incubated overnight at 4°C with anti-caspase-9
and anti-caspase-3 (dilution, 1:1,000). The immunoreactive proteins
following incubation at 37°C with the horseradish
peroxidase-conjugated goat anti-rabbit antibodies (dilution,
1:10,000) were detected using an enhanced chemiluminescence
detection kit. The results were normalized to those of β-actin
(dilution, 1:1,000).
Intracellular ATP evaluation
Cells (~1×105) were cultured in 96-well
plates and incubated with 0, 7, 9 or 11 µM oleanolic acid
derivative or vehicle (0.11% DMSO) alone for 36 h. Intracellular
ATP levels were determined using an ATP determination kit,
according to the manufacturer's protocol. The entire cell
population, including any floating cells, were assayed.
Luminescence was determined using a multimode microplate reader.
The experiment was repeated in triplicate.
Evaluation of mitochondrial membrane
potential (ΔΨm)
The lipophilic cationic fluorescent probe, JC-1, a
mitochondrial dye that stains mitochondria in living cells in a
membrane potential-dependent fashion, was used to quantify the ΔΨm.
The reduction in the red/green fluorescence intensity ratio of JC-1
is directly proportional to the loss of ΔΨm. In brief, cells were
treated with or without the oleanolic acid derivative (7, 9 or 11
µM) in 6-well plates for 12, 24 and 36 h. The medium was removed
and replaced with 1 ml fresh culture medium (RPMI-1640 medium
supplemented with 2% heat-inactivated fetal bovine serum and 1%
penicillin-streptomycin), followed by the addition of 1 ml JC-1
staining solution (final concentration of 2 mM) and incubation at
37°C for 20 min. The cells were then harvested and washed twice
with JC-1 Dyeing buffer (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany). Finally, 2 ml cell culture medium was added to each well,
and JC-1 fluorescence was determined using a fluorescence
microscope (×100).
Analysis of Cyt-C release
SMMC-7721 cells were harvested following treatment
with various concentrations of the oleanolic acid derivative (7, 9
and 11 µM) for 12 h. Cytoplasmic and mitochondrial proteins were
isolated using a Cytoplasmic and Mitochondrial Protein Extraction
kit, according to the manufacturer's protocol. The concentrations
of cytoplasmic and mitochondrial proteins were detected using the
BCA assay. The concentrations of Cyt-C in the cytoplasm and
mitochondria were detected according to the manufacturer's
instructions using a Human Cyt-C ELISA kit, In order to ensure that
the Cyt-C concentration was accurate, the cytoplasmic and
mitochondrial protein were adjusted to the same level and then the
sample was added to 50 µl of diluent to each well at the end. The
Cyt-C content of the sample was determined by the regression
equation of the standard curve of the reference standard.
Statistical analysis
All experiments were performed at least three times.
All data were analyzed using SPSS version 19.0 statistical software
(IBM Corp., Armonk, NY, USA). Data are presented as the mean ±
standard deviation. Statistical analysis of the data for multiple
groups was performed using one-way analysis of variance and
Dunnett's test. GraphPad Prism version 5.02 (GraphPad Software,
Inc., La Jolla, CA, USA) and Excel (2010) were used for statistical
analysis. P<0.05 was considered to indicate a statistically
significant difference.
Results
Oleanolic acid derivative dose- and
time-dependently inhibits the proliferation of SMMC-7721 and
HL-7702 cells
To determine the cytotoxicity of the oleanolic acid
derivative, the present study treated SMMC-7721 cells and HL-7702
normal liver cells with various concentrations of the oleanolic
acid derivative (0, 3, 5, 7, 9, 11, 13 and 15 µM) for 24, 48 and 72
h, and analyzed the cell viability by performing an MTT assay. In
comparison with the DMSO control, the oleanolic acid derivative
inhibited the proliferation of SMMC-7721 and HL-7702 cells in a
dose- and time-dependent manner (Fig.
1B). The maximum inhibition rates for the oleanolic acid
derivative on SMMC-7721 cells were 30.30, 63.00 and 82.46%, and on
HL-7702 cells were 37.20, 64.45 and 84.68% at 24, 48 and 72 h,
respectively. The inhibition rates demonstrated that the oleanolic
acid derivative had the same inhibitory effect on SMMC-7721 cells
and HL-7702 normal liver cells (Fig.
1B). These results indicated that the oleanolic acid derivative
dose- and time-dependently inhibited the proliferation of SMMC-7721
and HL-7702 cells.
Oleanolic acid derivative induces
membrane disintegrity of SMMC-7721 cells
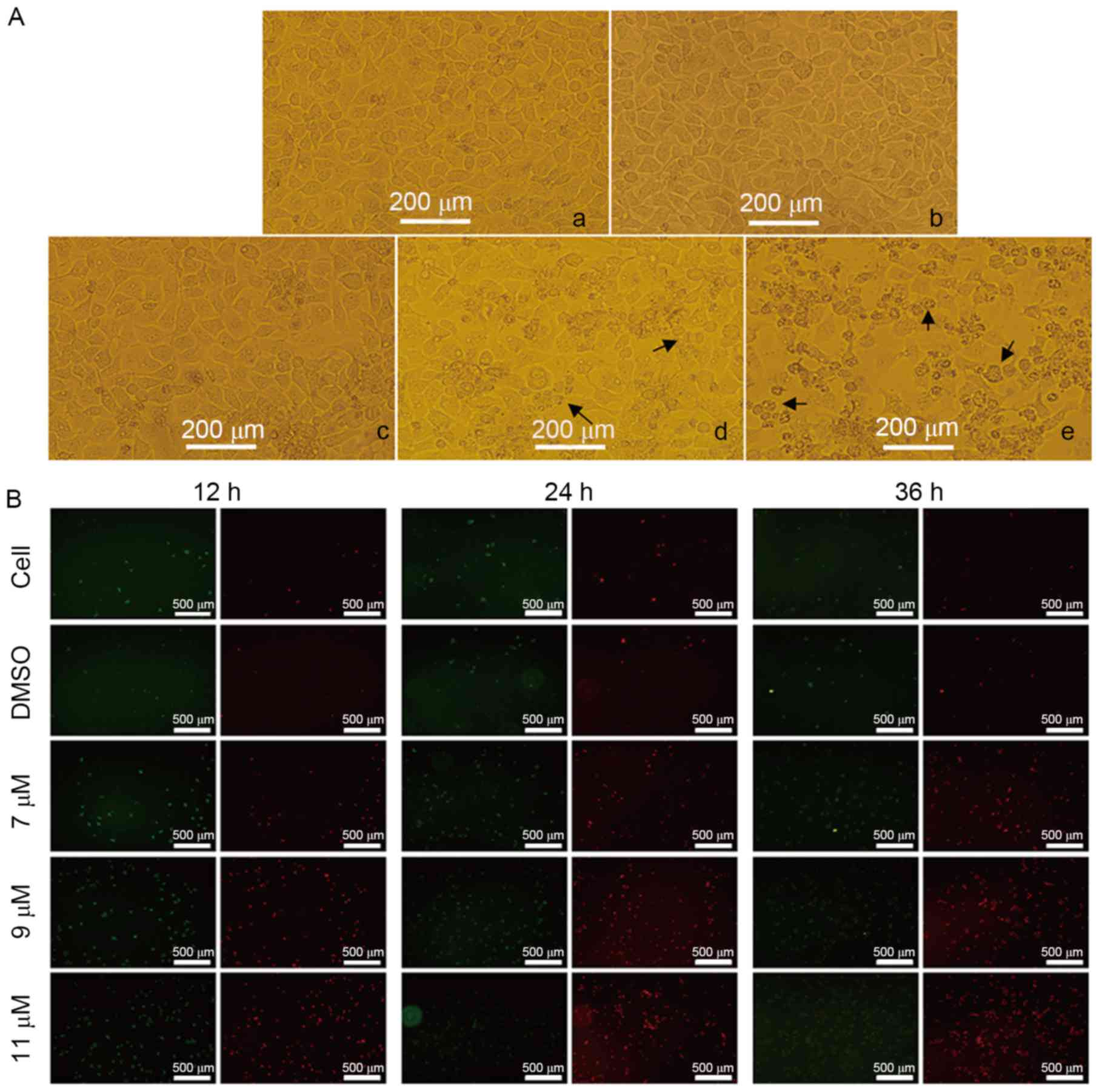
To determine whether the oleanolic acid derivative
induces cell apoptosis, the present study treated SMMC-7721 cells
with 0, 7, 9 or 11 µM oleanolic acid derivative for 36 h and
observed cell morphology under a microscope. Compared with the
DMSO-treated cells, the SMMC-7721 cells underwent the typical
morphological changes of apoptosis, including cell shrinkage,
membrane blebbing and accumulation of small apoptotic bodies (as
indicated by the arrows in Fig. 2A).
The induction of apoptosis was more evident when the oleanolic acid
derivative concentration was 9 or 11 µM. These results demonstrated
that the oleanolic acid derivative induced apoptosis of SMMC-7721
cells in a dose-dependent manner.
Annexin V is a protein that interacts strongly and
specifically with phosphatidylserine residues on the cell membrane
and is widely used for the detection of apoptosis (8). PI, a nucleic acid dye, cannot cross the
intact cell membrane; however, in the late-apoptotic cells and dead
cells, PI is able to penetrate the cell membrane and interact with
DNA, revealing red staining under a microscope. Thus, PI has been
widely applied to assess cell viability. To further support the
evidence that the oleanolic acid derivative induced apoptosis of
SMMC-7721 cells, the present study treated SMMC-7721 cells with 0,
7, 9 or 11 µM oleanolic acid derivative for 12, 24 and 36 h and
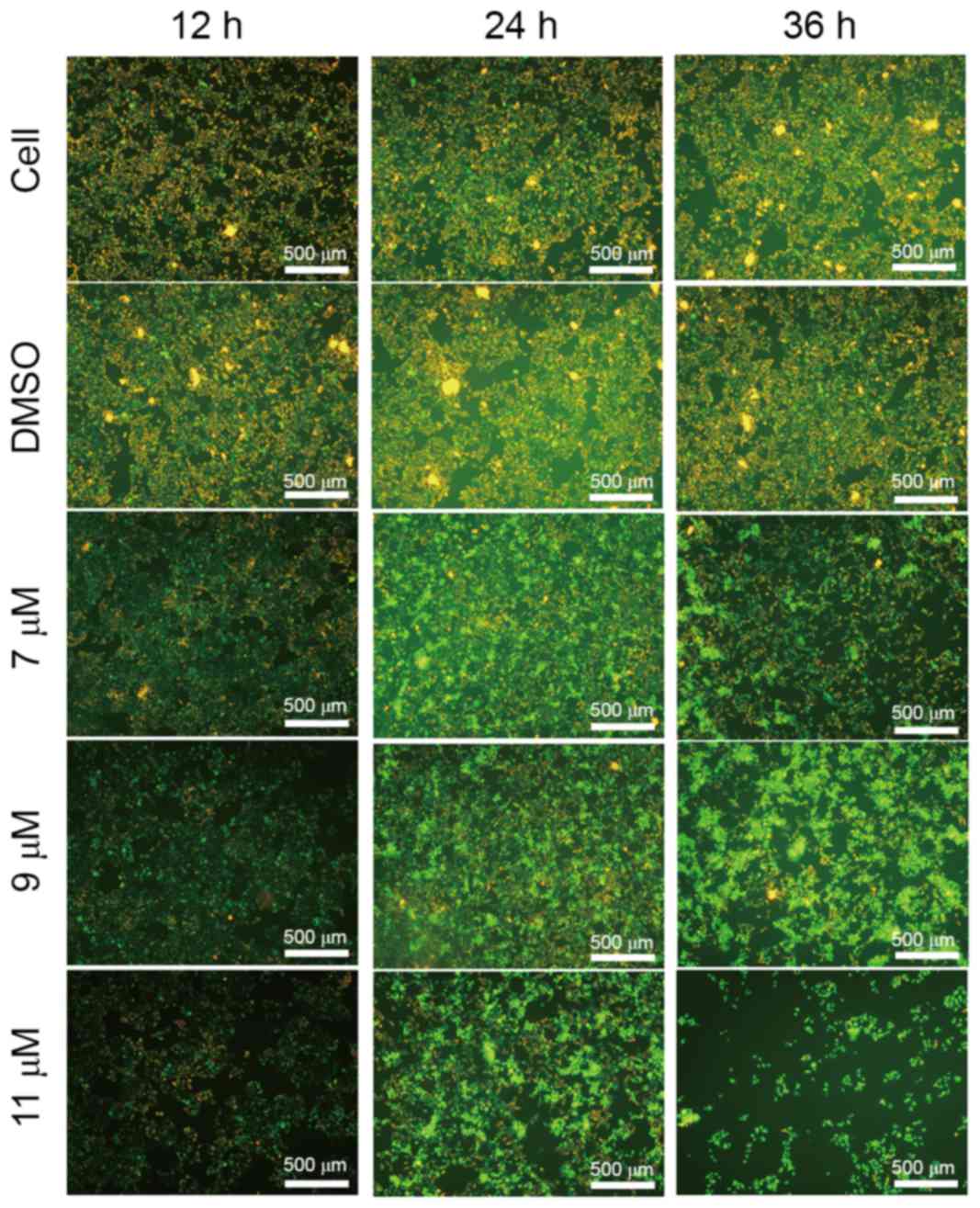
performed annexin V/PI double staining. As presented in Fig. 2B, there were markedly few red
(PI-stained) and green (annexin V-stained) fluorescent cells in the
control and DMSO groups, whereas the red fluorescence was increased
with the increase of oleanolic acid derivative concentration and
the extension of treatment time. The red fluorescence was highest
at 11 µM oleanolic acid derivative treatment for 36 h. These
results revealed that the oleanolic acid derivative induced
membrane disintegrity of SMMC-7721 cells in a dose- and
time-dependent manner.
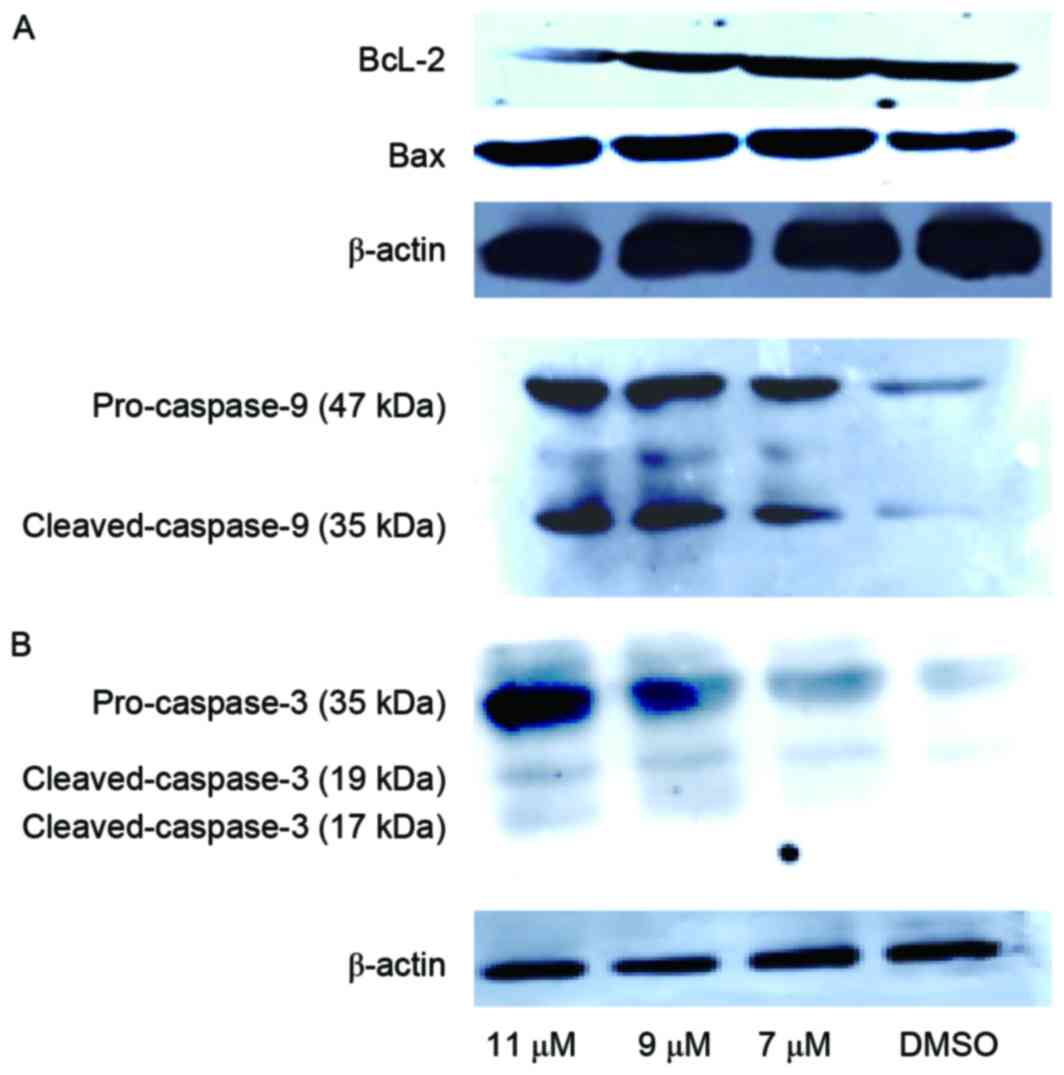
Oleanolic acid derivative induces activation of
the intrinsic apoptosis pathway in SMMC-7721 cells. To explore
the mechanism underlying apoptosis induction by the oleanolic acid
derivative, the present study determined the change of
apoptosis-associated protein expression levels in SMMC-7721 cells
following treatment with various concentrations of the oleanolic
acid derivative (5, 9 and 11 µM) for 48 h by western blotting. The
results revealed that anti-apoptotic protein, Bcl-2, was
downregulated following 11 µM oleanolic acid derivative treatment
for 48 h. Conversely, the proapoptotic protein, Bax, was
upregulated following treatment of 5, 9 or 11 µM oleanolic acid
derivative for 48 h (Fig. 3A). It is
well known that caspase-3 is the ultimate executioner caspase of
apoptosis and that activated caspase-9 cleaves downstream caspases,
including caspase-3, to initiate the caspase cascade (9). Indeed, treatment of SMMC-7721 cells with
the oleanolic acid derivative for 48 h induced obvious cleavage of
caspase-9 and caspase-3 in a concentration-dependent manner
(Fig. 3B). These results indicated
that the oleanolic acid derivative induced the intrinsic pathway of
apoptosis in SMMC-7721 cells.
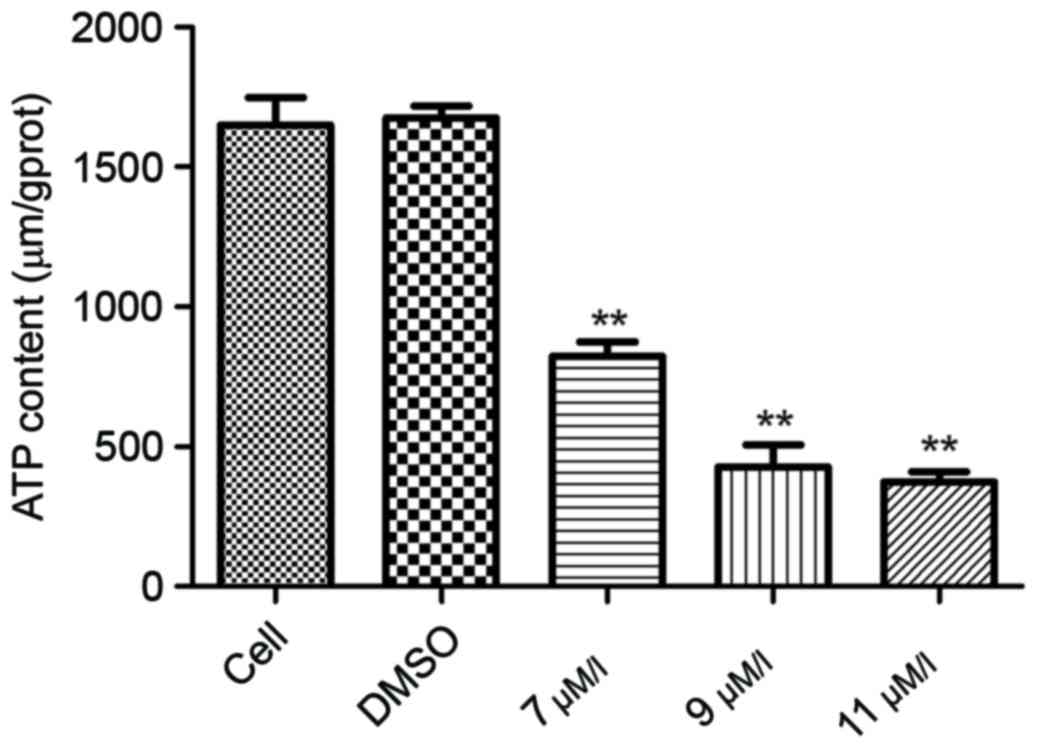
Oleanolic acid derivative reduces the
intracellular ATP expression level
Mitochondria serve a key role in activation of the
intrinsic cell apoptosis pathway. The activation of caspase-9
following oleanolic acid derivative treatment suggested that the
oleanolic acid derivative may disrupt mitochondrial function. To
investigate this hypothesis, the present study first assessed the
intracellular ATP expression level, a decrease of which indicates
an impairment of mitochondrial function. As presented Fig. 4, the ATP expression levels were
significantly decreased in a dose-dependent manner following
oleanolic acid derivative treatment. These results demonstrated
that the oleanolic acid derivative impaired mitochondrial
function.
Oleanolic acid derivative treatment
results in loss of ΔΨm
Impairment of mitochondrial function is closely
associated with the loss of ΔΨm. Subsequently, the present study
treated SMMC-7721 cells with 0, 7, 9 or 11 µM oleanolic acid
derivative for 12, 24 and 36 h, and determined the ΔΨm using the
lipophilic cationic fluorescent probe JC-1 assay. Compared with the
blank and DMSO controls, the oleanolic acid derivative
significantly decreased the red/green fluorescence ratio in
SMMC-7721 cells in a dose- and time-dependent manner (Fig. 5). Furthermore, the cell adherence rate
declined markedly when the cells were exposed to 11 µM oleanolic
acid derivative. Numerous floating cells were removed during the
experiments (data not shown). These results indicated that the
oleanolic acid derivative induced a decrease of ΔΨm in SMMC-7721
cells.
Oleanolic acid derivative induces
Cyt-C release from the mitochondria
It is well known that Cyt-C release from the
mitochondria during apoptosis occurs upstream of DEVD-specific
caspase activation and independently of mitochondrial transmembrane
depolarization, indicating that a reduction in ΔΨm and caspase
activation occurs considerably later than Cyt-C release (10). In the present study, depolarization of
ΔΨm was revealed in SMMC-7721 cells exposed to the oleanolic acid
derivative for 12 h (Fig. 5).
Therefore, the present study detected the mitochondrial and
cytoplasmic content of Cyt-C in SMMC-7721 cells treated with
various concentrations of the oleanolic acid derivative for 12 h.
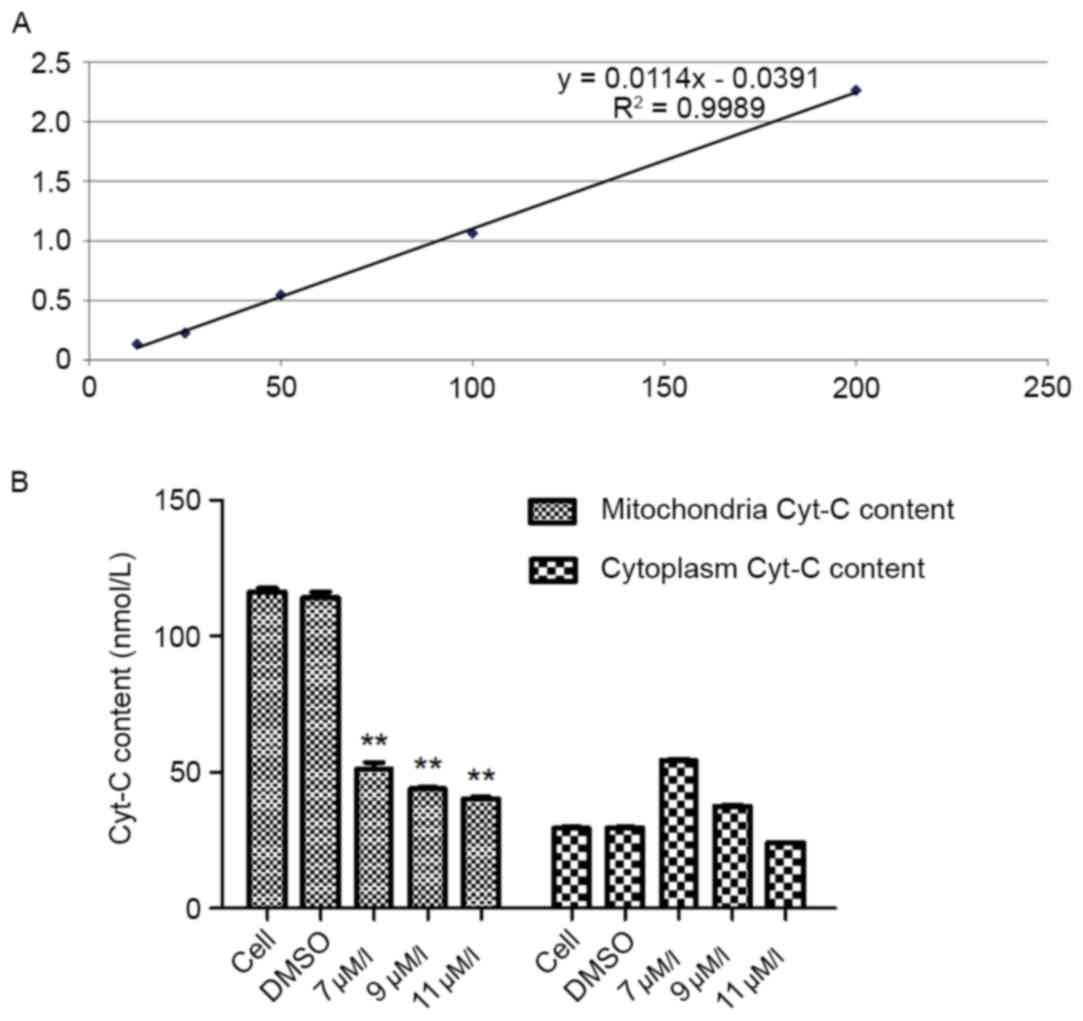
The standard curve regression equation was y=0.0114×-0.0391
(R2=0.9989; Fig. 6A). The
content of the sample was determined by the regression equation of
the standard curve of the reference standard. The results
demonstrated that when SMMC-7721 cells were treated with various
concentrations of the oleanolic acid derivative for 12 h, the
mitochondrial content of Cyt-C was decreased significantly in a
dose-dependent manner, with a 65.48% decrease by 11 µM oleanolic
acid derivative compared with the controls. In the cytoplasm, the
content of Cyt-C was increased when the cells were treated with 7
µM oleanolic acid derivative and then gradually decreased with 9
and 11 µM oleanolic acid derivative (Fig.
6B). These results demonstrated that the oleanolic acid
derivative induced Cyt-C release from the mitochondria in SMMC-7721
cells.
Discussion
The present study revealed that the oleanolic acid
derivative dose- and time-dependently inhibited the proliferation
of SMMC-7721, and HL-7702 cells. Furthermore, treatment of
SMMC-7721 cells with the oleanolic acid derivative resulted in cell
shrinkage, membrane blebbing and accumulation of small apoptosis
bodies, accompanied with upregulation of Bax and downregulation of
Bcl-2, and cleavage of caspase-9 and −3. Of note, treatment of
SMMC-7721 cells with the oleanolic acid derivative induced a
reduction in the intracellular ATP expression level, loss of ΔΨm
and Cyt-C release from the mitochondria. The results of the present
study suggested that the oleanolic acid derivative inhibited the
viability of SMMC-7721 cells by inducing activation of the
intrinsic cell apoptosis signaling pathway.
Firstly, the present study investigated the effect
of the oleanolic acid derivative on the proliferation of human
SMMC-7721 cells and revealed that the oleanolic acid derivative
induced apoptosis as demonstrated by the typical signs of
apoptosis, including cell shrinkage, chromatin condensation,
vacuolization in the cytoplasm and apoptotic bodies. Translocation
of phosphatidylserine to the outside of the cellular membrane is a
key step in apoptosis (11). The
annexin V-FITC/PI double-staining results revealed that oleanolic
acid derivative treatment resulted in the translocation of
phosphatidylserine. These results demonstrated that oleanolic acid
derivative treatment resulted in characteristic morphological
alterations of apoptosis.
Activation of caspases is the hallmark of apoptosis.
The present study revealed that the expression levels of the
proapoptotic protein, Bax, were upregulated, whereas the
anti-apoptotic protein, Bcl-2, was downregulated following
oleanolic acid derivative treatment. In addition, it was
demonstrated that caspase-3 and caspase-9 were activated, and
cleaved by the oleanolic acid derivative. Caspase-9 is one of the
most representative initiators of mitochondrial apoptosis. These
observations suggested that the oleanolic acid derivative triggers
activation of the intrinsic pathway of apoptosis (mitochondrial
apoptosis). It has been well established that mitochondria serve an
important role in the regulation of programmed cell death
(apoptosis) (12). There are
candidate natural compounds, in various stages of drug development,
that have been described to interfere with mitochondrial functions
in tumor cells, leading to cell cycle arrest and/or death by
apoptosis or necrosis. Therefore, the present study aimed to focus
on mitochondrial function to elucidate the anticancer molecular
mechanism underlying the oleanolic acid derivative.
It is well known that a decrease of the ΔΨm and
redistribution of Cyt-C is associated with mitochondrial function.
In addition, mitochondrial dysfunction is associated with cell
apoptosis (12). It has been reported
that certain anticancer compounds induce cancer cell apoptosis by
reducing the ΔΨm (12). Within tumor
cell subpopulations, a higher ΔΨm contributes to enhanced tumor
progression and expansion (13). JC-1
exhibits ΔΨm-dependent accumulation in the normal mitochondria and
emits strong red fluorescence; whereas in unhealthy mitochondria,
JC-1 emits a strong green fluorescence. The present study
determined whether the mitochondria were healthy or not by
observing the change in the red/green fluorescence ratio. The
results of the present study indicated that the oleanolic acid
derivative-induced cell death was associated with a loss of
ΔΨm.
Released Cyt-C from the mitochondria to the
cytoplasm may initiate caspase activation (14). Previous studies have revealed the
decreased content of Cyt-C in the mitochondrial fractions and that
the increased content of Cyt-C in the cytosolic fractions are
involved in cell death (15–18). The results of the present study
indicated that the content of Cyt-C in the cytosolic fraction
decreased in a dose-dependent manner at 12 h after oleanolic acid
derivative treatment. In addition, it has been reported that the
Cyt-C content in the cytosolic fraction increased at 2 h, but was
subsequently decreased at 6 h due to heat treatment in Tobacco
Bright-Yellow 2 cells (19). Similar
findings have been reported in cerebellar granule cells (20). However, it has been demonstrated that
there is a dynamic change in Cyt-C expression levels in the
cytosolic and mitochondrial fractions. Vacca et al (19) revealed that caspase-like proteases are
able to degrade Cyt-C in the cytoplasm. Therefore, it is possible
that Cyt-C in the cytosolic fraction was degraded by caspase-like
proteases, which were increased by the oleanolic acid derivative at
12 h after treatment.
In the presence of ATP/deoxyadenosine triphosphate,
Cyt-C binding to apoptotic protease activating factor-1 triggers
the activation of caspase-9 and ATP accelerates caspase-3
activation (21,22). Once activated, caspase-9 cleaves
caspase-3 to induce cell death. Furthermore, the mitochondria serve
an important role in the production of ATP. The results of the
present study demonstrated that the ATP content decreased
significantly following oleanolic acid derivative treatment in a
dose-dependent manner, which is consistent with the previous
observation in bladder cancer cells treated with high-dose
chemotherapeutics (23). The majority
of cytotoxic anticancer agents induce cell death via the
mitochondrial signaling pathway (24). The decrease in ΔΨm, redistribution of
Cyt-C and decrease of the intracellular ATP expression level
indicate that the oleanolic acid derivative induces mitochondrial
dysfunction, thereby reducing the cell viability of cancer
cells.
In conclusion, the oleanolic acid derivative
promotes cell apoptosis of SMMC-7721 cells by inducing
mitochondrial dysfunction. The results of the present study suggest
that oleanolic acid derivatives may serve as potential therapeutic
agents for human HCC.
Acknowledgements
The present study was supported by the Agricultural
science and technology achievements transformation projects (grant
no. 2014GB2A300003).
References
|
1
|
El-Serag HB: Epidemiology of viral
hepatitis and hepatocellular carcinoma. Gastroenterology.
142:1264–1273.e1. 2012. View Article : Google Scholar
|
|
2
|
Bruix J and Sherman M: American
Association for the Study of Liver Diseases: Management of
hepatocellular carcinoma: An update. Hepatology. 53:1020–1022.
2011. View Article : Google Scholar
|
|
3
|
Ng IO, Liu CL, Fan ST and Ng M: Expression
of P-glycoprotein in hepatocellular carcinoma. A determinant of
chemotherapy response. Am J Clin Pathol. 113:355–363. 2000.
View Article : Google Scholar
|
|
4
|
Vitale A, Volk ML, Pastorelli D, Lonardi
S, Farinati F, Burra P, Angeli P and Cillo U: Use of sorafenib in
patients with hepatocellular carcinoma before liver
transplantation: A cost-benefit analysis while awaiting data on
sorafenib safety. Hepatology. 51:165–173. 2010. View Article : Google Scholar
|
|
5
|
Liu L, Fu J, Li T, Cui R, Ling J, Yu X, Ji
H and Zhang Y: NG, a novel PABA/NO-based oleanolic acid derivative,
induces human hepatoma cell apoptosis via a ROS/MAPK-dependent
mitochondrial pathway. Eur J Pharmacol. 691:61–68. 2012. View Article : Google Scholar
|
|
6
|
Wei J, Liu M, Liu H, Wang H, Wang F, Zhang
Y, Han L and Lin X: Oleanolic acid arrests cell cycle and induces
apoptosis via ROS-mediated mitochondrial depolarization and
lysosomal membrane permeabilization in human pancreatic cancer
cells. J Appl Toxicol. 33:756–765. 2013. View Article : Google Scholar
|
|
7
|
Zheng QY, Li PP, Jin FS, Yao C, Zhang GH,
Zang T and Ai X: Ursolic acid induces ER stress response to
activate ASK1-JNK signaling and induce apoptosis in human bladder
cancer T24 cells. Cell Signal. 25:206–213. 2013. View Article : Google Scholar
|
|
8
|
Arur S, Uche UE, Rezaul K, Fong M,
Scranton V, Cowan AE, Mohler W and Han DK: Annexin I is an
endogenous ligand that mediates apoptotic cell engulfment. Dev
Cell. 4:587–598. 2003. View Article : Google Scholar
|
|
9
|
Kuida K: Caspase-9. Int J Biochem Cell
Biol. 32:121–124. 2000. View Article : Google Scholar
|
|
10
|
Bossy-Wetzel E, Newmeyer DD and Green DR:
Mitochondrial cytochrome c release in apoptosis occurs upstream of
DEVD-specific caspase activation and independently of mitochondrial
transmembrane depolarization. EMBO J. 17:37–49. 1998. View Article : Google Scholar
|
|
11
|
Mourdjeva M, Kyurkchiev D, Mandinova A,
Altankova I, Kehayov I and Kyurkchiev S: Dynamics of membrane
translocation of phosphatidylserine during apoptosis detected by a
monoclonal antibody. Apoptosis. 10:209–217. 2005. View Article : Google Scholar
|
|
12
|
Joza N, Susin SA, Daugas E, Stanford WL,
Cho SK, Li CY, Sasaki T, Elia AJ, Cheng HY, Ravagnan L, et al:
Essential role of the mitochondrial apoptosis-inducing factor in
programmed cell death. Nature. 410:549–554. 2001. View Article : Google Scholar
|
|
13
|
Houston MA, Augenlicht LH and Heerdt BG:
Stable differences in intrinsic mitochondrial membrane potential of
tumor cell subpopulations reflect phenotypic heterogeneity. Int J
Cell Biol. 2011:9785832011. View Article : Google Scholar
|
|
14
|
Hao Z, Duncan GS, Chang CC, Elia A, Fang
M, Wakeham A, Okada H, Calzascia T, Jang Y, You-Ten A, et al:
Specific ablation of the apoptotic functions of cytochrome C
reveals a differential requirement for cytochrome C and Apaf-1 in
apoptosis. Cell. 121:579–591. 2005. View Article : Google Scholar
|
|
15
|
Samuel S, Tumilasci VF, Oliere S, Nguyên
TL, Shamy A, Bell J and Hiscott J: VSV oncolysis in combination
with the BCL-2 inhibitor obatoclax overcomes apoptosis resistance
in chronic lymphocytic leukemia. Mol Ther. 18:2094–2103. 2010.
View Article : Google Scholar
|
|
16
|
Barbu EM, Shirazi F, McGrath DM, Albert N,
Sidman RL, Pasqualini R, Arap W and Kontoyiannis DP: An
antimicrobial peptidomimetic induces Mucorales cell death through
mitochondria-mediated apoptosis. PLoS One. 8:e769812013. View Article : Google Scholar
|
|
17
|
Jang JH, Cho YC, Kim KH, Lee KS, Lee J,
Kim DE, Park JS, Jang BC, Kim S, Kwon TK and Park JW: BAI, a novel
Cdk inhibitor, enhances farnesyltransferase inhibitor
LB42708-mediated apoptosis in renal carcinoma cells through the
downregulation of Bcl-2 and c-FLIP (L). Int J Oncol. 45:1680–1690.
2014. View Article : Google Scholar
|
|
18
|
Wang X, Beitler JJ, Wang H, Lee MJ, Huang
W, Koenig L, Nannapaneni S, Amin AR, Bonner M, Shin HJ, et al:
Honokiol enhances paclitaxel efficacy in multi-drug resistant human
cancer model through the induction of apoptosis. PLoS One.
9:e863692014. View Article : Google Scholar
|
|
19
|
Vacca RA, Valenti D, Bobba A, Merafina RS,
Passarella S and Marra E: Cytochrome c is released in a reactive
oxygen species-dependent manner and is degraded via caspase-like
proteases in tobacco Bright-Yellow 2 cells en route to heat
shock-induced cell death. Plant Physiol. 141:208–219. 2006.
View Article : Google Scholar
|
|
20
|
Bobba A, Atlante A, Giannattasio S,
Sgaramella G, Calissano P and Marra E: Early release and subsequent
caspase-mediated degradation of cytochrome c in apoptotic
cerebellar granule cells. FEBS Lett. 457:126–130. 1999. View Article : Google Scholar
|
|
21
|
Li P, Nijhawan D, Budihardjo I,
Srinivasula SM, Ahmad M, Alnemri ES and Wang X: Cytochrome c and
dATP-dependent formation of Apaf-1/caspase-9 complex initiates an
apoptotic protease cascade. Cell. 91:479–489. 1997. View Article : Google Scholar
|
|
22
|
Zou H, Li Y, Liu X and Wang X: An
APAF1.cytochrome c multimeric complex is a functional apoptosome
that activates procaspase-9. J Biol Chem. 274:11549–11556. 1999.
View Article : Google Scholar
|
|
23
|
Yoshida T, Okuyama H, Nakayama M,
Nishimura K, Nonomura N and Inoue M: Rapid decrease of ATP followed
by necrosis-like cell death in bladder cancer cells after exposure
to high-dose chemotherapeutics used in intravesical therapy. Cancer
Res. 74:13412014. View Article : Google Scholar
|
|
24
|
Galluzzi L and Kroemer G: Necroptosis: A
specialized pathway of programmed necrosis. Cell. 135:1161–1163.
2008. View Article : Google Scholar : PubMed/NCBI
|