Introduction
Multimodal treatment consisting of surgery,
chemotherapy and radiotherapy is used to treat advanced, recurrent
cancer patients, yet a definitive cure remains difficult to
achieve. However, a new treatment method, cancer immunotherapy,
which uses the host's immune system to fight against cancer, is
being researched and developed. Tumor antigens recognized by
CD8-positive cytotoxic T lymphocytes (T cells) were discovered in
the 1990s (1). This discovery has
advanced the development of cancer-specific immunostimulatory
treatments, including cancer vaccine therapy, adoptive immune cell
therapy and antibody therapy (2).
Immunotherapy studies in animal models have demonstrated induction
of tumor antigen-specific immunity and antitumor effects. However,
Rosenberg et al (3) reported
that only a response rate of a few percent has been demonstrated in
human clinical trials. Multiple immune escape mechanisms, including
the presence of immunosuppressive cells, loss of human leukocyte
antigen (HLA) class I antigens and immunological tolerance, have
been suggested as possible causes of the low efficacy of
immunostimulatory treatments in humans. Therefore, combined cancer
vaccines and adjuvant immunotherapies, including OK-432 and
poly-ICLC is widely performed to enhance its effect. Furthermore,
OK-432 has been reported to enhance the effect of chemotherapy
(4), while poly-ICLC has been
reported to rapidly and potently induce NY-ESO-1-specific immune
responses (5).
Since 2009, in search of biomarkers for predicting
therapeutic effect, a cancer vaccine clinical trial has been
conducted with the cancer antigen, melanoma antigen gene-A4
(MAGE-A4), using the full-length protein as an immunogenic agent
(clinical trial registration no. UMIN000001999). Numerous studies
have used antigen-specific humoral immune response as a biomarker
for cancer vaccines (6–8). However, the majority of these studies
have only evaluated IgG antibodies directed against an immunogenic
agent. To the best of our knowledge, there are no studies that have
reported on biomarkers that reflect therapeutic effect. From the
beginning of the cancer vaccine clinical trial, the levels of IgG
subclass (IgG1, IgG2, IgG3 and IgG4) and IgE antibodies were
measured and used as biomarkers for predicting therapeutic effect.
Antigen-specific IgG1, IgG2 and IgG3 antibodies are produced by B
lymphocytes (B cells) when the type 1 T helper (Th1) cell-mediated
immune response, including tumor immunity, is favored.
Antigen-specific IgG4 and IgE antibodies are produced by B cells
when the type 2 T helper (Th2) cell-mediated humoral immune
response, including allergic reactions, is favored (9,10).
Therefore, IgG1, IgG2 and IgG3 were classified as Th1-associated
antibodies, while IgG4 and IgE were classified as Th2-associated
antibodies.
The objective of the present study was to assess the
effects of cancer vaccination on the Th1-associated MAGE-A4
specific antibodies IgG1, IgG2 and IgG3 as well as on
Th2-associated MAGE-A4 specific antibodies IgG4 and IgE, in
patients vaccinated with the cancer vaccine. This was evaluated by
measuring: i) Levels of MAGE-A4 specific IgG subclass and IgE
antibodies as biomarkers and ii) time-course changes of the
antigen-specific humoral immune response using enzyme-linked
immunosorbent assay (ELISA).
Materials and methods
Study design
The clinical trial was an open-label trial. The
subjects of this trial were patients with locally advanced,
recurrent or metastatic tumors that were histologically confirmed
as malignant and were resistant to standard therapy. Eligibility
criteria were as follows: i) Patients with tumors expressing
MAGE-A4 antigen, assessed by immunohistochemistry; ii) an Eastern
Cooperative Oncology Group performance status of 0–2 (11); iii) an age of ≥20 years; iv) >4
months survival expected; v) adequate bone-marrow, cardiac,
pulmonary, hepatic and renal functions; and vi) the patient had no
desire to become pregnant. Exclusion criteria were as follow: i)
Positive for human immunodeficiency virus antibody; ii) multiple
malignant diseases; iii) concurrent autoimmune disease; iv) a past
history of anaphylaxis; v) active metastasis to the central nervous
system; vi) concurrent anticancer therapy during the 4 weeks prior
to the initiation of the trial (except with an anticancer drug that
does not require drug breaks or hormone agents), including systemic
steroids, immunosuppressive agents, irradiation or surgery for
primary lesions; vii) pregnancy or breastfeeding and viii) a
decision by the principal investigator or physician in charge that
the patient was unsuitable. The patient recruitment began in August
2009 and ended in March 2013. It was confirmed that the patients'
tumors expressed the MAGE-A4 antigen, which was assessed using
immunohistochemistry (IHC), as described later. The patients were
divided into 3 groups in the order of registration: Group 1
patients (n=3) received 100 µg cholesteryl pullulan (CHP)-MAGE-A4
vaccine; group 2 (n=3) received 300 µg CHP-MAGE-A4 vaccine; group 3
(n=12) received 300 µg CHP-MAGE-A4 vaccine and 50 µg OK-432 (Chugai
Pharmaceutical Co., Ltd., Tokyo, Japan) that was used as an immune
adjuvant. Patients were injected subcutaneously for a total of 6
cycles at 2-week intervals (one injection per one cycle). Complete
written informed consent was obtained from all patients at the time
of enrollment. The study was approved by the Ethics Committee of
Hokkaido University Graduate School of Medicine (Sapporo, Japan).
Patient characteristics of the patients in Groups 1 and 2 were
reported previously (12).
Evaluation of clinical responses
To evaluate the clinical response, computed
tomography (CT) scans were taken prior to the first vaccination and
after the fourth vaccination. All measurable lesions were
classified using the modified Response Evaluation Criteria in Solid
Tumors (mRECIST). mRECIST are original criteria consisting of
adaptations to the RECIST based upon the recommendations of the
Cancer Vaccine Clinical Trial Working Group (13). mRECIST were as follows: i) Complete
response (CR), disappearance of all target lesions; ii) partial
response (PR), compared with the sum of the largest diameters of
the target lesions at the time of registration in this trial, the
total sum of the largest diameters of the target lesions, new
target lesions and non-target lesions (increased to ≥10 mm),
decreased by ≥30%; iii) stable disease (SD), tumor shrinkage is
inadequate to be PR and tumor growth is insufficient to be
progressive disease (PD), compared with the minimum sum of the
largest diameters of the target lesions following treatment; and
iv) PD, compared with the minimum sum of the largest diameters of
target lesions following treatment, the total sum of the largest
diameter of target lesions, new lesions and non-target lesions
(increased to ≥10 mm), increased by ≥20%. mRECIST do not provide a
PD classification when new lesions appear alone.
Subject patients of the study
The subjects of the present study were the 12
patients who were enrolled in group 3.
Serum samples
Patient peripheral blood was collected prior to the
initial vaccination (baseline), at 2 weeks after each subsequent
vaccination and at 4 weeks after the last (6th) vaccination. Plasma
was collected using EDTA as an anticoagulant and was centrifuged
for 6 min at 240 × g at 18°C. The supernatant was then centrifuged
for 10 min at 670 × g at 4°C. Plasma was then stored at −80°C until
analysis.
Preparation of CHP-MAGE-A4
Full length MAGE-A4 cDNA was cloned into a pET
vector (ImmunoFrontier, Inc., Tokyo, Japan) and introduced into
Escherichia coli. Expression of His-MAGE-A4 protein was
induced by the addition of isopropyl-L-thio-β-D-galactopyranoside
to the bacterial cell culture; produced protein was recovered and
highly purified using a combination of chromatographic techniques,
including metal chelating affinity, anion exchange, size exclusion
and hydroxyapatite chromatography. CHP was synthesized by a
chemical reaction between pullulan (average molecular weight, 100
kDa) and cholesterol isocyanate in pyridine/dimethyl sulfoxide
solution (Nippon Oil and Fats Co., Ltd., Tokyo, Japan). After
purification by extraction and precipitation, resultant CHP was
emulsified in water and subsequently freeze-dried. When redissolved
in water or buffers, CHP spontaneously forms nanoparticles
(14–17). These nanoparticles (20–50 nm) contain
the hydrophobic domains of cholesterol groups internally, which
associate with hydrophobic regions of the MAGE-A4 protein, forming
a stable complex in solution (14–17). This
complex of protein and CHP was used as the CHP-MAGE-A4 vaccine.
These vaccines were produced by ImmunoFrontier, Inc. (Tokyo, Japan)
and kindly provided by the Department of Immuno-Gene Therapy at Mie
University Graduate School of Medicine (Tsu, Japan).
Detection of MAGE-A4 expression in
tumors
To investigate MAGE-A4 antigen expression in tumors
to determine whether patients could be enrolled in the present
study, each patient's formalin-fixed (in 10–15% formalin overnight
at room temperature), paraffin-embedded tissue sections (thickness,
1.5 µm) were subjected to IHC analysis. These tissues were provided
by the hospital where patient had previously received treatment.
Immunohistochemical reactions were performed using the
streptavidin-biotin-peroxidase method (18). The primary antibody, molluscum
contagiosum virus 1 (MCV-1; 2.8 mg/ml), provided by Mie University,
was used diluted to 1:2,000 in Dako Antibody Diluent and Protein
Block Serum-Free (Dako; Agilent Technologies, Inc., Santa Clara,
CA, USA). MCV-1 is a monoclonal antibody generated in mice
immunized with a human MAGE-A4 recombinant protein. Archival tissue
sections were deparaffinized in xylene, and rehydrated in a graded
series of ethanol solutions. After washing in deionized water,
antigens were unmasked by incubation for 7 min with citric acid
buffer (pH 6.0) in a pressure cooker at 120°C. After washing in
deionized water, endogenous peroxidase activity was blocked by
incubation for 5 min with 3% hydrogen peroxide in methanol at room
temperature. After washing in deionized water and high-wash-PBS-T
(pH 7.7, 0.44 M NaCl, 0.1% Tween-20 in PBS), specimens were
incubated overnight at 4°C with the primary antibody described
above. After washing in high-wash-PBS-T, sections were incubated
with peroxidase-labeled goat anti-mouse and anti-rabbit IgG (Fab')
polyclonal antibody [Histofine Simple Stain MAX PO (MULTI);
Nichirei Biosciences, Inc., Tokyo, Japan] for 30 min at room
temperature. After washing in high-wash-PBS-T, immunohistochemical
reactions were visualized with freshly prepared
3,3′-diaminobenzidine tetrahydrochloride (Histofine SAB-PO [M] kit;
Nichirei Biosciences, Inc.). Subsequently, slides were
counterstained with hematoxylin (undiluted solution) for 20 sec at
room temperature and mounted with coverslips. All slides were
observed using a light microscope at magnifications of ×40, ×100
and ×400.
If stained cells were observed, the specimen was
labeled ‘positive’, regardless of the degree of positivity, or the
localization of staining in the stained cells (nucleus, cytoplasm
or nucleus and cytoplasm). MAGE-A4 expression was confirmed with
positive identification by two physicians, one being a
pathologist.
Detection of antibody responses to the
MAGE-A4 protein
Specific whole IgG antibodies against MAGE-A4 in the
sera were measured using ELISA. The MAGE-A4 recombinant protein in
PBS was adsorbed onto immuno plates (442404; Nunc; Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany) at a concentration of 20 ng/50
µl/well overnight at 4°C. Plates were washed in PBS with 0.05%
Tween-20 and were then blocked for 2 h at room temperature with 200
µl/well 1% bovine serum albumin (Sigma-Aldrich; Merck KGaA)
(BSA)/PBS. Serum samples were diluted 1:100, 1:400 and 1:1,600 in
1% BSA/PBS. After washing, 100 µl serum/well was added as the
primary antibody and was incubated overnight at 4°C. After washing,
100 µl/well of 1:4,000 diluted goat anti-human IgG (H+L
chain)-horseradish peroxidase (HRP) (cat. no. 206; Medical &
Biological Laboratories Co., Ltd., Nagoya, Japan) in 1% BSA/PBS was
added as the secondary antibody and was incubated for 5 h at 4°C.
Plates were washed and incubated with 100 µl/well TMB Substrate
(Pierce; Thermo Fisher Scientific, Inc., Waltham, MA, USA) for 3
min at room temperature. Subsequently, 100 µl/well 0.18 M
H2SO4 was added and the optical density (OD)
of the sample was immediately read in a microplate
spectrophotometer at a wavelength of 450 nm (SpectraMax 190;
Molecular Devices, LLC, Sunnyvale, CA, USA). The cutoff OD 450
absorption value was calculated according to the following
equation: Cutoff value=mean OD value of a 1:400 diluted pooled
serum sample from healthy donors (n=24)+1.645× standard deviation
(SD). The cutoff value was determined to be 0.288. Similarly,
healthy donor sera were used as controls to correct errors in every
examination and patients' OD values were corrected using the
resultant calculated error ratios. The healthy donors comprised
doctors and other medical staff from the department of
Gastroenterological Surgery II, Division of Surgery, Hokkaido
University Graduate School of Medicine, Sapporo, Japan (age range,
20–60 years; 18 male and 6 female). Healthy donor accrual began and
finished in April 2009. Written informed consent was obtained from
these donors.
For cases with negative serum response before
vaccination (referred to as ‘baseline negative’ hereafter),
positivity was defined when OD values of the serum samples at
400-fold dilution exceeded the aforementioned cutoff value before
the end of the 6th vaccination. For cases with positive serum
response before vaccination (referred to as ‘baseline positive’
hereafter), positivity was defined when OD values of the serum
samples at 400-fold dilution increased by >100% before the end
of the 6th vaccination.
Detection of IgG subclass antibody
responses to the MAGE-A4 protein
The IgG subclass antibody response to the MAGE-A4
protein was detected with ELISA using the aforementioned method.
The secondary antibodies were polyclonal sheep anti-human IgG1
(dilution, 1:25,600; cat. no. AP006), IgG2 (dilution, 12,800; cat.
no. AP007), IgG3 (dilution, 1:12,800; cat. no. AP008) and IgG4
(dilution, 1:12,800; cat. no. AP009) (H+L chain)-HRP (Binding Site,
Birmingham, UK).
The following cutoff values of IgG subclass
antibodies were calculated using the aforementioned method: IgG1,
0.192; IgG2, 0.140; IgG3, 0.076; and IgG4, 0.004. Similarly,
healthy donor plasma samples were used as controls to correct
errors in every examination, and patient OD values were corrected
using the resultant calculated error ratios.
As in the aforementioned method, positivity in
baseline negative cases was determined when OD values exceeded the
cut-off values before the end of the 6th vaccination. Likewise,
positivity in baseline positive cases was determined when OD values
increased by >100% before the end of the sixth vaccination.
In all of the tests, the serum samples of the three
aforementioned donors close to the median OD value, collected while
calculating the cutoff value, were used as controls. For IgG1 and
IgG2, the OD values were adjusted according to the error ratio.
The OD values of IgG subclass antibodies are lower
compared with the OD values of IgG when the dilution concentration
of the patient's serum samples is the same. Therefore, the
calculation of the IgG subclass cutoff value and the
post-vaccination evaluation were conducted using OD values of serum
samples at 100-fold dilution.
Detection of IgE antibody responses to
the MAGE-A4 protein
Specific IgE antibodies against MAGE-A4 in the
plasma were measured using ELISA, according to the aforementioned
method, with the following differences: As the primary antibody,
the collected plasma samples were diluted from 1:40 to 1:640 in 1%
BSA/PBS. As the secondary antibody, 100 µl/well of 1:1,000 diluted
rabbit polyclonal anti-human IgE (A0094; Dako; Agilent
Technologies, Inc.) in 1% BSA/PBS was added and incubated for 5 h
at 4°C. After washing, 100 µl/well of 1:100 diluted goat polyclonal
anti-rabbit immunoglobulin (K1491; EnVision kit-HRP; Dako; Agilent
Technologies, Inc.) in 1% BSA/PBS was added as a tertiary antibody.
Samples were incubated for 40 min at room temperature. After
washing, the OD was measured as described above. Since there were
non-specific reactions in the negative control wells, the
evaluation of IgE was performed qualitatively rather than
quantitatively. Specific IgE antibodies were considered to be
positive when the OD values of 40-fold diluted serum samples
following vaccination were >150% of the OD values of baseline
serum sample.
Statistical analysis
Data are presented as the median (range).
Correlation was assessed using Pearson's product-moment correlation
coefficient. The degree of correlation was determined according to
the absolute value of the correlation coefficient R as follows: No
correlation, 0≤R<0.2; weak correlation, 0.2≤R<0.4; moderate
correlation, 0.4≤R<0.7 and strong correlation, 0.7≤R<1.0. The
Kruskal-Wallis test followed by a Games-Howel post hoc test was
used to detect significant differences between the ELISA results.
The Kaplan-Meier method was used to estimate overall survival and
survival differences were analyzed by the log-rank test. P<0.05
was considered to indicate a statistically significant difference.
All statistical analyses were performed using StatView J version
5.0 (SAS Institute, Inc., Cary, NC, USA) and Microsoft Excel 2011
(Microsoft Corporation, Redmond, WA, USA).
Results
Patient characteristics
For the purpose of matching the administration
conditions (dose of CHP-MAGE-A4 and presence or absence of OK-432)
of the cancer vaccine, the focus of the present study was limited
to the 12 patients in group 3. Patient characteristics are
presented in Table I. There were 12
patients (9 males and 3 females) who were diagnosed with locally
advanced, recurrent or metastatic tumors that were histologically
confirmed as malignant and that were resistant to standard therapy
(7 colorectal, 1 breast, 1 pancreas, 1 bile duct, 1 malignant
mesothelioma and 1 gallbladder). The median age was 63 years
(range, 34–79 years).
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
|
|
|
|
| Anti MAGE-A4
antibody response |
|
|
|---|
|
|
|
|
|
|
|
|
|---|
| Case | Sex | Age, years | Tumor type | IgG | IgG1 | IgG2 | IgG3 | IgG4 | IgE | Clinical response
(mRECIST) | Survival time after
vaccination, days |
|---|
| 1 | M | 62 | Colon Ca | + | + | + | + | + | + | PD | 96 |
| 2 | F | 63 | Breast Ca |
|
|
|
|
|
| PD | 88 |
| 3 | F | 48 | Pancreas Ca | + | + |
| + |
|
| SD | 148 |
| 4 | M | 79 | Bile duct Ca | B |
| B |
|
|
| SD | 470 |
| 5 | M | 63 | Rectal Ca | +B | +B | + | +B | + |
| SD | 469 |
| 6 | M | 58 | Mesothelioma | + | + |
| + | + |
| SD | 509 |
| 7 | M | 78 | Gallbladder Ca | + | + | + | +B | + |
| SD | 141 |
| 8 | M | 34 | Rectal Ca | + | B |
|
| + |
| SD | 317 |
| 9 | F | 63 | Colon Ca | B | + |
| B |
|
| SD | 255 |
| 10 | M | 60 | Colon Ca | + | + | + | + | + |
| PD | 257 |
| 11 | M | 71 | Colon Ca | B | +B |
| B | + | + | PD | 105 |
| 12 | M | 71 | Colon Ca | + | + | + | + | + |
| PD | 167 |
| Positive conversion
ratio, % |
|
|
| 66.7 | 75.0 | 41.7 | 58.3 | 66.7 | 16.7 |
|
|
Time-dependent transition of
MAGE-A4-specific antibody responses
The positive conversion rates of anti-MAGE-A4 IgG
subclass and anti-MAGE-A4 IgE in the patient serum samples at the
time of completing the 6th vaccination are presented in Table I. The positive rate of Th1-associated
antibodies IgG1, IgG2 and IgG3 was 75% (9/12), 41.7% (5/12) and
58.3% (7/12), respectively. The positive rate of Th2-associated
antibodies IgG4 and IgE was 66.7% (8/12) and 16.7% (2/12),
respectively. Th1- and Th2-associated antibodies were induced
together in 7 cases (patients 1, 5, 6, 7, 10, 11 and 12); only
Th1-associated antibodies were induced in 2 cases (patients 3 and
9); only Th2-associated antibodies were induced in 1 case (patient
8); neither Th1- nor Th2-associated antibodies were induced in 2
cases (patients 2 and 4; Table I).
The pre- and post-vaccination time-dependent changes of OD values
obtained with ELISA for each IgG subclass of antibodies were
examined using the Kruskal-Wallis test followed by the Games-Howel
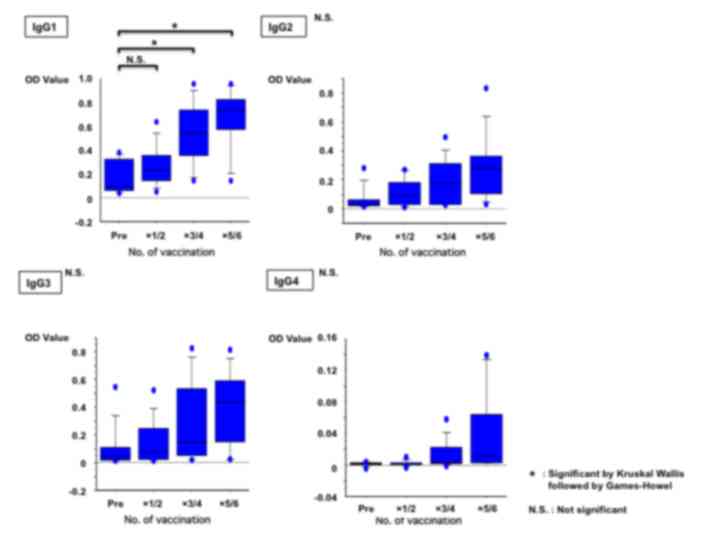
post hoc test. There was a significant increase in the OD values of
IgG1 following the completion of the scheduled vaccinations
(P=0.0001; Fig. 1).
The timing of the rise in antibody levels of the
Th1-associated antibodies, IgG1, IgG2 and IgG3, is described below.
Firstly, the median of the rising period for the 9 cases with
increased IgG1 level was observed upon completion of the 3rd
vaccination. Secondly, the median of the rising period for the 5
cases with increased IgG2 level was observed upon completion of the
2nd vaccination. Lastly, the median of the rising period for the 7
cases with increased IgG3 level was observed upon completion of the
4th vaccination. For the Th2-associated antibodies, IgG4 and IgE,
the median of the rising period for the 8 cases with increased IgG4
level was observed between the 4th and 5th vaccinations. The median
of the rising period for the 2 cases with positive IgE response was
observed upon completion of the 5th vaccination.
Correlations between antibody response
patterns elicited by MAGE A4 vaccinations
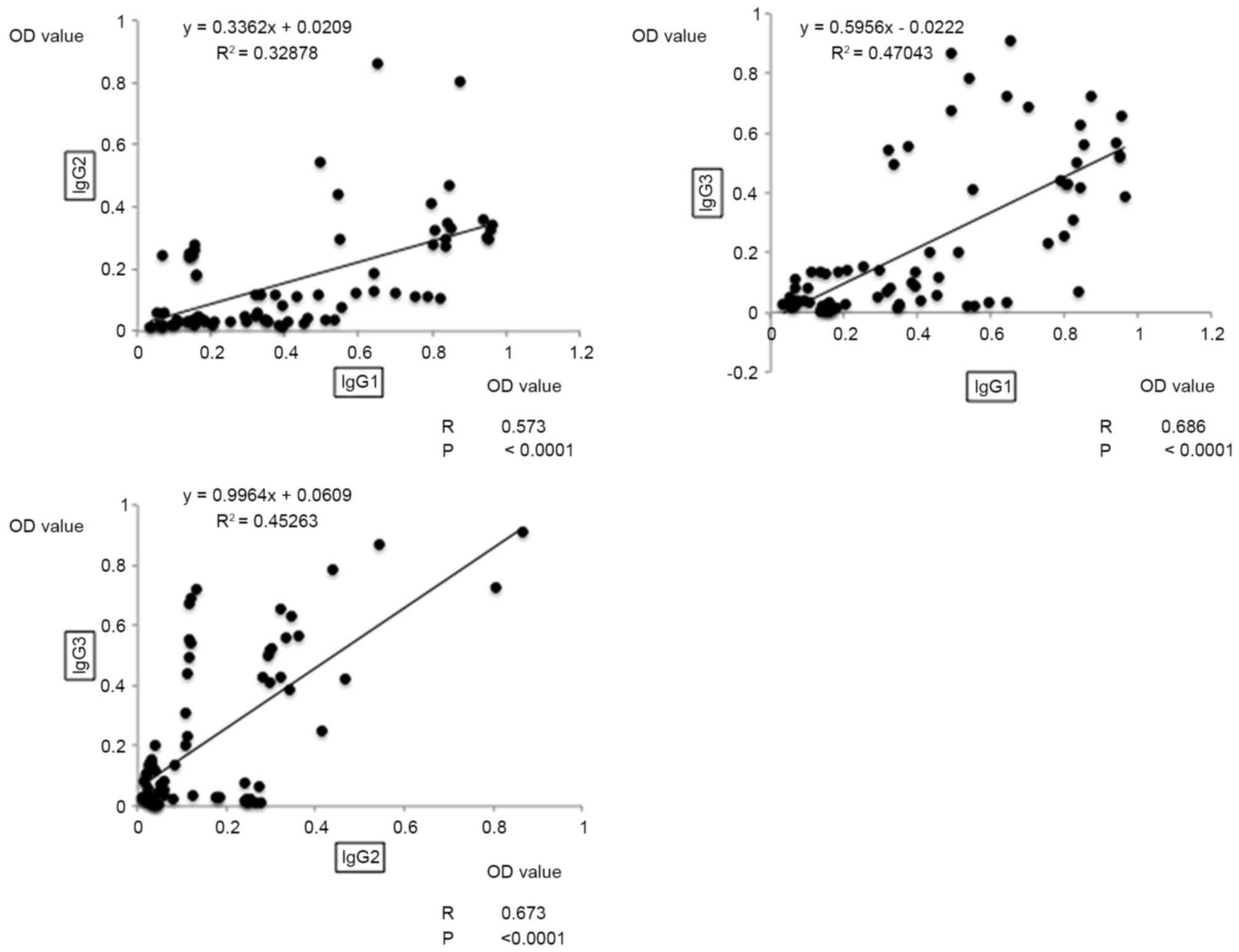
The correlations between each of the antibodies in
the patients' serum samples were examined using correlation
analysis of OD values based on the ELISA results. Among the
Th1-asssociated antibodies, the OD values of IgG1 and IgG3
presented the highest correlation, with a moderate positive
correlation coefficient of 0.686 (P<0.0001; Fig. 2). Additionally, a moderate positive
correlation was observed between IgG1 and IgG2 (R=0.573;
P<0.0001), and between IgG2 and IgG3 (R=0.673; P<0.001;
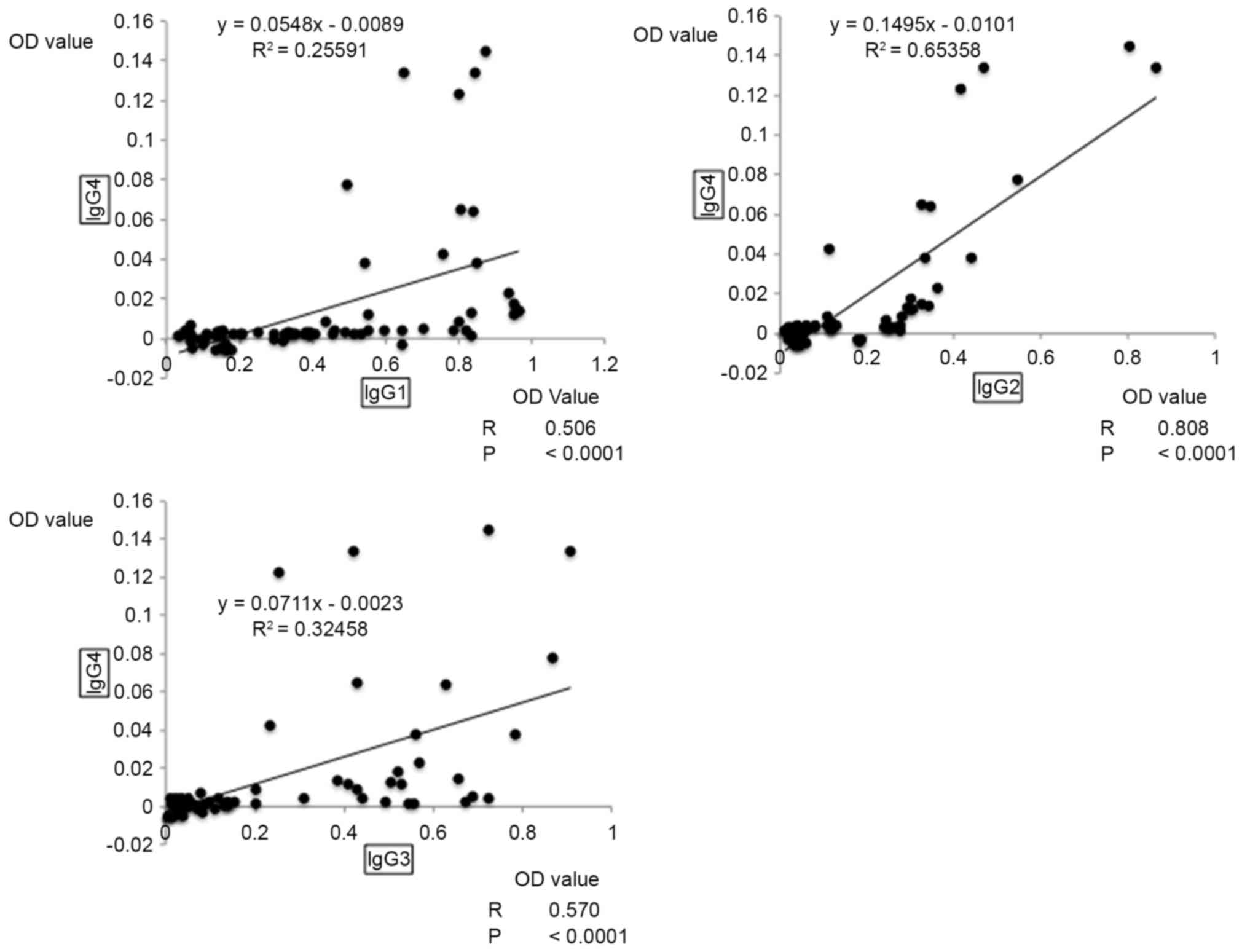
Fig. 2). As for the OD values of
antibodies associated with Th1 and Th2, a moderate positive
correlation was observed between IgG1 and IgG4 (R=0.506;
P<0.0001), and between IgG3 and IgG4 (R=0.570; P<0.001;
Fig. 3). When Th1-asssociated
antibodies were compared with Th2-associated antibodies, the
highest correlation was observed between IgG2 and IgG4, with a
strong positive correlation coefficient of 0.808 (P<0.0001;
Fig. 3).
Analysis of overall survival
The median survival time after vaccination was 211
days (range, 88–509 days). All 12 cases were classified as
cause-specific cases of mortality (Table
I).
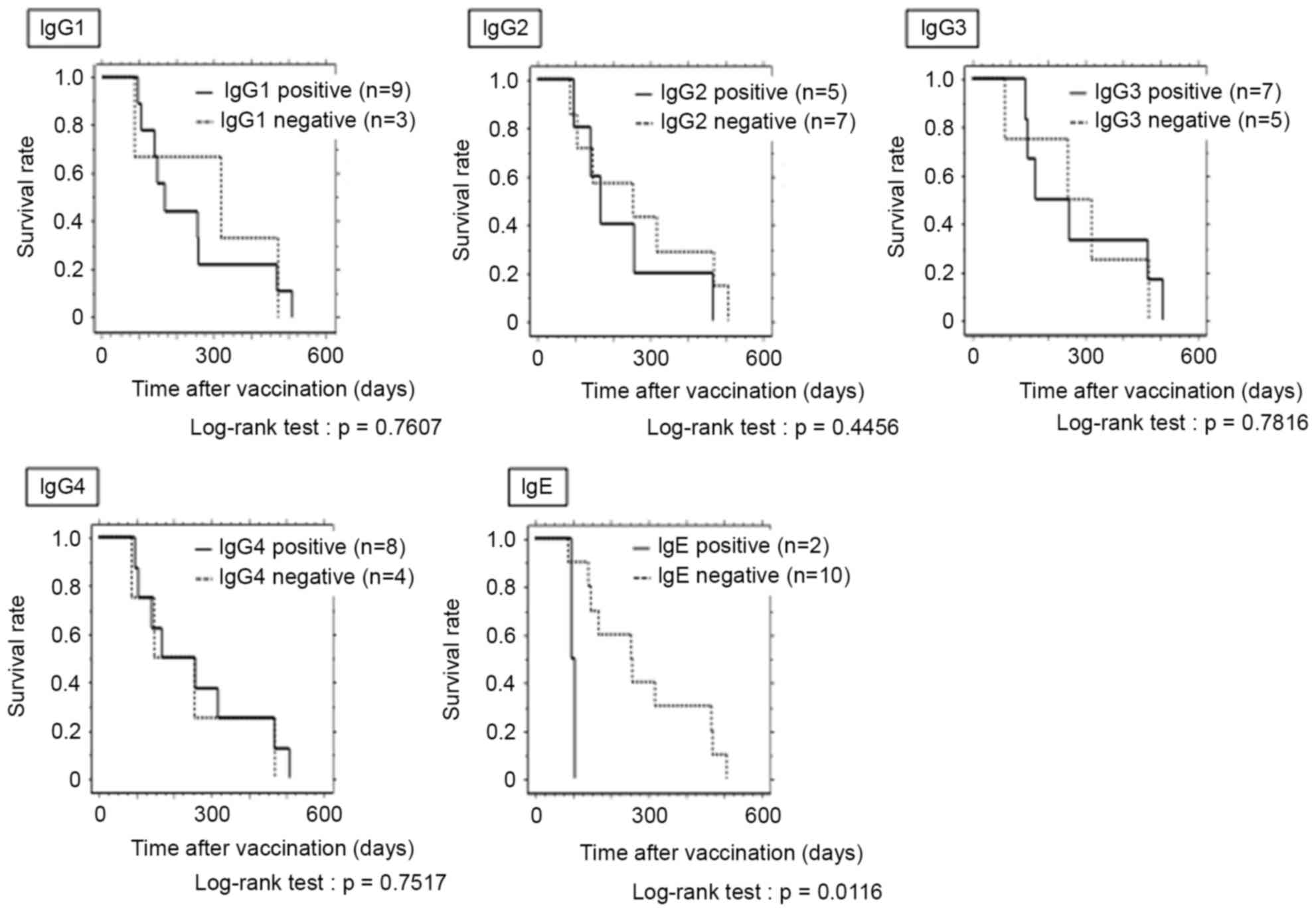
In terms of Th1-associated antibodies, IgG1, IgG2
and IgG3, the survival rate for positive patients (IgG1, n=9; IgG2,
n=5; IgG3, n=7) was not significantly higher compared with negative
patients (Fig. 4). In terms of
Th2-associated antibodies, IgG4 and IgE, no significant difference
in survival time was observed between IgG4 positive (n=8) and
negative patients. However, for IgE, the survival rate for positive
patients (n=2) was significantly lower compared with negative
patients (P=0.0116; Fig. 4).
Evaluation of therapeutic effect based
on the mRECIST classification
The evaluation of therapeutic effect based on the
mRECIST classification identified no cases of complete response or
partial response. There were 5 cases of progressive disease (PD)
and 7 cases of stable disease (SD).
No significant association was identified between
the presence or absence of positive conversion of IgG subclasses
and IgE on the one hand, and the therapeutic effect evaluated using
the mRECIST on the other (data not shown). There was also no
association between baseline positive cases and therapeutic effect
evaluated using the mRECIST (data not shown).
Discussion
The clinical efficacy of therapeutic cancer vaccines
has not been sufficiently demonstrated to date (19–21).
According to the RECIST criteria, which are used to evaluate the
pre- and post-treatment effect on tumor regression, the majority of
cases are classified as SD or PD (19–21). On
the other hand, traditional biomarkers of antitumor efficacy are
not necessarily reliable indicators in immunotherapy. In
immunotherapy, cases have been reported where a decrease in lesion
size was observed when therapy was continued despite the appearance
of a new lesion with PD classification (22). Therefore, biomarkers, including the
immune-related response criteria (19), which does not give a PD classification
when new lesions appear alone and which evaluates the sum of the
longest diameters of measurable lesions, have emerged (22). Since therapeutic efficacy cannot be
determined through imaging at present, the ideal approach is to
estimate the therapeutic efficacy of cancer vaccines using
biomarkers, including the antigen-specific cellular immune response
[whether there is an induction of antigen-specific cytotoxic T
lymphocytes (CTL)] and the antigen-specific humoral immune response
(production of antigen-specific antibodies), in order to select
cases with favorable responses to the treatment. Since tumor cells
are directly impaired by CTL, and antibodies have an auxiliary
function, ideally an assessment of the antigen-specific cellular
immune response should be conducted. However, there is currently no
universal standard for the detection of antigen-specific cellular
immune response by enzyme-linked immunospot, intracellular cytokine
and HLA-multimer staining methods. Furthermore, there is a problem
of low repeatability and sensitivity, as well as extensive issues
associated with inter-laboratory validation (23). By contrast, measurement methods for
antigen-specific humoral immune responses, including ELISA and
Luminex assays, are well established, and the problems of
repeatability and sensitivity can be resolved. There are pros and
cons to using antigen-specific cellular immune responses and
antigen-specific humoral immune responses as biomarkers of cancer
vaccines. However, in the present study, repeatability and
sensitivity were given priority and therefore antigen-specific
humoral immune response was used for the evaluation.
When the induction of humoral immunity after
vaccination was investigated in the present study, the positive
conversion rates of Th1-associated antibodies IgG1, IgG2 and IgG3
were 75% (9/12), 41.7% (5/12) and 58.3% (7/12), respectively. These
values indicated high rates of induction. By contrast, the positive
conversion rates of the Th2-associated antibodies, IgG4 and IgE,
were 66.7% (8/12) and 16.7% (2/12), respectively.
Previously, the current authors reported that a type
III allergic reaction is triggered when there is an excessive
induction of antigen-specific immunity with a cancer vaccine
(12). When Th1-associated antibodies
are excessively induced, there is a possible shift from a
Th1-biased cytokine environment to a Th2-biased cytokine
environment (12). In the present
study, the comparison of Th1-asssociated antibodies revealed the
strongest correlation between IgG1 and IgG3, with a correlation
coefficient of R=0.686 (P<0.0001). Th1 and Th2 cells are known
to suppress and balance each other. Th1 cells secrete IFNγ and
activate inflammatory pathways, while Th2 cells secrete IL-4 and
IL-5, which upregulate antibody formation. Therefore, Th1 cells and
Th2 cells are able to cross-inhibit each other (24); the results of the present study
revealed a strong positive correlation between the Th1-associated
antibody IgG2 and the Th2-associated antibody IgG4, with a
correlation coefficient of R=0.808 (P<0.0001). Th2-associated
antibody IgG4 may be induced in direct proportion to the degree of
induction of Th1-associated antibody IgG2.
When the association between increased antibody
levels and survival time was examined for the vaccination in the
present study, no significant difference in survival time was
observed between patients with ≥1 induced Th1-associated antibody
and patients without induction. Although an antigen-specific immune
reaction was triggered, survival time was not affected due to the
following: i) Sufficient therapeutic effect was not observed since
an antigen-specific CTL was not induced in spite of the induction
of an antigen-specific humoral immune response; and ii) target
patients had unresectable advanced recurrent cancers and
controlling their condition with immunotherapy alone may have been
difficult from the start.
By contrast, the survival time of the patients in
which the Th2-associated antibody IgE was induced was significantly
shorter compared with the survival time of patients without IgE
induction. Previous studies of IgE levels and tumors have reported
a negative correlation between past history of allergies and
various cancers (25–27). From a molecular biology perspective,
the antitumor effect of antigen-specific IgE antibodies has been
demonstrated in vivo and in vitro (28–35). Since
its effect is known to be stronger and to last for a longer period
of time compared with IgG, IgE immunotherapy has gained acceptance
as a new concept in cancer treatment (28–35). This
idea conflicts with the current findings and the authors intend to
investigate the cause of the discrepancy in the future. A study has
reported on the association between induction of antigen-specific
IgE in cancer immunotherapy of humans and survival rate (36). When the survival rate of colorectal
cancer patients vaccinated with a recombinant carcinoembryonic
antigen protein was examined, there was a significant positive
improvement in survival rate in the group with IgA antibody
induction, even though the presence or absence of IgE antibody
induction did not affect the survival rate (36). Future studies will involve additional
investigation using IgA fractions. The present study, a clinical
trial of CHP-MAGE-A4 cancer vaccine, does not explain whether
survival time was shortened as a result of IgE induction or whether
IgE is induced when life expectancy is short. However, the results
of the present study suggest that caution is required when
antigen-specific IgE responses are observed during cancer
vaccination therapy.
In the present study, no significant difference in
survival time was observed between patients with Th2-associated
IgG4 antibody induction and patients without IgG4 induction. IgG4
is the least abundant IgG subclass in healthy adult serum,
accounting for ~3% of the total IgG level (37,38). It is
primarily induced as a response to chronic antigen stimulation and
inflammation (39). It is also
associated with immunological tolerance under chronic antigen
stimulation as exemplified by the desensitization therapies used
for allergies (39). From the
perspective of antitumor immunity, IgG4 and IgE are known to
inhibit the antitumor effect (40).
In fact, previous studies have indicated an association between the
elevation of serum IgG4 levels and an unfavorable prognosis of
biliary cancer and malignant melanomas (41–43). The
adverse effect of IgG4 antibodies on antitumor immunity could not
be confirmed based on the survival curve from the present study. In
terms of association between the induction of a humoral response
and the clinical response, the current data did not reveal any
significant associations, including IgG, IgG subclass, IgE and
baseline positivity.
Jäger et al (44) reported in a clinical trial utilizing
NY-ESO1 that baseline positivity of the antigen-specific antibody
affected patient survival. The presence of anti-MAGE-A4 antibodies
prior to MAGE-A4 cancer vaccination is likely to depend on
background factors such as the history and duration of chemotherapy
and radiotherapy before enrollment in the clinical trial. Also,
prognosis may be affected by the immune environment of the advanced
cancer patients. According to data from the present study, the
presence or absence of baseline antibodies does not affect
prognosis. Further investigation is required to clarify the
influence of baseline antibody on prognosis by examining more cases
with additional study variables, including pretreatment and
suppressor cells.
Further study is warranted that should be based on
addressing the limitations of this study; these include analysis of
an increased number of patients, and additional data regarding the
elicited specific T cell response and immune suppressor cells.
In conclusion, the results of the present study
suggest that, in patients who have been vaccinated with the
CHP-MAGE-A4 cancer vaccine, it may be possible to predict the
induction of Th2-associated antibody IgG4 by monitoring
Th1-associated antibody IgG2, and that serum IgE response may be a
marker for an unfavorable prognosis.
References
|
1
|
Boon T, Coulie PG, Van den Eynde BJ and
van der Bruggen P: Human T cell responses against melanoma. Annu
Rev Immunol. 24:175–208. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rosenberg SA: Progress in human tumour
immunology and immunotherapy. Nature. 411:380–384. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rosenberg SA, Yang JC and Restifo NP:
Cancer immunotherapy: Moving beyond current vaccines. Nat Med.
10:909–915. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Oba K, Teramukai S, Kobayashi M, Matsui T,
Kodera Y and Sakamoto J: Efficacy of adjuvant immunochemotherapy
with polysaccharide K for patients with curative resections of
gastric cancer. Cancer Immunol Immunother. 56:905–911. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Takeoka T, Nagase H, Kurose K, Ohue Y,
Yamasaki M, Takiguchi S, Sato E, Isobe M, Kanazawa T, Matsumoto M,
et al: NY-ESO-1 protein cancer vaccine with poly-ICLC and OK-432:
Rapid and strong induction of NY-ESO-1-specific immune responses by
poly-ICLC. J Immunother. 40:140–147. 2017. View Article : Google Scholar
|
|
6
|
Hoon DS, Yuzuki D, Hayashida M and Morton
DL: Melanoma patients immunized with melanoma cell vaccine induce
antibody responses to recombinant MAGE-1 antigen. J immunol.
154:730–737. 1995.PubMed/NCBI
|
|
7
|
Noguchi M, Mine T, Komatsu N, Suekane S,
Moriya F, Matsuoka K, Yutani S, Shichijo S, Yamada A, Toh U, et al:
Assessment of immunological biomarkers in patients with advanced
cancer treated by personalized peptide vaccination. Cancer Biol
Ther. 10:1266–1279. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yoshida K, Noguchi M, Mine T, Komatsu N,
Yutani S, Ueno T, Yanagimoto H, Kawano K, Itoh K and Yamada A:
Characteristics of severe adverse events after peptide vaccination
for advanced cancer patients: Analysis of 500 cases. Oncol Rep.
25:57–62. 2011.PubMed/NCBI
|
|
9
|
Romagnani S: TH1 and TH2 in human
diseases. Clin Immunol Immunopathol. 80:225–235. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shaw DR, Khazaeli MB and LoBuglio AF:
Mouse/human chimeric antibodies to a tumor-associated antigen:
Biologic activity of the four human IgG subclasses. J Natl Cancer
Inst. 80:1553–1559. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Oken MM, Creech RH, Tormey DC, Horton J,
Davis TE, McFadden ET and Carbone PP: Toxicity and response
criteria of the Eastern Cooperative Oncology Group. Am J Clin
Oncol. 5:649–655. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kyogoku N, Ikeda H, Tsuchikawa T, Abiko T,
Fujiwara A, Maki T, Yamamura Y, Ichinokawa M, Tanaka K, Imai N, et
al: Time-dependent transition of the immunoglobulin G subclass and
immunoglobulin E response in cancer patients vaccinated with
cholesteryl pullulan-melanoma antigen gene-A4 nanogel. Oncol Lett.
12:4493–4504. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hoos A, Parmiani G, Hege K, Szno M,
Loibner H, Eggermont A, Urba W, Blumenstein B, Sacks N, Keilholz U,
et al: A clinical development paradigm for cancer vaccine and
related biologics. J Immunother. 30:1–15. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Aoki M, Ueda S, Nishikawa H, Kitano S,
Hirayama M, Ikeda H, Toyoda H, Tanaka K, Kanai M, Takabayashi A, et
al: Antibody responses against NY-ESO-1 and HER2 antigens in
patients vaccinated with combinations of cholesteryl pullulan
(CHP)-NY-ESO-1 and CHP-HER2 with OK-432. Vaccine. 27:6854–6861.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kageyama S, Kitano S, Hirayama M, Nagata
Y, Imai H, Shiraishi T, Akiyoshi K, Scott AM, Murphy R, Hoffman EW,
et al: HUmoral immune responses in patients vaccinated with 1–146
HER2 protein complexed with cholesteryl pullulan nanogel. Cancer
Sci. 99:601–607. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kageyama S, Wada H, Muro K, NIwa Y, Ueda
S, Miyata H, Takiguchi S, Sugino SH, Miyahara Y, Ikeda H, et al:
Dose-dependent effects of NY-ESO-1 protein vaccine complexed with
cholesteryl pullulan (CHP-NY-ESO-1) on immune responses and
survival benefits of esophageal cancer patients. J Transl Med.
11:2462013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gu XG, Schmitt M, Hiasa A, Nagata Y, Ikeda
H, Sasaki Y, Akiyoshi K, Sunamoto J, Nakamura H, Kuribayashi K and
Shiku H: A novel hydrophobized polysaccharide/oncoprotein complex
vaccine induces in vitro and in vivo cellular and humoral immune
responses against HER2-expressing murine sarcoma. Cancer Res.
58:3385–3390. 1998.PubMed/NCBI
|
|
18
|
Hsu SM, Raine L and Fanger H: The use of
antiavidin antibody and avidin-biotin-peroxidase complex in
immunoperoxidase technics. Am J Clin Pathol. 75:816–821. 1981.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Goydos JS, Elder E, Whiteside TL, Finn OJ
and Lotze MT: A phase I trial of a synthetic mucin peptide vaccine.
Induction of specific immune reactivity in patients with
adenocarcinoma. J syrg Res. 63:298–304. 1996. View Article : Google Scholar
|
|
20
|
Miyagi Y, Imai N, Sasatomi T, Yamada A,
Mine T, Katagiri K, Nakagawa M, Muto A, Okouchi S, Isomoto H, et
al: Induction of cellular immune responses to tumor cells and
peptides in colorectal cancer patients by vaccination with SART3
peptides. Clin Cancer Res. 7:3950–3962. 2001.PubMed/NCBI
|
|
21
|
Tsuruma T, Hata F, Torigoe T, Furuhata T,
Idenoue S, Kurotaki T, Yamamoto M, Yagihashi A, Ohmura T, Yamaguchi
K, et al: Phase I clinical study of anti-apoptosis protein,
survivin-derived peptide vaccine therapy for patients with advanced
or recurrent colorectal cancer. J Transl Med. 2:192004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wolchok JD, Hoos A, O'Day S, Weber JS,
Hamid O, Lebbé C, Maio M, Binder M, Bohnsack O, Nichol G, et al:
Guidelines for the evaluation of immune therapy activity in solid
tumors: Immune-related response criteria. Clin Cancer Res.
15:7412–7420. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Janetzki S, Britten CM, Kalos M, Levitsky
HI, Maecker HT, Melief CJ, Old LJ, Romero P, Hoos A and Davis MM:
‘MIATA’-minimal information about T cell assays. Immunity.
31:527–528. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kidd P: Th1/Th2 balance: The hypothesis,
its limitations, and implications for health and disease. Altern
Med Rev. 8:223–246. 2003.PubMed/NCBI
|
|
25
|
Turner MC, Chen Y, Krewski D, Ghadirian P,
Thun MJ and Calle EE: Cancer mortality among US men and women with
asthma and hay fever. Am J Epidemiol. 162:212–221. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang H and Diepgen TL: Is atopy a
protective or a risk factor for cancer? A review of epidemiological
studies. Allergy. 60:1098–1111. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Turner MC, Chen Y, Krewski D and Ghadirian
P: An overview of the association between allergy and cancer. Int J
Cancer. 118:3124–3132. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nagy E, Berczi I and Sehon AH: Growth
inhibition of murine mammary carcinoma by monoclonal IgE antibodies
specific for the mammary tumor virus. Cancer Immunol Immunother.
34:63–69. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kershaw MH, Darcy PK, Trapani JA,
MacGregor D and Smyth MJ: Tumor-specific IgE-mediated inhibition of
human colorectal carcinoma xenograft growth. Oncol Res. 10:133–142.
1998.PubMed/NCBI
|
|
30
|
Gould HJ, Mackay GA, Karagiannis SN,
O'Toole CM, Marsh PJ, Daniel BE, Coney LR, Zurawski VR Jr, Joseph
M, Capron M, et al: Comparison of IgE and IgG antibody-dependent
cytotoxicity in vitro and in a SCID mouse xenograft model of
ovarian carcinoma. Eur J Immunol. 29:3527–3537. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Reali E, Greiner JW, Corti A, Gould HJ,
Bottazzoli F, Paganelli G, Schlom J and Siccardi AG: IgEs targeted
on tumor cells: Therapeutic activity and potential in the design of
tumor vaccines. Cancer Res. 61:5517–5522. 2001.PubMed/NCBI
|
|
32
|
Riemer AB, Untersmayr E, Knittelfelder R,
Duschl A, Pehamberger H, Zielinski CC, Scheiner O and
Jensen-Jarolim E: Active induction of tumor-specific IgE antibodies
by oral mimotope vaccination. Cancer Res. 67:3406–3411. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jensen-Jarolim E, Achatz G, Turner MC,
Karagiannis S, Legrand F, Capron M, Penichet ML, Rodríguez JA,
Siccardi AG, Vangelista L, et al: AllergoOncology: The role of
IgE-mediated allergy in cancer. Allergy. 63:1255–1266. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Singer J and Jensen-Jarolim E: IgE-based
immunotherapy of cancer: Challenges and chances. Allergy.
69:137–149. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Josephs DH, Spicer JF, Karagiannis P,
Gould HJ and Karagiannis SN: IgE immunotherapy: A novel concept
with promise for the treatment of cancer. MAbs. 6:54–72. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Staff C, Magnusson CG, Hojjat-Farsangi M,
Mosolits S, Liljefors M, Frodin JE, Wahrén B, Mellstedt H and
Ullenhag GJ: Induction of IgM, IgA and IgE antibodies in colorectal
cancer patients vaccinated with a recombinant CEA protein. J Clin
Lmmunol. 32:855–865. 2012. View Article : Google Scholar
|
|
37
|
Schur PH: IgG subclasses. A historical
perspective. Monogr Allergy. 23:1–11. 1988.PubMed/NCBI
|
|
38
|
Vidarsson G, Dekkers G and Rispens T: IgG
subclasses and allotypes: From structure to effector function.
Front Immunol. 5:5202014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Shamji MH, Ljørring C, Francis JN,
Calderon MA, Larché M, Kimber I, Frew AJ, Ipsen H, Lund K, Würtzen
PA and Durham SR: Functional rather than immunoreactive levels of
IgG4 correlate closely with clinical response to grass pollen
immunotherapy. Allergy. 67:217–226. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Crescioli S, Correa I, Karagiannis P,
Davies AM, Sutton BJ, Nestle FO and Karagiannis SN: IgG4
characteristics nad functions in cancer immunity. Curr Allergy
Asthma Rep. 16:72016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Karagiannis P, Gilbert AE, Josephs DH, Ali
N, Dodev T, Saul L, Correa I, Roberts L, Beddowes E, Koers A, et
al: IgG4 subclass antibodies impair antitumor immunity in melanoma.
J Clin Invest. 123:1457–1474. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
42
|
Karagiannis P, Villanova F, Josephs DH,
Correa I, Van Hemelrijck M, Hobbs C, Saul L, Egbuniwe IU, Tosi I,
Ilieva KM, et al: Elevated IgG4 in patient circulation is
associated with the risk of disease progression in melanoma.
Oncoimmunology. 4:e10324922015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Harada K, Shimoda S, Kimura Y, Sato Y,
Ikeda H, Igarashi S, Ren XS, Sato H and Nakanuma Y: Significance of
immunoglobulin G4 (IgG4)-positive cells in extrahepatic
cholangiocarcinoma: Molecular mechanism of IgG4 reaction in cancer
tissue. Hepatology. 56:157–164. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Jäger E, Gnjatic S, Nagata Y, Stockert E,
Jäger D, Karbach J, Neumann A, Rieckenberg J, Chen YT, Ritter G, et
al: Induction of primary NY-ESO-1 immunity: CD8+ T lymphocyte and
antibody responses in peptide-vaccinated patients with NY-ESO-1+
cancers. Proc Natl Acad Sci USA. 97:pp. 12198–12203. 2000;
View Article : Google Scholar : PubMed/NCBI
|