Introduction
Lung adenocarcinoma is a common type of lung cancer
with high incidence (40%) (1). It has
high risk of distant metastasis, which poses a severe threat to the
survival rates of patients (2).
However, prognosis varies considerably at different stages of this
disease (3). Therefore, an effective
screening tool is imperative and of critical importance in the
clinical management of lung adenocarcinoma, which provides early
and accurate detection thereof.
DNA methylation markers are effective biomarkers and
promising candidates in the diagnosis of disease (4,5). Aberrant
methylation in cancer usually occurs at CpG islands, which results
in changes in the transcription of tumor suppressor genes (6). For lung adenocarcinoma, several in
vitro studies have shown the difference of the DNA methylation
level between patients and normal controls. For example,
F2RL3 methylation has been demonstrated to be a strong
predictor for the incidence of lung adenocarcinoma (7). The p16 gene promoter methylation
is also associated with smoking-induced lung adenocarcinoma
(7). Tomizawa et al (8) suggested that RASSF1A methylation was
significantly associated with pleural involvement, vascular
invasion and decreased patient survival time, which may be a
powerful marker for the prognosis of patients with lung
adenocarcinoma at an early stage. Previous findings have shown the
role of DNA methylation in a potential diagnosis of lung
adenocarcinoma. However, the pathogenesis involved in lung
adenocarcinoma has not been clearly understood.
In the present study, microarray data (GSE66386)
were downloaded and a comprehensive bioinformatics approach was
performed to screen differentially expressed methylation genes in
lung adenocarcinoma samples compared with normal controls. The aim
was to identify potential DNA methylation biomarkers and to
determine the possible pathogenesis for lung adenocarcinoma.
Materials and methods
Microarray data and preprocessing
The gene expression dataset (GSE66386) was collected
from the Gene Expression Omnibus (GEO, https://www.ncbi.nlm.nih.gov/geo/) database. The
dataset included 183 samples, of which 164 were lung adenocarcinoma
samples, and 19 were matched normal lung tissue samples. The
dataset was uploaded by Bjaanæs et al and cited in their
study (9). The platform of this
microarray was Illumina Infinium 450 K Human Methylation BeadChip.
The differentially methylated genes were identified using the limma
package available at http://www.bioconductor.org-packages/release/bioc/html/limma.html.
As a result, the DNA methylation data with 456947 CpGs were used
for analysis. For preprocessing of the row data, the following
probes were removed: i) The distance value (from CpG to SNP) <2;
ii) the minor allele frequency <0.05; iii) the cross-hybridising
probes and sex chromosome-specific DNA probes.
Identification of genes with
methylation differences
The percentage methylation values were expressed as
β values. The differential methylation of CpGs between lung
adenocarcinoma and control samples were identified if the absolute
mean difference between the two groups was <0.05, and the
P-value was <0.05. Consequently, the P-value was calculated by
t-test.
Functional enrichment analysis of
differential methylation of genes
Gene ontology (GO) analysis for screening of
differential methylation of genes was conducted to search the vital
functions related to the differential gene methylation in lung
adenocarcinoma (10). The P-values
was adjusted into false discovery rate (FDR) by Benjaminiand
Hochberg study (11). FDR <0.01
was considered as the cutoff value. The proteins of annotated
differential gene methylation were compared with their homologous
proteins based on COG database (http://www.ncbi.nlm.nih.gov/COG). P-value <0.05 was
regarded as the cut off threshold value. By this strategy, the
screened genes with methylation differences were then assigned to
different gene functions including cellular component (CC),
molecular function (MF) and biological process (BP).
Pathway analysis
A Kyoto Encyclopedia of Genes and Genomes (KEGG)
pathway (12) analysis was performed
to evaluate the associations between different proteins in the
regulation of cell function and metabolism. Fisher's test was used
to identify significant pathways that were enriched by genes with
methylation differences with the threshold of FDR <0.05.
Results
Identification of methylation
genes
After standardized pretreatment, we collected 413043
CpGs for the subsequent analysis. After further screening, 112662
differentially methylated regions (DMRs) in lung adenocarcinoma
patients compared with normal controls, including 57235
upmethylated CpGs and 55427 downmethylated ones. These CpGs were
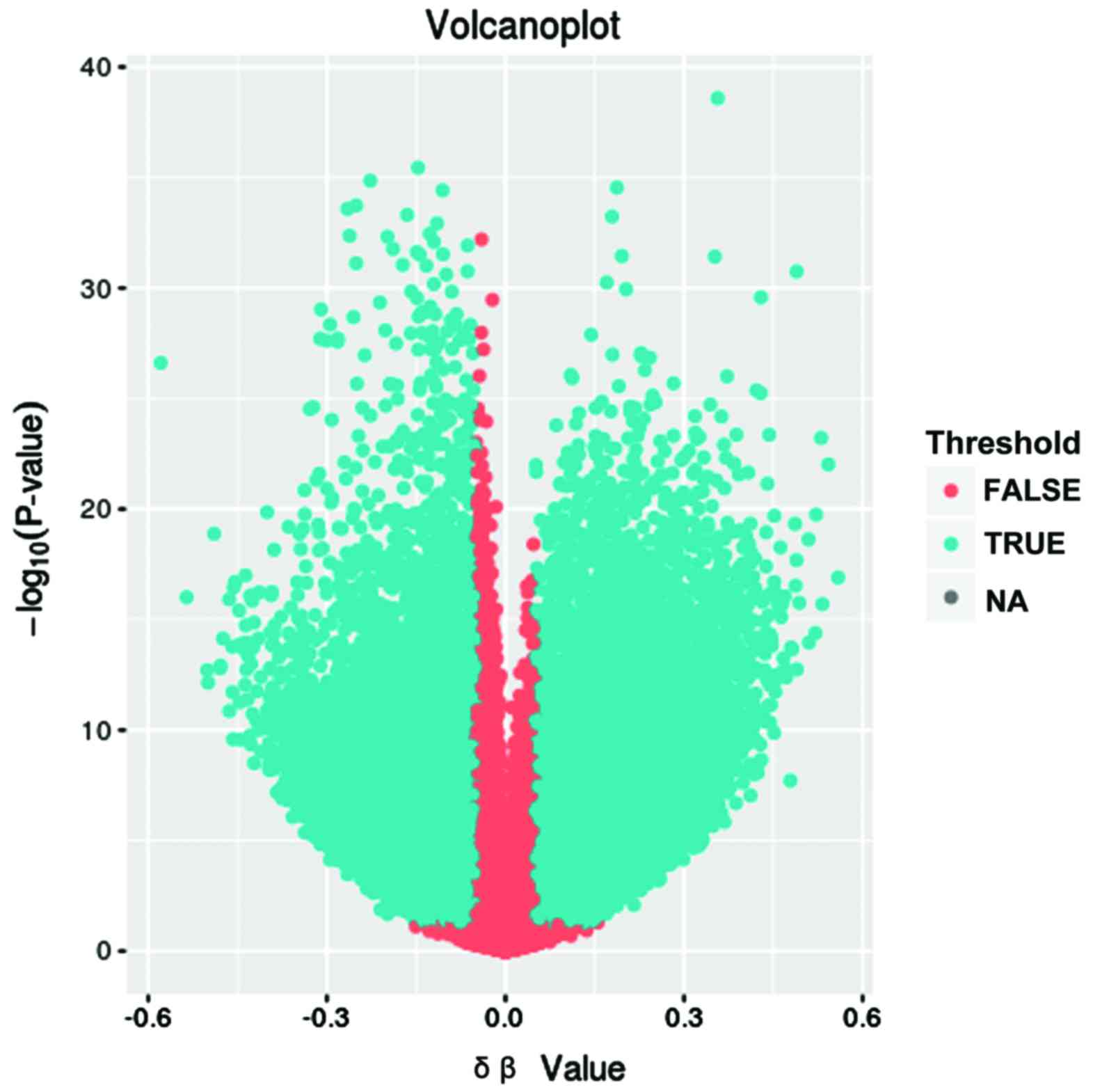
involved in 16,705 genes. The results are summarized as volcano
plots (Fig. 1). The difference of the
differentially methylated genes between lung adenocarcinoma and
normal control is shown on the x-axis, and the negative P-value was
shown on the y-axis.
To obtain more important methylation genes, we
initially removed 109 CpGs with β value ≤0.2 or ≥0.8, and then
excluded 99954 CpGs with an absolute value of difference between
the average β values of the two groups ≥0.2. As a result, 12599
CpGs related to 5,489 genes were obtained.
Functional enrichment analysis
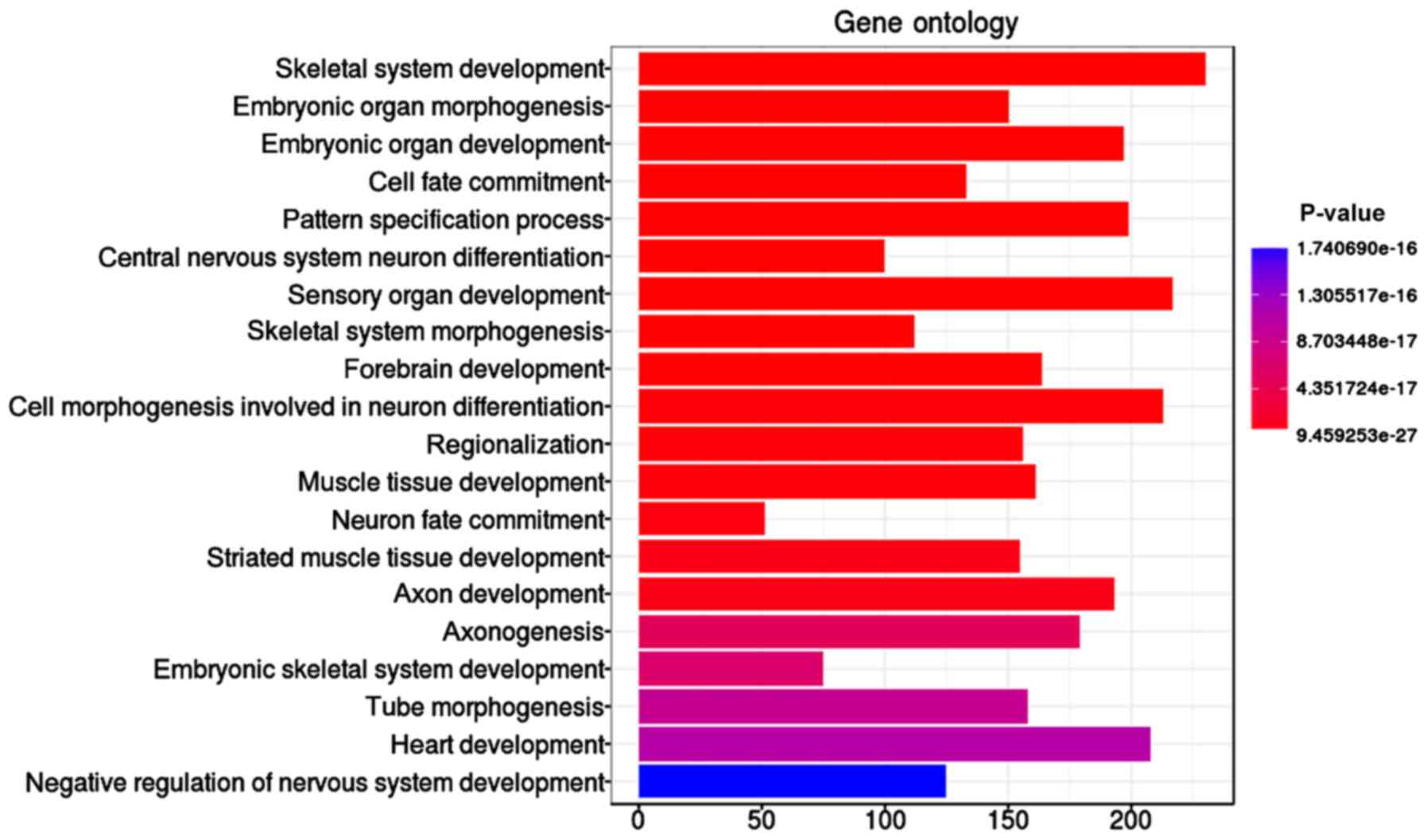
The ‘skeletal system development’ function,
including 230 significant genes with methylation differences, was
identified as the most significant (P=9.46E-27) GO term with 5,489
methylation genes (Table I and
Fig. 2). In addition, embryonic organ
morphogenesis (P=8.67E-24), embryonic organ development
(P=1.20E-23), and cell fate commitment (P=5.90E-23) were found to
be involved in the lung adenocarcinoma.
 | Table I.The top 10 enriched GO descriptions
for differentially expressed methylation genes. |
Table I.
The top 10 enriched GO descriptions
for differentially expressed methylation genes.
| ID | Description | P-value | P-adjust | Count |
|---|
| GO:0001501 | Skeletal system
development | 9.46E-27 | 5.19E-23 | 230 |
| GO:0048562 | Embryonic organ
morphogenesis | 8.67E-24 | 2.20E-20 | 150 |
| GO:0048568 | Embryonic organ
development | 1.20E-23 | 2.20E-20 | 197 |
| GO:0045165 | Cell fate
commitment | 5.90E-23 | 8.10E-20 | 133 |
| GO:0007389 | Pattern specification
process | 5.27E-21 | 5.79E-18 | 199 |
| GO:0021953 | Central nervous
system neuron differentiation | 1.81E-20 | 1.66E-17 | 100 |
| GO:0007423 | Sensory organ
development | 1.87E-19 | 1.47E-16 | 217 |
| GO:0048705 | Skeletal system
morphogenesis | 4.66E-19 | 3.20E-16 | 112 |
| GO:0030900 | Forebrain
development | 1.11E-18 | 6.75E-16 | 164 |
| GO:0048667 | Cell morphogenesis
involved in neuron differentiation | 1.79E-18 | 9.50E-16 | 213 |
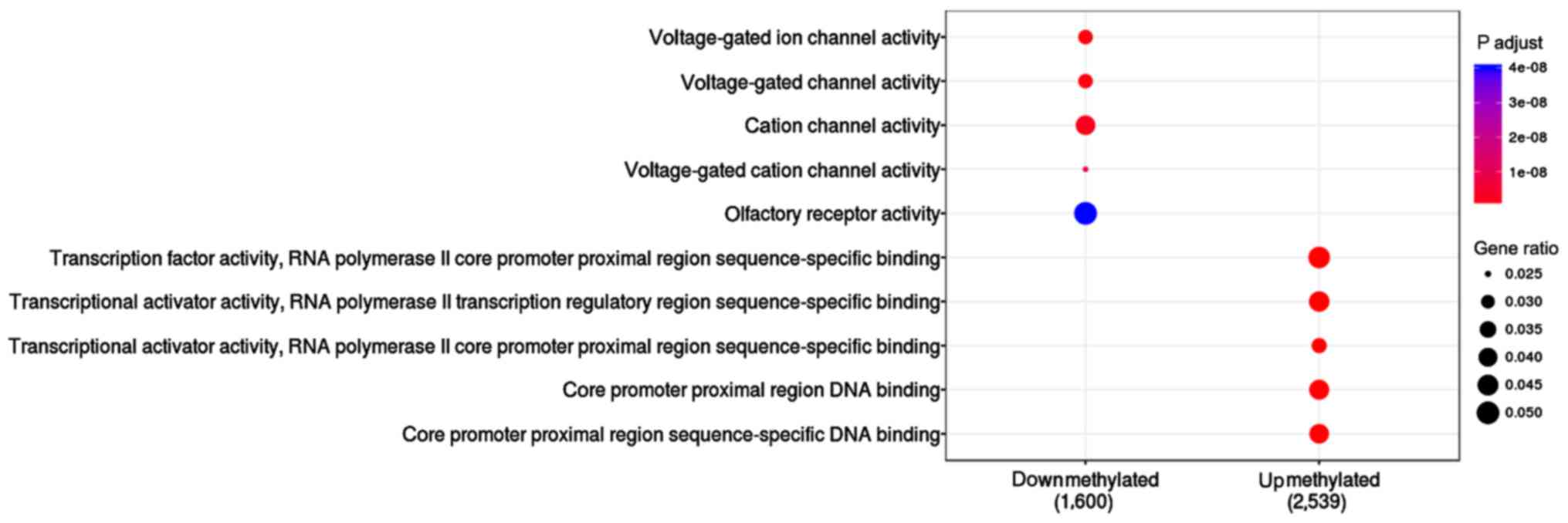
The function of upmethylated and downmethylated gene
sets were compared in this study (Table
II and Fig. 3). Results showed
that 2,539 upmethylated genes were enriched in the following
pathways: transcription factor activity (P=4.13E-19), RNA
polymerase II core promoter proximal region sequence-specific
binding (P=1.82E-17), and transcriptional activator activity
(P=4.43E-13). A total of 1,600 differential downmethylated genes
were mainly enriched in voltage-gated ion channel activity
(P=1.13E-09), voltage-gated channel activity (P=1.13E-09) and
cation channel activity (P=2.66E-09).
 | Table II.The top 5 enriched down- and
upmethylated pathways involved in the development of lung
adenocarcinoma. |
Table II.
The top 5 enriched down- and
upmethylated pathways involved in the development of lung
adenocarcinoma.
| Cluster | ID | P-value | P-adjust | Count |
|---|
| Downmethylated | GO:0005244 | 2.52E-12 | 1.13E-09 | 52 |
| Downmethylated | GO:0022832 | 2.52E-12 | 1.13E-09 | 52 |
| Downmethylated | GO:0005261 | 8.86E-12 | 2.66E-09 | 69 |
| Downmethylated | GO:0022843 | 5.10E-11 | 1.15E-08 | 40 |
| Downmethylated | GO:0004984 | 2.64E-10 | 4.00E-08 | 84 |
| Upmethylated | GO:0000982 | 4.19E-22 | 4.13E-19 | 124 |
| Upmethylated | GO:0001228 | 3.7E-20 | 1.82E-17 | 116 |
| Upmethylated | GO:0001077 | 1.35E-15 | 4.43E-13 | 85 |
| Upmethylated | GO:0001159 | 1.92E-13 | 4.74E-11 | 113 |
| Upmethylated | GO:0000987 | 1.55E-12 | 3.05E-10 | 110 |
Pathway analysis
Pathway analysis was used to evaluate the possible
pathway associated with lung adenocarcinoma induced by DNA
methylation (Table III). The
results showed that significant genes with methylation differences
were involved in 74 pathways, among which, the cancer pathways
(P=3.64E-07), Rap1 signaling pathway (P=9.21E-05) and calcium
signaling pathway (P=9.21E-05) were the important pathways
associated with lung adenocarcinoma.
 | Table III.The top 10 enriched pathways involved
in development of lung adenocarcinoma. |
Table III.
The top 10 enriched pathways involved
in development of lung adenocarcinoma.
| ID | Pathway name | P-value | P-adjust | Count |
|---|
| hsa05200 | Pathways in
cancer | 1.28E-09 | 3.64E-07 | 152 |
| hsa04015 | Rap1 signaling
pathway | 9.70E-07 | 9.21E-05 | 85 |
| hsa04020 | Calcium signaling
pathway | 7.74E-07 | 9.21E-05 | 75 |
| hsa04921 | Oxytocin signaling
pathway | 1.78E-06 | 0.000127156 | 67 |
| hsa04024 | cAMP signaling
pathway | 2.75E-06 | 0.000156673 | 80 |
| hsa03008 | Ribosome biogenesis
in eukaryotes | 6.25E-06 | 0.000296837 | 5 |
| hsa04010 | MAPK signaling
pathway | 9.25E-06 | 0.000376788 | 96 |
| hsa00190 | Oxidative
phosphorylation | 1.08E-05 | 0.000384468 | 13 |
| hsa04550 | Signaling pathways
regulating pluripotency of stem cells | 1.34E-05 | 0.000423855 | 59 |
| hsa04022 | cGMP-PKG signaling
pathway | 1.73E-05 | 0.000448535 | 54 |
Discussion
Alterations of epigenetics such as DNA methylation
are of great importance in carcinogenesis. Increased knowledge has
shown the great importance of DNA methylation in lung cancer
(13). Thus it is of great importance
to evaluate the connection between the level of methylation and the
increased expression in epigenetic diagnosis and therapy. In the
present study, we identified a great amount of genomic regions that
showed different methylations in tumor and normal lung
adenocarcinoma tissues by microarray based on the methylation
screening approach. A total of 12599 DMRs associated with 5,489
genes were eventually screened, including PTPRF,
HOXD3, HOXD13 and CACNA1A. These genes are
promising in the development of biomarkers for diagnostic or
therapy purposes.
PTPRF gene is a member of the protein
tyrosine phosphatase (PTP) family, which is known to be a signaling
molecule involved in the regulation of cell processes (14). It was previously reported to be
expressed as a potential predictive marker for treatment with
erlotinib in non-small-cell lung cancer (15), and has been found to promote cell
migration in several types of cancer (16,17). The
present study showed that the methylated PTPRF was focused
on the role of regulation of protein binding. In lung
adenocarcinomas, CRBP-1 high expression was suggested to be
an aggressive phenotype (18).
CPBP-1 is known as a 15 kDa cytosolic binding protein that
is crucial in retinol uptake and esterification (19,20).
CPBP-1 was also highly expressed in lung adenocarcinoma
(18), and played an important role
in protein binding. Therefore, we consider that the function of
methylated PTPRF associated with protein binding is of great
importance in lung adenocarcinomas.
Homeobox (HOX) genes, belonging to the
homeobox family, are important in the regulation of transcription
which controls the expression of morphogenesis-related genes
(21) In the present study,
HOXD3 was upmethylated in lung adenocarcinoma patients
compared to the normal controls. In addition, HOXD3 was
involved in two possible functions associated with lung
adenocarcinoma, positive regulation of cell development and
cell-substrate adhesion. In an in vitro experiment, Hamada
et al (22) have shown that
the overexpression of HOXD3 gene significantly promoted
migration and invasion in human lung cancer A549 cells. Authors of
that study suggested that HOXD3 overexpression prominently
induced a high expression of integrin β3, which promotes pulmonary
metastasis via increased endothelium and adhesion (22). Another study identified that in A549
cells, overexpressing HOXD3 gene accelerated the expression
of N-cadherin and integrins (23).
These results suggest that the aberrant methylation of HOXD3
plays an important role in the regulation of genes involved in the
metastasis and invasion of lung adenocarcinoma via the function of
cell-substrate adhesion.
Another HOX gene, HOXD13, was screened
with differential hypermethylation enriched in the transcriptional
activator activity in lung adenocarcinoma in the present study.
Hypermethylated HOXD13 has been reported in malignant
melanomas (24), breast cancer
(25) and extrahepatic
cholangiocarcinoma (26), with
methylation rates of 30.8, 94.38 and 57.7%, respectively. Thus, the
upmethylation of HOXD13 is a potential diagnostic biomarker
and therapeutic target.
There is insufficient information regarding the
relationship between CACNA1A and cancer. However, we found
that CACNA1A was downmethylated in patients with lung
adenocarcinoma compared to normal controls. As a novel possible
tumor-methylated candidate involved in lung cancer, the
CACNA1A methylation has been identified from microarray
experiment (27,28). However, the methylated level as well
as its possible carcinogenic mechanism have not been clearly
understood. Further analysis in the present study showed that
downmethylated CACNA1A was involved in several functions
such as the calcium channel and was also voltage-dependent. The
intracellular calcium overload is regarded as an important factor
inducing mitochondrial-dependent apoptosis directly and indirectly
(29). The function of mitochondrial
transmembrane potential may be interrupted by calcium phosphate in
the matrix (30). In a previous
study, it was shown that in TCM, curcumin exerted an
anti-proliferative effect by calcium overload via activating the
calcium channel in lung cancer cells (31). Therefore, downmethylated
CACNA1A may be involved in the development of lung
adenocarcinoma via the calcium channel signaling pathway.
In conclusion, from the DNA methylation profiling,
we achieved two purposes: i) identification of DNA methylation
changes that may be associated with lung adenocarcinoma. ii)
Identification of function and pathway of methylated genes that may
be involved in lung adenocarcinomas. From these data, we concluded
that screened genes with methylation differences, including
PTPRF, HOXD3, HOXD13 and CACNA1A serve
as potential markers and may be used in the diagnosis and therapy
of patients with lung adenocarcinoma.
Acknowledgements
The present study was funded by the Science and
Technology Research projects of Guizhou province [SY: (2012)3101]
and Guizhou Province Science and Technology Research
ProjectsGuizhou Branch SY Word [2012] no. 3101: Induced sputum
Runx3 gene promoter region methylation in value for early diagnosis
of lung cancer.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Shi J, Hua X, Zhu B, Ravichandran S, Wang
M, Nguyen C, Brodie SA, Palleschi A, Alloisio M, Pariscenti G, et
al: Somatic genomics and clinical features of lung adenocarcinoma:
A retrospective study. PLoS Med. 13:e10021622016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Consonni D, Pierobon M, Gail MH, Rubagotti
M, Rotunno M, Goldstein A, Goldin L, Lubin J, Wacholder S, Caporaso
NE, et al: Lung cancer prognosis before and after recurrence in a
population-based setting. J Natl Cancer Inst. 107:djv0592015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Siegel R, DeSantis C, Virgo K, Stein K,
Mariotto A, Smith T, Cooper D, Gansler T, Lerro C, Fedewa S, et al:
Cancer treatment and survivorship statistics, 2012. CA Cancer J
Clin. 62:220–241. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Balgkouranidou I, Liloglou T and Lianidou
ES: Lung cancer epigenetics: Emerging biomarkers. Biomarkers Med.
7:49–58. 2013. View Article : Google Scholar
|
|
5
|
Mehta A, Dobersch S, Romero-Olmedo AJ and
Barreto G: Epigenetics in lung cancer diagnosis and therapy. Cancer
Metastasis Rev. 34:229–241. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Robertson KD: DNA methylation and human
disease. Nat Rev Genet. 6:597–610. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang Y, Schöttker B, Ordóñez-Mena J,
Holleczek B, Yang R, Burwinkel B, Butterbach K and Brenner H: F2RL3
methylation, lung cancer incidence and mortality. Int J Cancer.
137:1739–1748. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tomizawa Y, Kohno T, Kondo H, Otsuka A,
Nishioka M, Niki T, Yamada T, Maeshima A, Yoshimura K, Saito R, et
al: Clinicopathological significance of epigenetic inactivation of
RASSF1A at 3p21.3 in stage I lung adenocarcinoma. Clin Cancer Res.
8:2362–2368. 2002.PubMed/NCBI
|
|
9
|
Bjaanæs MM, Fleischer T, Halvorsen AR,
Daunay A, Busato F, Solberg S, Jørgensen L, Kure E, Edvardsen H,
Børresen-Dale AL, et al: Genome-wide DNA methylation analyses in
lung adenocarcinomas: Association with EGFR, KRAS and TP53 mutation
status, gene expression and prognosis. Mol Oncol. 10:330–343. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ashburner M, Ball CA, Blake JA, Botstein
D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT,
et al: The Gene Ontology Consortium: Gene Ontology: Tool for the
unification of biology. Nat Genet. 25:25–29. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Benjamini Y and Hochberg Y: Controlling
the false discovery rate - A practical and powerful approach to
multiple testing. JR Stat Soc Ser A Stat Soc. 57:289–300. 1995.
|
|
12
|
Huang W, Sherman BT and Lempicki RA:
Systematic and integrative analysis of large gene lists using DAVID
bioinformatics resources. Nat Protoc. 4:44–57. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Raungrut P, Petjaroen P, Geater SL,
Keeratichananont W, Phukaoloun M, Suwiwat S and Thongsuksai P:
Methylation of 14-3-3σ gene and prognostic significance of 14-3-3σ
expression in non-small cell lung cancer. Oncol Lett. 14:5257–5264.
2017.PubMed/NCBI
|
|
14
|
Chagnon MJ, Uetani N and Tremblay ML:
Functional significance of the LAR receptor protein tyrosine
phosphatase family in development and diseases. Biochem Cell Biol.
82:664–675. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Soulières D, Hirsch FR, Shepherd FA,
Bordogna W, Delmar P, Shames DS and Klughammer B: PTPRF Expression
as a potential prognostic/predictive marker for treatment with
erlotinib in non-small-cell lung cancer. J Thorac Oncol.
10:1364–1369. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wu R, Levin AM, Kardia SLR and Cho KR:
Identification of novel amplicons in ovarian cancer using a
model-based scan statistic for identifying increased chromosomal
regions of gene expression. Cancer Res. 65:847. 2005.
|
|
17
|
Park J, Lee J and Choi C: Evaluation of
drug-targetable genes by defining modes of abnormality in gene
expression. Sci Rep. 5:135762015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Doldo E, Costanza G, Ferlosio A, Pompeo E,
Agostinelli S, Bellezza G, Mazzaglia D, Giunta A, Sidoni A and
Orlandi A: High expression of cellular retinol binding protein-1 in
lung adenocarcinoma is associated with poor prognosis. Genes
Cancer. 6:490–502. 2015.PubMed/NCBI
|
|
19
|
Napoli JL: Biosynthesis and metabolism of
retinoic acid: roles of CRBP and CRABP in retinoic acid: roles of
CRBP and CRABP in retinoic acid homeostasis. J Nutr. 123 Suppl
2:362–366. 1993.PubMed/NCBI
|
|
20
|
Doldo E, Costanza G, Ferlosio A, Passeri
D, Bernardini S, Scioli MG, Mazzaglia D, Agostinelli S, Del Bufalo
D, Czernobilsky B, et al: CRBP-1 expression in ovarian cancer: A
potential therapeutic target. Anticancer Res. 34:3303–3312.
2014.PubMed/NCBI
|
|
21
|
Gehring WJ and Hiromi Y: Homeotic genes
and the homeobox. Annu Rev Genet. 20:147–173. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hamada Ji, Omatsu T, Okada F, Furuuchi K,
Okubo Y, Takahashi Y, Tada M, Miyazaki YJ, Taniguchi Y, Shirato H,
et al: Overexpression of homeobox gene HOXD3 induces coordinate
expression of metastasis-related genes in human lung cancer cells.
Int J Cancer. 93:516–525. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ohta H, Hamada J, Tada M, Aoyama T,
Furuuchi K, Takahashi Y, Totsuka Y and Moriuchi T:
HOXD3-overexpression increases integrin alpha v beta 3 expression
and deprives E-cadherin while it enhances cell motility in A549
cells. Clin Exp Metastasis. 23:381–390. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Furuta J, Nobeyama Y, Umebayashi Y, Otsuka
F, Kikuchi K and Ushijima T: Silencing of peroxiredoxin 2 and
aberrant methylation of 33 CpG islands in putative promoter regions
in human malignant melanomas. Cancer Res. 66:6080–6086. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhong Z, Shan M, Wang J, Liu T, Xia B, Niu
M, Ren Y and Pang D: HOXD13 methylation status is a prognostic
indicator in breast cancer. Int J Clin Exp Pathol. 8:10716–10724.
2015.PubMed/NCBI
|
|
26
|
Shu Y, Wang B, Wang J, Wang JM and Zou SQ:
Identification of methylation profile of HOX genes in extrahepatic
cholangiocarcinoma. World J Gastroenterol. 17:3407–3419. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sánchez-Peña ML, Isaza CE, Pérez-Morales
J, Rodríguez-Padilla C, Castro JM and Cabrera-Ríos M:
Identification of potential biomarkers from microarray experiments
using multiple criteria optimization. Cancer Med. 2:253–265. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Castro M, Grau L, Puerta P, Gimenez L,
Venditti J, Quadrelli S and Sánchez-Carbayo M: Multiplexed
methylation profiles of tumor suppressor genes and clinical outcome
in lung cancer. J Transl Med. 8:862010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pinton P, Giorgi C, Siviero R, Zecchini E
and Rizzuto R: Calcium and apoptosis: ER-mitochondria
Ca2+ transfer in the control of apoptosis. Oncogene.
27:6407–6418. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kurskii MD, Tugai VA and Fedoriv AN:
Effect of serotonin and calcium on separate components of
respiratory chain of mitochondria in some rabbit tissues). Ukr
Biokhim Zh. 42:584–588. 1970.(In Ukrainian). PubMed/NCBI
|
|
31
|
Xu X, Chen D, Ye B, Zhong F and Chen G:
Curcumin induces the apoptosis of non-small cell lung cancer cells
through a calcium signaling pathway. Int J Mol Med. 35:1610–1616.
2015. View Article : Google Scholar : PubMed/NCBI
|