Introduction
Oncolytic virotherapy is not a new concept; this
area of research first arose as an observational science in the
early 20th century, when it was occasionally reported that cancer
regression occurred spontaneously in patients following certain
viral infections (1). Numerous
experiments were carried out following these reports; however, the
topic of anticancer viral therapy languished, due to the deficiency
of specificity and the harmful side effects, as well as the limited
knowledge regarding the mechanisms of how cancer cells succumb to
oncolytic viruses (OVs) (2). Since
the 1990s, the genomes of wild-type viruses have been selectively
engineered as a result of the development of molecular virology;
this has reignited interest in the use of replicating viruses as
cancer therapeutics. The manufactured viruses are capable of
preferential replication and proliferation in cancerous cells,
which may cause the cancerous cells to die at the end of
replication cycles via lysis or the activation of an antitumor
immune response, with minimal damage to normal cells (3). Several OVs have been tested in humans,
and although the safety results were optimistic, their efficacy as
single agents was limited (4).
Administration of chemotherapy or radiotherapy in addition to the
viruses is vital for creating an effective treatment. Over the past
two decades, the study of oncolytic virotherapy has grown
exponentially alongside the advancement of molecular biology,
virology, immunology and, particularly, genetic engineering
(5). In addition, combinations with
other treatment options, particularly cancer gene therapy, has been
demonstrated the potential to strengthen the antitumor efficacy
through viral amplification of the therapeutic transgene inside
tumor cells and tissues (6). Various
cancer-targeted OVs carry a number of exogenous genes that have
transitioned from preclinical studies into early phase clinical
testing and more recently into randomized clinical trials (Table I) (1).
These include vaccinia, adenovirus, herpes simplex virus, reovirus
and Newcastle disease virus (7,8);
additionally, novel OV species are being explored. The current
status of clinical trials for such therapeutic OVs has recently
been reviewed by Russell et al (9). Compared with conventional treatments for
cancer, including chemotherapy and radiotherapy, the engineered OV
strain expressing transgenes exhibits a contrasting mechanism of
action, specificity and cross-resistance, among other
characteristics (Fig. 1) (10–12).
 | Table I.A listing of clinical trials of
oncolytic viruses provided by a previous review (1). |
Table I.
A listing of clinical trials of
oncolytic viruses provided by a previous review (1).
| Virus family | Genetic
modifications | Target types of
cancer | Clinic trial
sponsor |
|---|
| Adenovirus |
|
|
|
|
Oncorine (H101) | Ad-E1b− | Liver, lung,
head/neck and pancreas | Shanghai Sunway
(approved in China) |
|
CGTG-102 | Ad-GMCSF+ | Solid tumors | Oncos |
|
DNX-2401 | Ad-d24RGD | Brain | DNAtrix/Erasmus
Medical Center |
|
ICOVIR-5 |
Ad-DM-E2F-K-d24RGD | Melanoma | Institut Catala
d'Oncologia |
|
CG0070 | Ad-GMCSF+ | Bladder | Cold Genesys |
| Colo
Ad1 | Ad3:Ad11p
hybrid | Metastatic solid
tumors | PsiOxus |
| Herpesvirus |
|
|
|
|
T-VEC |
HSV1-ICP34.5−/4−GMCSF+ | Melanoma | Amgen/BioVex
(completed Phase III) |
|
Seprehvir |
HSV1716-ICP34.5− | Lung, various solid
tumors | Virttu Biologics
and Children's Hospital |
|
G207 |
HSV1-ICP34.5−/6− | Brain | MediGene |
|
HF10 | HSV-HF strain | Head/neck, skin,
breast and melanoma | Takara Bio |
| Poxvirus |
|
|
|
|
Pexa-Vec/JX594 | Vaccinia Wyeth
TK−/GMCSF+ | Liver, colorectal,
head/neck, others | Jennerex (multiple
trials) |
| Paramyxovirus |
|
|
|
| Measles
virus | MV-NIS+,
MV-CEA+ | Ovarian,
peritoneal, myeloma, others | Mayo Clinic/NCI
(multiple trials) |
|
Newcastle disease virus | Natural isolate
(HUJ) | Glioblastoma
multiforme, neuroblastoma, sarcomas | Hadassah Medical
Organization |
| Reovirus |
|
|
|
|
Reolysin | Reovirus-serotype
3 | Diverse types of
cancer | Oncolytics Biotech
Inc. (multiple trials) |
| Rhabdovirus |
|
|
|
|
Vesicular somatitis virus | VSV-IFNb+ | Hepatocellular
carcinoma | Mayo Clinic |
| Maraba
virus | MAGE A3+/Matrix
glycoprotein mutations | Lung, colon,
melanoma | NCIC Clinical
Trials Group |
| Picornavirus |
|
|
|
|
CAVATAK |
Coxsackievirus-A21 | Melanoma | Viralytics
(multiple trials) |
|
PVS-RIPO | Polio:Rhinovirus
chimera | Glioblastoma | Duke
University |
| Seneca
Valley V. | Natural isolate
(NTX-010) | Neuroendocrine
tumors | Children's Oncology
Group |
| Parvovirus |
|
|
|
| H-1
PV | Natural
isolate | Glioblastoma | Heidelberg
University Hospital |
Specificity of OV in succumbing tumor
cells
The progression of a tumor is generally considered a
stochastic, dynamic process; genetic and epigenetic changes are
also involved, including in the limitless proliferative potential,
evasion of apoptosis, enhanced angiogenic capacity, tissue invasion
and metastasis, and modification of the intracellular signaling
pathway. These changes may make malignant cells susceptible to an
infection, due to a number of the pathways subverted by the tumor
also being necessary for efficient antiviral responses. Once a
virus penetrates into a tumor cell, the tumor microenvironment
provides abundant support for viral replication; therefore, these
changes create the conditions required for oncolytic viral
selective replication in cancer cells (11,12). The
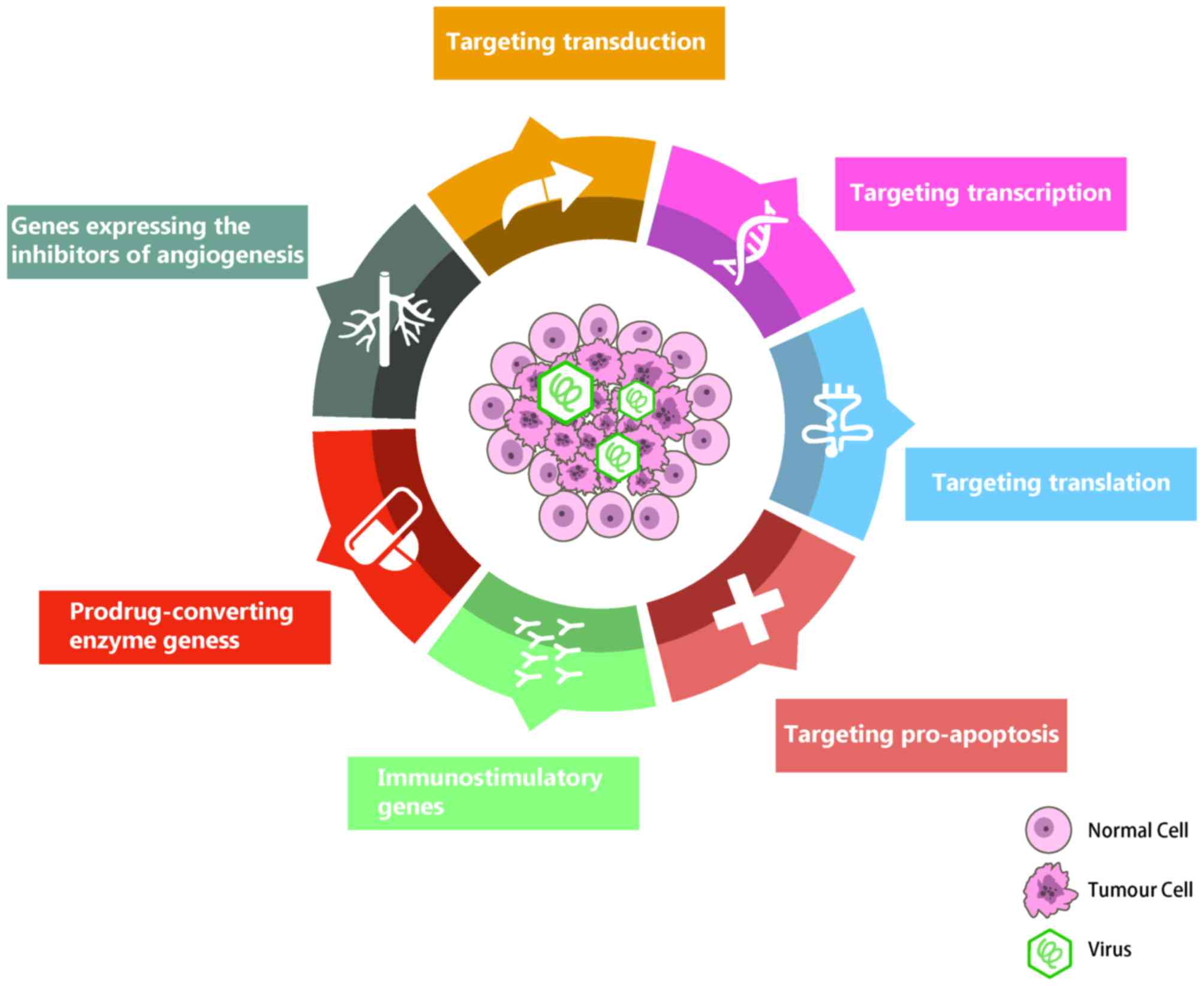
oncolytic viral targeting mechanism is divided into the following
categories: Targeting transduction, targeting transcription,
targeting translation and targeting pro-apoptosis mechanisms.
Targeting transduction
Tumor cells have specific and high surface
expression of the oncolytic viral primary receptor, which is
capable of binding to viral surface proteins via the
receptor-ligand pathway, essential for transduction. The first step
is absorption, followed by the viral surface adhesion protein
combining with the target cell surface receptor, which initiates
endocytic signaling pathways (13). A
number of viruses have a natural tropism for tumor cells. This
principle is illustrated by the susceptibility of malignant glioma
cells to poliovirus, depending on the presence of CD155 on glial
neoplasms (14,15). Alternatively, in order to infect the
targeted tumor cells, the viral surface binding protein, which has
the ability to serve as a receptor, must transform by introducing a
single chain antibody or a polypeptide binding ligand: For example,
the urokinase receptor (uPAR) is overexpressed in multiple
malignancies and serves a role in tumor invasion and angiogenesis
(16,17). Measles virus retargeted against human
(MV-h-uPA) or mouse (MV-m-uPA) uPAR were constructed by
superinducing the aminoterminal fragment of either human or mouse
urokinase in the C-terminus of recombinant measles virus. In
vitro experiments indicated that MV-h-uPA and MV-m-uPA were
able to specifically infect cancer cells that overexpressed uPAR
via the receptor-ligand pathway (18).
Targeting transcription
The approach to achieving tumor-selective viral
replication has been to alter the function of the control of
genetic transcription, which is essential in viral replication, to
a tissue- or tumor-specific promoter. These promoters include human
telomerase reverse transcription (hTERT) (19), hypoxia-inducible factor-1 (20), prostate-specific antigen (21) and α-fetoprotein (22), among others. hTERT has been identified
as a major protein that functions to maintain telomere length in
tumors; however, it demonstrates little or no expression in normal
cells, allowing cancer cells to subvert the Hayflick limit
(23). An attenuated adenovirus 5
vector, OBP-301, was constructed, whereby the hTERT promoter
element drives the expression of E1 genes: Due to only tumor cells
with telomerase activity possessing the ability to activate this
promoter, selective viral replication and oncolytic cell death was
demonstrated (19).
In addition to this approach, a novel system, which
may regulate oncolytic viral gene-targeted expression by exploiting
microRNAs (miRNAs), has been developed. miRNAs are 20–22
nucleotides of small noncoding endogenously produced RNAs that can
base pair to their target mRNAs. This enables them to guide
post-transcriptional silencing of their target genes and determine
the differential expression between cancerous and normal tissues
(24). The negative regulation in
gene expression makes it possible to utilize these miRNAs to
inhibit viral replication in normal cells (25,26).
Aberrant expression of miRNAs has previously been observed in
numerous types of cancer (27). The
downregulation of miRNA (miR)-199 occurs consistently in almost all
hepatocellular carcinomas (HCC), when compared with a normal liver
(28). Additionally, a conditionally
replication-competent oncolytic adenovirus (OAd), ad-199T, was
generated by introducing miR-199 target sites within the
3′untranslated region of the E1A gene, essential for viral
replication, in order that miR-199 demonstrated the capacity to
negatively regulate E1A gene expression. As a result, ad-199T
replication was inhibited in normal miR-199-positive liver
parenchyma, and unaffected in tumor cells with low expression of
miR-199 (29).
Targeting translation
Type I interferons (IFNs), which are spontaneously
produced in response to a viral infection, are an important
cytokine (12). The mechanism of
cancer evolution that causes the loss of antiviral responsiveness
in the majority of cancer cell lines, particularly the activity of
IFN-regulated signaling pathways, is not completely understood.
Previous studies have revealed that tumor antiviral activity is
incompatible with their own efficient cell growth, as IFN and
IFN-responsive genes are known angiogenesis inhibitors (30), and are also recognized for their
capacity to induce apoptosis (31).
In addition, the deficiency of tumor antiviral activity renders it
more susceptible to an infection compared with normal cells, which
results in a survival advantage for viruses within tumor cells. The
vesicular stomatitis virus (VSV) is sensitive to IFN and therefore
has an advantage in cancer therapeutics, including using a
defective IFN pathway, to preferentially infect and kill tumor
cells (32). The targeting of cancer
cells by the VSV can be improved by introducing mutations in the
matrix (M) protein. The M protein of VSV is a major structural
protein that functions in virus assembly, as well as in the
suppression of host gene expression, causing inhibition of IFNs and
other antiviral proteins. The ability of the M protein to subdue
host gene expression is genetically distinct from its viral
assembly function. Furthermore, various mutations render the M
protein defective in its ability to suppress host gene expression,
without subverting its ability to function in viral assembly
(33). As a result, normal cells, due
to the activated IFN pathway, inhibit the M protein mutant VSV
replication; otherwise cancer cells with defects in the IFN pathway
are killed by M protein mutant VSV selective replication (34).
Targeting pro-apoptosis
mechanisms
TP53 serves a key function in inducing apoptosis by
prompting cell cycle arrest, due to activation in response to
oncogene activation, DNA damage and other stress signals (35). p53 is the most frequently
mutated gene in cancer, being altered in >50% of all types of
human malignancies (36). The mutated
p53 gene is an important hallmark of cancer (37). Viruses have evolved the ability to
inhibit the apoptosis of host cells, in order to accomplish viral
replication. The E1B55K adenovirus codes for the protein that
inactivates the p53 protein of host cells, contributing to
self-proliferation and virus replication (38). China approved the world's first OV
therapy for cancer treatment (39).
OV, also termed H101, has been approved for the treatment of
numerous types of cancer clinically (40), H101 is a type of OAd with E1B-55KD and
partial E3 deletion, and it cannot replicate in normal cells where
p53 is active; therefore, H101 can selectively infect and kill
tumor cells via the targeting of pro-apoptosis (41,42).
Lethal effect of OV on tumor
cells
An important reason why OVs are currently being
advanced as a promising antitumor modality is that they are able to
transfer and amplify therapeutic genes, whilst simultaneously
avoiding immunosurveillance by infected host cells (43). Virosomes are utilized as vectors for
the delivery of toxic genes, including immunostimulatory genes [for
instance, interleukin-12 (IL-12) (44)], anti-angiogenic genes (45), prodrug-converting enzyme genes and
pro-apoptotic genes, to advance its potency, which can be used for
oncotherapy (46).
Immunostimulatory genes
Delivery of immunostimulatory genes to cancer cells
should boost immune responses against tumor antigens and the
activity of cytotoxic effector cells, as a result of an abnormal
increase of inflammatory infiltrates in the tumor milieu (47). Currently, a variety of
immunostimulatory genes are used as part of recombinant oncolytic
virusal vectors, which are utilized in viral gene therapy,
including granulocyte-macrophage colony stimulating factor (GM-CSF)
(48), IL-2, IL-12, IL-15 and IL-18
(49,50), IFN-α and IFN-β (51,52).
Previously, Choi et al (49)
generated IL-12- and IL-18-expressing (Ad-ΔE1Bmt7/IL-12/IL-18) OAd.
IL-12 has been demonstrated to directly activate cytotoxic T cells
and natural killer (NK) cells, which then produce high levels of
IFN-γ, enhance their cytolytic activity and induce an antitumor
effect (46); however, despite
encouraging results in animal models, weaker antitumor effects of
IL-12 were recorded in early clinical trials, and frequently
accompanied by unacceptable levels of adverse events (53,54). This
markedly dampened the hopes of the clinical application of this
cytokine in patients with cancer. In order to minimize the adverse
events of an IL-12-based therapy, a decrease in the systemic
expression of IL-12 has been the selected approach. This approach
involves patients who are injected intratumorally with
Ad-RTS-hIL-12, an adenoviral vector engineered for the controlled
expression of IL-12. Preliminary results were encouraging, with
positive clinical efficacy observed in 5/7 patients treated with
Ad-RTS-IL-12. These responses were associated with intratumoral
IL-12 mRNA expression, reflected as a decrease in the size of the
injected and distant lesions (55).
IL-18 serves a function in inducing the differentiation of
antitumor effector T cells and the activation of NK cells, which
exhibit potent cytotoxicity against tumor cells (56). In a B16-F10 murine melanoma model,
IL-12, IL-18, IFN-γ and GM-CSF levels within the tumor tissues were
significantly increased when treated with the intratumoral
application of OAd co-expressing IL-12 and IL-18 compared with
control virus-treated mice. Higher numbers of CD4+ T
cells, CD8+ T cells and NK cells were detected via an
immunohistochemical staining and the histological evaluation of
tumor sections, which also exhibited large areas of necrosis
compared with control virus-treated mice. These results further
demonstrated the enhanced antitumor effects of OAd that express
IL-12 and IL-18. The results were also validated in a murine colon
adenocarcinoma model of MC38cea (48). IL-12- and IL-18-based immunotherapies
were particularly efficacious in patients with cancer that
contained inherited defects in IL-12 or IL-18 production, or with
downregulated expression of IL-12 or IL-18. Compared with control
virus-treated mice, the intratumoral administration of
GM-CSF-expressing oncolytic MV markedly prolonged the median
overall survival time (49).
Prodrug-converting enzyme genes
OVs were engineered to express prodrug-converting
enzymes, which caused the conversion of the non-toxic prodrug into
a toxic form, at the site of viral replication; therefore, causing
selective tumor cell death. The prodrug-converting enzymes applied
to the oncolytic virotherapy, including thymidine kinase (TK)
(57), cytosine deaminase (CD) and
purine-nucleoside phosphorylase (PNP) (58–60). The
mechanism of action involves the conversion of the prodrug
ganciclovir (GCV), via TK, into ganciclovir triphosphate (GCV-TP),
CD converts the prodrug 5-fluorocytosine (5-FC) into 5-fluorouracil
(5-FU) and PNP converts the prodrug fludarabine phosphate into
2-fluoroadenine. The transformed products are nucleoside analogs,
which interfere with DNA replication in actively dividing tumor
cells via the inhibition of DNA synthesis (61). A new recombinant herpes simplex virus
type 1 thymidine kinase (HSV-1-TK), containing BoHV-4, has been
constructed by Redaelli et al (62). The HSV-TK enzyme, which is an enzyme
homologous to that of TK, has 1,000 times more affinity for the
substrate GCV than the host cell TK (63). HSV-1-TK in combination with GCV has
been identified as a promising suicide gene system, whereby GCV is
phosphorylated by HSV-1-TK to form GCV-TP. GCV-TP has the ability
to inhibit DNA polymerases, resulting from competing with
deoxyguanosine triphosphate to bind to DNA polymerases, causing DNA
damage that leads to cell death (62,64). The
active metabolite of TK, PNP and 5-FU can directly kill infected
tumor cells. Furthermore, they promote a strong bystander effect on
neighboring tumor cells that are not actively infected (59,65,66). In a
previous study by Leveille et al (67), recombinant vesicular stomatitis virus
(VSV-MD51) expressing the cytosine deaminase/uracil
phosphoribosyl-transferase suicide gene with the 5-FC prodrug was
combined for the treatment of cancer. It was observed that there
was a synergistic effect on killing cancer cells with VSV-MD51 and
5-FU in several cancer cell lines. The high solubility of 5-FU is
lethal to non-infected bystander tumor cells, which is an important
advantage in using this combination.
Genes encoding the inhibitors of
angiogenesis
In 1971, Folkman (68), to the best of our knowledge, first
produced the hypothesis that tumor growth depends on angiogenesis,
which also serves an essential role in tumor invasion and
metastasis. Within four decades, anti-angiogenic therapy with broad
targeting has rapidly evolved to become an integral component of
current standard anticancer treatments (69,70).
Endostatin and angiostatin are two endogenous and broad-spectrum
angiogenesis inhibitors. These inhibitors only function when they
are continuously transported to the tumor microenvironment
(71,72). Additionally, it is difficult to make
the two endogenous inhibitors function in the traditional way, due
to characteristics of short serum half-lives, low solubility and
poor stability (73,74). Hutzen et al (73) developed recombinant MVs that express
human and mouse variants of endostatin: Angiostatin (E:A) fusion
proteins, known as MV-hE:A and MV-mE:A, respectively. The in
vitro study revealed that there is active MV replication and
concomitant continuous expression of target genes within the
medulloblastoma cells; therefore, angiogenic factors were
inhibited, leading to a significant decrease in endothelial cell
growth, viability and migration (73).
New trends in OV based approaches
Gene-based virotherapy mediated by OV is currently
the focus of numerous studies; however, little attention has been
given to the dual gene virotherapy strategy, which could be
utilized as a novel therapeutic approach for mediating triplex
anticancer combination effects, particularly if the two suitable
genes are well selected (75). A
number of the previously published reports have stated that a
single therapeutic modality, including those mediated by tumor
necrosis factor-associated apoptosis-inducing ligand (TRAIL) or
IL-12 alone, could not achieve sufficient antitumor responses;
therefore, combinations of anticancer therapeutics, including those
mediated by dual gene-based cancer therapy, hold great promise for
killing cancer cells in the future (76–78). The
anticancer therapeutic potential of OAd virotherapy
strategy-mediated co-delivery of TRAIL and IL-12 genes has not been
sufficiently investigated thus far. El-Shemi et al (79) suggested in their preclinical study
that dual therapy with Ad-ΔB/TRAIL plus Ad-ΔB/IL-12 markedly
suppressed human HCC via the promotion of antitumor apoptosis and
immune activity, as well as by inhibiting tumor angiogenesis and
neovascularization. Although further studies are warranted to
evaluate this therapeutic combination, and also to explore the
precise anti-tumor mechanisms, the OAd strategy-mediated
co-delivery of TRAIL and IL-12 genes may be a potential therapeutic
strategy for the treatment of human HCC, along with other types of
cancer (79). Co-therapy with
OAd-expressing TRAIL and another type of immunostimulant cytokine
(IL-24) has been previously reported to be associated with potent
activation in the caspase pathway, particularly of caspases-3 and
−8, and apoptosis promotion in HCC (80). Comparatively, Han et al
(81) demonstrated that treating
patients with pancreatic cancer with gemcitabine and OAd armed with
survivin shRNA and TRAIL greatly enhanced the cytotoxic death of
pancreatic cancer cells (81). In
addition, OVs utilized to simultaneously express dual anticancer
genes have attracted an increased interest, as it has provided a
multimodal method of killing cancer cells with an increased
effectiveness, via a selective viral lytic effect on cancer cells
and the additive or synergistic interaction between the two
expressed anticancer genes (82,83).
Conclusions
The oncolytic virotherapy area of study for cancer
therapeutics has experienced considerable progress in recent years.
Preclinical models have demonstrated an anticancer activity against
numerous types of cancer (84).
Currently, several recombinant viruses, including adenovirus,
herpes simplex virus, vaccinia virus, reovirus, Newcastle disease
virus and Parvovirus are in late phase clinical trials. A number of
the clinical trials have indicated promising results, including in
the safety and reliability of the treatment, particularly when
combined with standard antineoplastic therapies (85). There is hope that oncolytic
virotherapy will be included in the armamentarium of anticancer
agents, and that certain groups of patients will benefit from the
treatment. Despite the renewed hope, the following key challenges
remain: OV cannot be viewed as a stand-alone therapy for any type
of cancer; the safety of systemic administration remains untested;
and the optimal arming strategies that combine chemo-, radio- and
immuno-therapies require investigation (2). With the rapid development of molecular
biology and cell biology techniques, cancer is viewed as a systemic
and heterogeneous disease; therefore, using OV as a single therapy
would be difficult when attempting to achieve the desired
therapeutic effect. Individual and comprehensive therapies have
become new trends in cancer therapy. Conventional therapies,
including radiotherapy and chemotherapy, have various
disadvantages, such as limited efficacy low specificity,
cross-resistance, severe adverse effects and an eventual inability
to meet the requirements of the patient (2). Oncolytic virotherapy as a new modality
of cancer therapy is promising, and may be used to complement
conventional therapies; however, the efficacy of combination
therapy regimens must be validated. In the foreseeable future, an
ever-growing number of recombinant OV may be used in clinical
trials and applied in clinical practice.
Glossary
Abbreviations
Abbreviations:
|
OV
|
oncolytic viruses
|
|
uPAR
|
urokinase receptor
|
|
GM-CSF
|
granulocyte-macrophage colony
stimulating factor
|
|
OAd
|
oncolytic adenovirus
|
References
|
1
|
Bell J and McFadden G: Viruses for tumor
therapy. Cell Host Microbe. 15:260–265. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fukuhara H, Ino Y and Todo T: Oncolytic
virus therapy: A new era of cancer treatment at dawn. Cancer Sci.
107:1373–1379. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liu TC, Galanis E and Kirn D: Clinical
trial results with oncolytic virotherapy: A century of promise, a
decade of progress. Nat Clin Pract Oncol. 4:101–117. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nemunaitis J, Ganly I, Khuri F, Arseneau
J, Kuhn J, McCarty T, Landers S, Maples P, Romel L, Randlev B, et
al: Selective replication and oncolysis in p53 mutant tumors with
ONYX-015, an E1B-55kD gene-deleted adenovirus, in patients with
advanced head and neck cancer: A phase II trial. Cancer Res.
60:6359–6366. 2000.PubMed/NCBI
|
|
5
|
Ruf B and Lauer UM: Assessment of current
virotherapeutic application schemes: ‘Hit hard and early’ versus
‘killing softly’? Mol Ther Oncolytics. 4:150182015. View Article : Google Scholar
|
|
6
|
Breitbach CJ, Lichty BD and Bell JC:
Oncolytic viruses: Therapeutics with an identity crisis.
EBioMedicine. 9:31–36. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cross D and Burmester JK: Gene therapy for
cancer treatment: Past, present and future. Clini Med Res.
4:218–227. 2006. View Article : Google Scholar
|
|
8
|
Liu TC and Kirn D: Gene therapy progress
and prospects cancer: Oncolytic viruses. Gene Ther. 15:877–884.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Russell SJ, Peng KW and Bell JC: Oncolytic
virotherapy. Nat Biotechnol. 30:658–670. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Galanis E, Atherton PJ, Maurer MJ, Knutson
KL, Dowdy SC, Cliby WA, Haluska P Jr, Long HJ, Oberg A, Aderca I,
et al: Oncolytic measles virus expressing the sodium iodide
symporter to treat drug-resistant ovarian cancer. Cancer Res.
75:22–30. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hanahan D and Weinberg RA: The hallmarks
of cancer. Cell. 100:57–70. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ilkow CS, Swift SL, Bell JC and Diallo JS:
From scourge to cure: Tumour-selective viral pathogenesis as a new
strategy against cancer. PLoS Pathog. 10:e10038362014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cattaneo R, Miest T, Shashkova EV and
Barry MA: Reprogrammed viruses as cancer therapeutics: Targeted,
armed and shielded. Nature Rev Microbiol. 6:529–540. 2008.
View Article : Google Scholar
|
|
14
|
Gromeier M, Lachmann S, Rosenfeld MR,
Gutin PH and Wimmer E: Intergeneric poliovirus recombinants for the
treatment of malignant glioma. Proc Natl Acad Sci the USA. 97:pp.
6803–6808. 2000; View Article : Google Scholar
|
|
15
|
Merrill MK, Bernhardt G, Sampson JH,
Wikstrand CJ, Bigner DD and Gromeier M: Poliovirus receptor
CD155-targeted oncolysis of glioma. Neuro Oncol. 6:208–217. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Blasi F and Carmeliet P: uPAR: A versatile
signalling orchestrator. Nat Rev Mol Cell Biol. 3:932–943. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lester RD, Jo M, Montel V, Takimoto S and
Gonias SL: uPAR induces epithelial-mesenchymal transition in
hypoxic breast cancer cells. J Cell Biol. 178:425–436. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jing Y, Zaias J, Duncan R, Russell SJ and
Merchan JR: In vivo safety, biodistribution and antitumor effects
of uPAR retargeted oncolytic measles virus in syngeneic cancer
models. Gene Ther. 21:289–297. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sato D, Kurihara Y, Kondo S, Shirota T,
Urata Y, Fujiwara T and Shintani S: Antitumor effects of
telomerase-specific replication-selective oncolytic viruses for
adenoid cystic carcinoma cell lines. Oncol Rep. 30:2659–2664. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Longo SL, Griffith C, Glass A, Shillitoe
EJ and Post DE: Development of an oncolytic herpes simplex virus
using a tumor-specific HIF-responsive promoter. Cancer Gene Ther.
18:123–134. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lu Y, Zhang Y, Chang G and Zhang J:
Comparison of prostate-specific promoters and the use of PSP-driven
virotherapy for prostate cancer. Biomed Res Int. 2013:6246322013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang KJ, Zhang J, Wu YM, Qian J, Liu XJ,
Yan LC, Zhou XM, Xiao RJ, Wang YG, Cao X, et al: Complete
eradication of hepatomas using an oncolytic adenovirus containing
AFP promoter controlling E1A and an E1B deletion to drive IL-24
expression. Cancer Gene Ther. 19:619–629. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Verdun RE and Karlseder J: Replication and
protection of telomeres. Nature. 447:924–931. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Giandomenico V, Thirlwell C and Essand M:
Other Novel Therapies: Biomarkers, microRNAs and microRNA
inhibitors, DNA methylation, epigenetics, immunotherapy and
virotherapy. Front Horm Res. 44:248–262. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ruiz AJ and Russell SJ: MicroRNAs and
oncolytic viruses. Curr Opin Virol. 13:40–48. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yao W, Guo G, Zhang Q, Fan L, Wu N and Bo
Y: The application of multiple miRNA response elements enables
oncolytic adenoviruses to possess specificity to glioma cells.
Virology. 458–459, 1-82. 2014.
|
|
27
|
Negrini M, Ferracin M, Sabbioni S and
Croce CM: MicroRNAs in human cancer: From research to therapy. J
Cell Sci. 120:1833–1840. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Murakami Y, Yasuda T, Saigo K, Urashima T,
Toyoda H, Okanoue T and Shimotohno K: Comprehensive analysis of
microRNA expression patterns in hepatocellular carcinoma and
non-tumorous tissues. Oncogene. 25:2537–2545. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Callegari E, Elamin BK, D'Abundo L,
Falzoni S, Donvito G, Moshiri F, Milazzo M, Altavilla G, Giacomelli
L, Fornari F, et al: Anti-tumor activity of a miR-199-dependent
oncolytic adenovirus. PLoS One. 8:e739642013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Indraccolo S: Interferon-alpha as
angiogenesis inhibitor: Learning from tumor models. Autoimmunity.
43:244–247. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kotredes KP and Gamero AM: Interferons as
inducers of apoptosis in malignant cells. J Interferon Cytokine
Res. 33:162–170. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Balachandran S and Barber GN: Vesicular
stomatitis virus (VSV) therapy of tumors. IUBMB Life. 50:135–138.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ahmed M, Cramer SD and Lyles DS:
Sensitivity of prostate tumors to wild type and M protein mutant
vesicular stomatitis viruses. Virology. 330:34–49. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Stewart JH IV, Ahmed M, Northrup SA,
Willingham M and Lyles DS: Vesicular stomatitis virus as a
treatment for colorectal cancer. Cancer Gene Ther. 18:837–849.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bradner JE: Cancer: An essential passenger
with p53. Nature. 520:626–627. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liu J, Zhang C and Feng Z: Tumor
suppressor p53 and its gain-of-function mutants in cancer. Acta
Biochim Biophys Sin (Shanghai). 46:170–179. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Duffy MJ, Synnott NC, McGowan PM, Crown J,
O'Connor D and Gallagher WM: p53 as a target for the treatment of
cancer. Cancer Treat Rev. 40:1153–1160. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bischoff JR, Kirn DH, Williams A, Heise C,
Horn S, Muna M, Ng L, Nye JA, Sampson-Johannes A, Fattaey A and
McCormick F: An adenovirus mutant that replicates selectively in
p53-deficient human tumor cells. Science. 274:373–376. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Garber K: China approves world's first
oncolytic virus therapy for cancer treatment. J Natl Cancer Inst.
98:298–300. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Cheng PH, Wechman SL, McMasters KM and
Zhou HS: Oncolytic replication of E1b-Deleted adenoviruses.
Viruses. 7:5767–5779. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Song X, Zhou Y, Jia R, Xu X, Wang H, Hu J,
Ge S and Fan X: Inhibition of retinoblastoma in vitro and in vivo
with conditionally replicating oncolytic adenovirus H101. Invest
Ophthalmol Vis Sci. 51:2626–2635. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yu W and Fang H: Clinical trials with
oncolytic adenovirus in China. Curr Cancer Drug Targets. 7:141–148.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kaneda Y: A non-replicating oncolytic
vector as a novel therapeutic tool against cancer. BMB Rep.
43:773–780. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Gil-Farina I, Di Scala M, Vanrell L,
Olagüe C, Vales A, High KA, Prieto J, Mingozzi F and
Gonzalez-Aseguinolaza G: IL12-mediated liver inflammation reduces
the formation of AAV transcriptionally active forms but has no
effect over preexisting AAV transgene expression. PLoS One.
8:e677482013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Guse K, Sloniecka M, Diaconu I,
Ottolino-Perry K, Tang N, Ng C, Le Boeuf F, Bell JC, McCart JA,
Ristimäki A, et al: Antiangiogenic arming of an oncolytic vaccinia
virus enhances antitumor efficacy in renal cell cancer models. J
Virol. 84:856–866. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Jeyaretna DS and Kuroda T: Recent advances
in the development of oncolytic HSV-1 vectors: ‘Arming’ of HSV-1
vectors and application of bacterial artificial chromosome
technology for their construction. Curr Opin Mol Ther. 9:447–466.
2007.PubMed/NCBI
|
|
47
|
Tsun A, Miao XN, Wang CM and Yu DC:
Oncolytic immunotherapy for treatment of cancer. Adv Exp Med Biol.
909:241–283. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Grossardt C, Engeland CE, Bossow S, Halama
N, Zaoui K, Leber MF, Springfeld C, Jaeger D, von Kalle C and
Ungerechts G: Granulocyte-macrophage colony-stimulating
factor-armed oncolytic measles virus is an effective therapeutic
cancer vaccine. Hum Gene Ther. 24:644–654. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Choi IK, Lee JS, Zhang SN, Park J, Sonn
CH, Lee KM and Yun CO: Oncolytic adenovirus co-expressing IL-12 and
IL-18 improves tumor-specific immunity via differentiation of T
cells expressing IL-12Rβ2 or IL-18Rα. Gene Ther. 18:898–909. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
van Rikxoort M, Michaelis M, Wolschek M,
Muster T, Egorov A, Seipelt J, Doerr HW and Cinatl J Jr: Oncolytic
effects of a novel influenza A virus expressing interleukin-15 from
the NS reading frame. PLoS One. 7:e365062012. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Li H, Peng KW, Dingli D, Kratzke RA and
Russell SJ: Oncolytic measles viruses encoding interferon beta and
the thyroidal sodium iodide symporter gene for mesothelioma
virotherapy. Cancer Gene Ther. 17:550–558. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Wang CJ, Xiao CW, You TG, Zheng YX, Gao W,
Zhou ZQ, Chen J, Xue XB, Fan J and Zhang H: Interferon-α enhances
antitumor activities of oncolytic adenovirus-mediated IL-24
expression in hepatocellular carcinoma. Mol Cancer. 11:312012.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Cao L, Zeng Q, Xu C, Shi S, Zhang Z and
Sun X: Enhanced antitumor response mediated by the codelivery of
paclitaxel and adenoviral vector expressing IL-12. Mol Pharm.
10:1804–1814. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Gollob JA, Mier JW, Veenstra K, McDermott
DF, Clancy D, Clancy M and Atkins MB: Phase I trial of twice-weekly
intravenous interleukin 12 in patients with metastatic renal cell
cancer or malignant melanoma: Ability to maintain IFN-gamma
induction is associated with clinical response. Clin Cancer Res.
6:1678–1692. 2000.PubMed/NCBI
|
|
55
|
Lasek W, Zagożdżon R and Jakobisiak M:
Interleukin 12: Still a promising candidate for tumor
immunotherapy? Cancer Immunol Immunother. 63:419–435. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Tomura M, Zhou XY, Maruo S, Ahn HJ,
Hamaoka T, Okamura H, Nakanishi K, Tanimoto T, Kurimoto M and
Fujiwara H: A critical role for IL-18 in the proliferation and
activation of NK1.1+ CD3-cells. J Immunol. 160:4738–4746.
1998.PubMed/NCBI
|
|
57
|
Chen C, Fang H, Han Z, Ye F, Ji T, Gong D,
Li F, Zhou J, Ma D and Gao Q: Novel permissive murine
immunocompetent orthotopic colon carcinoma model for comparison of
the antitumoral and safety profiles of three Adv-TKs. Gene Ther.
22:702015. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Graepler F, Lemken ML, Wybranietz WA,
Schmidt U, Smirnow I, Gross CD, Spiegel M, Schenk A, Graf H, Lauer
UA, et al: Bifunctional chimeric SuperCD suicide gene-YCD: YUPRT
fusion is highly effective in a rat hepatoma model. World J
Gastroenterol. 11:6910–6919. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Lampe J, Bossow S, Weiland T, Smirnow I,
Lehmann R, Neubert W, Bitzer M and Lauer UM: An armed oncolytic
measles vaccine virus eliminates human hepatoma cells independently
of apoptosis. Gene Ther. 20:1033–1041. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Moriuchi S, Wolfe D, Tamura M, Yoshimine
T, Miura F, Cohen JB and Glorioso JC: Double suicide gene therapy
using a replication defective herpes simplex virus vector reveals
reciprocal interference in a malignant glioma model. Gene Ther.
9:584–591. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Yamada S, Kuroda T, Fuchs BC, He X, Supko
JG, Schmitt A, McGinn CM, Lanuti M and Tanabe KK: Oncolytic herpes
simplex virus expressing yeast cytosine deaminase: Relationship
between viral replication, transgene expression, prodrug
bioactivation. Cancer Gene Ther. 19:160–170. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Redaelli M, Franceschi V, Capocefalo A,
D'Avella D, Denaro L, Cavirani S, Mucignat-Caretta C and Donofrio
G: Herpes simplex virus type 1 thymidine kinase-armed bovine
herpesvirus type 4-based vector displays enhanced oncolytic
properties in immunocompetent orthotopic syngenic mouse and rat
glioma models. Neuro Oncol. 14:288–301. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Elion GB, Furman PA, Fyfe JA, de Miranda
P, Beauchamp L and Schaeffer HJ: Selectivity of action of an
antiherpetic agent, 9-(2-hydroxyethoxymethyl) guanine. Proc Natl
Acad Sci USA. 74:pp. 5716–5720. 1977; View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Boucher PD, Ostruszka LJ and Shewach DS:
Synergistic enhancement of herpes simplex virus thymidine
kinase/ganciclovir-mediated cytoxicity by hydroxyurea. Cancer Res.
60:1631–1636. 2000.PubMed/NCBI
|
|
65
|
Freeman SM, Abboud CN, Whartenby KA,
Packman CH, Koeplin DS, Moolten FL and Abraham GN: The ‘bystander
effect’: Tumor regression when a fraction of the tumor mass is
genetically modified. Cancer Res. 53:5274–5283. 1993.PubMed/NCBI
|
|
66
|
Hong JS, Waud WR, Levasseur DN, Townes TM,
Wen H, McPherson SA, Moore BA, Bebok Z, Allan PW, Secrist JA III,
et al: Excellent in vivo bystander activity of fludarabine
phosphate against human glioma xenografts that express the
escherichia coli purine nucleoside phosphorylase gene. Cancer Res.
64:6610–6615. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Leveille S, Samuel S, Goulet ML and
Hiscott J: Enhancing VSV oncolytic activity with an improved
cytosine deaminase suicide gene strategy. Cancer Gene Ther.
18:435–443. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Folkman J: Tumor angiogenesis: Therapeutic
implications. N Engl J Med. 285:1182–1186. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Folkman J: What is the evidence that
tumors are angiogenesis dependent? J Natl Cancer Inst. 82:4–6.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Wang Z, Dabrosin C, Yin X, Fuster MM,
Arreola A, Rathmell WK, Generali D, Nagaraju GP, El-Rayes B,
Ribatti D, et al: Broad targeting of angiogenesis for cancer
prevention and therapy. Semin Cancer Biol. 35 Suppl:S224–S243.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
O'Reilly MS, Boehm T, Shing Y, Fukai N,
Vasios G, Lane WS, Flynn E, Birkhead JR, Olsen BR and Folkman J:
Endostatin: An endogenous inhibitor of angiogenesis and tumor
growth. Cell. 88:277–285. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
O'Reilly MS, Holmgren L, Shing Y, Chen C,
Rosenthal RA, Cao Y, Moses M, Lane WS, Sage EH and Folkman J:
Angiostatin: A circulating endothelial cell inhibitor that
suppresses angiogenesis and tumor growth. Cold Spring Harb Symp
Quant Biol. 59:471–482. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Hutzen B, Bid HK, Houghton PJ, Pierson CR,
Powell K, Bratasz A, Raffel C and Studebaker AW: Treatment of
medulloblastoma with oncolytic measles viruses expressing the
angiogenesis inhibitors endostatin and angiostatin. BMC Cancer.
14:2062014. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Shin SU, Cho HM, Merchan J, Zhang J,
Kovacs K, Jing Y, Ramakrishnan S and Rosenblatt JD: Targeted
delivery of an antibody-mutant human endostatin fusion protein
results in enhanced antitumor efficacy. Mol Cancer Ther.
10:603–614. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Refaat A, Abd-Rabou A and Reda A: TRAIL
combinations: The new ‘trail’ for cancer therapy (Review). Oncol
Lett. 7:1327–1332. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Lichty BD, Breitbach CJ, Stojdl DF and
Bell JC: Going viral with cancer immunotherapy. Nat Rev Cancer.
14:559–567. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Hao C, Song JH, Hsi B, Lewis J, Song DK,
Petruk KC, Tyrrell DL and Kneteman NM: TRAIL inhibits tumor growth
but is nontoxic to human hepatocytes in chimeric mice. Cancer Res.
64:8502–8506. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Poutou J, Bunuales M, Gonzalez-Aparicio M,
Garcia-Aragoncillo E, Quetglas JI, Casado R, Bravo-Perez C,
Alzuguren P and Hernandez-Alcoceba R: Safety and antitumor effect
of oncolytic and helper-dependent adenoviruses expressing
interleukin-12 variants in a hamster pancreatic cancer model. Gene
Ther. 22:696–706. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
El-Shemi AG, Ashshi AM, Na Y, Li Y,
Basalamah M, Al-Allaf FA, Oh E, Jung BK and Yun CO: Combined
therapy with oncolytic adenoviruses encoding TRAIL and IL-12 genes
markedly suppressed human hepatocellular carcinoma both in vitro
and in an orthotopic transplanted mouse model. J Exp Clin Cancer
Res. 35:742016. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Cai Y, Liu X, Huang W and Liu XY:
Synergistic antitumor effect of TRAIL and IL-24 with complete
eradication of hepatoma in the CTGVT-DG strategy. Acta Biochim
Biophys Sin (Shanghai). 44:535–543. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Han Z, Lee S, Je S, Eom CY, Choi HJ, Song
JJ and Kim JH: Survivin silencing and TRAIL expression using
oncolytic adenovirus increase anti-tumorigenic activity in
gemcitabine-resistant pancreatic cancer cells. Apoptosis.
21:351–364. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Liu X, Cao X, Wei R, Cai Y, Li H, Gui J,
Zhong D, Liu XY and Huang K: Gene-viro-therapy targeting liver
cancer by a dual-regulated oncolytic adenoviral vector harboring
IL-24 and TRAIL. Cancer Gene Ther. 19:49–57. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Gao Y, Zhu Y, Huang X, Ai K, Zheng Q and
Yuan Z: Gene therapy targeting hepatocellular carcinoma by a
dual-regulated oncolytic adenovirus harboring the focal adhesion
kinase shRNA. Int J Oncol. 47:668–678. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Lawler SE, Speranza MC, Cho CF and Chiocca
EA: Oncolytic viruses in cancer treatment: A review. JAMA Oncol.
3:841–849. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Warner SG, O'Leary MP and Fong Y:
Therapeutic oncolytic viruses: Clinical advances and future
directions. Curr Opin Oncol. 29:359–365. 2017. View Article : Google Scholar : PubMed/NCBI
|