Introduction
Gastric adenocarcinoma is a major contributor to the
global health burden, ranking as the fifth most common type of
malignancy worldwide (1). Gastric
adenocarcinoma is the second-leading cause of cancer death owing to
its poor prognosis (2). The
identification of novel biomarkers with potential prognostic value
would aid the assessment of patient prognosis and the selection of
therapeutic treatments for individual patients with gastric
adenocarcinoma.
Scavenger receptor class B type I (SR-BI) is a
well-documented high-density lipoprotein (HDL) receptor, which is
most abundantly expressed in liver and the steroidogenic tissues,
including the ovaries, testes and adrenal glands (3). SR-BI mediates the selective uptake of
HDL cholesteryl esters and the bidirectional transfer of
unesterified cholesterol between cells and HDL. In addition, SR-BI
serves a role in sepsis, adaptive immune and hepatitis C virus
entry (4–6). It is becoming increasingly apparent that
SR-BI serves a role in cancer development (7,8).
Pronounced expression of SR-BI was observed in a variety of
carcinomas, including hepatoma, prostate, breast, colorectal,
pancreatic, ovarian, and nasopharyngeal cancer (9–12).
Moreover, SR-BI has been demonstrated to exert a profound influence
on the proliferation, migration and invasion of breast and prostate
cancer cells (7,8). In human stomach, Lobo et al
(13) reported that SR-BI was not
detected in epithelial, parietal, mucous and endocrinal cells.
However, to the best of our knowledge, the status of SR-BI
expression in gastric adenocarcinoma and its clinical significance
have not been reported to date.
The present study evaluated the expression of SR-BI
using a high-throughput tissue microarray containing 90 cases of
gastric carcinomas, with the aim of investigating its association
with clinicopathological variables and patient outcome.
Materials and methods
Human gastric adenocarcinoma tissue
microarray
The commercial gastric adenocarcinoma tissue
microarray (cat. no. HStm-Ade180Sur-06; Shanghai Outdo Biotech Co.,
Ltd., Shanghai, China) contained samples from 90 individual cases,
with each adjacent non-cancerous tissue placed next to its matched
cancer tissue. Of the 90 invasive carcinoma samples used to
construct the tissue microarray, 84 were available for evaluation,
excluding the uninformative tissue microarray cores that were
either lost or fragmented during the immunohistochemical procedure.
The clinical characteristics of the 84 patients are listed in
Table I. The cohort included 50 male
and 34 female patients, with a median age of 63 years (range 28–88
years) at the time of surgery. These specimens were collected from
patients who underwent primary surgical resection between August
2008 and March 2009. No chemotherapy or radiotherapy was conducted
in these patients prior to surgery. All the gastric adenocarcinoma
patients included had well-documented clinicopathological data and
follow-up information. The histological grade was determined
according to the World Health Organization classification criteria
(14). The histotype was based on the
criteria of Lauren's classification (15). The pathological Tumor-Node-Metastasis
(pTNM) stage was assessed according to the 7th Edition of the
staging system set out by American Joint Committee on Cancer (AJCC)
(16). Overall survival (OS) time,
defined as the time from the date of surgery to death or last
follow-up, was used as a measure of prognosis. All patients were
followed-up until death or until September 2014 with a median of 24
months (range, 1–73 months). The research was approved by the
Ethical Committee of Shandong Provincial Hospital affiliated to
Shandong University (Jinan, China).
 | Table I.Clinicopathological characteristics of
the patient cohort (n=84). |
Table I.
Clinicopathological characteristics of
the patient cohort (n=84).
| Variable | Patients, % (n) |
|---|
| Age, years |
|
|
<60 | 38 (32) |
| ≥60 | 62 (52) |
| Gender |
|
|
Female | 40 (34) |
| Male | 60 (50) |
| Tumor size, cm |
|
|
<4 | 26 (22) |
| ≥4 | 74 (62) |
| Grade |
|
|
Well/moderate | 23 (19) |
|
Poor | 77 (65) |
| Lauren type |
|
|
Intestinal type | 57 (48) |
| Diffuse
type | 43 (36) |
| Tumor location |
|
|
Cardia | 11 (9) |
|
Body/antrum | 89 (75) |
| Lymphatic
invasion |
|
|
Negative | 73 (61) |
|
Positive | 27 (23) |
| pTNM stage |
|
|
I–II | 39 (33) |
|
III–IV | 61 (51) |
| T
classification |
|
|
T1-T2 | 14 (12) |
|
T3-T4 | 86 (72) |
| N
classification |
|
| N0 | 26 (22) |
|
N1-N3 | 74 (62) |
| Distant
metastasis |
|
| M0 | 95 (80) |
| M1 | 5 (4) |
Immunohistochemistry
The tissue microarray slide was deparaffinized and
rehydrated in graded solutions of ethanol (100, 95, 80, and 70%)
and distilled water for 5 min in room temperature. For antigen
retrieval, the slide was immersed in 10 mM citrate buffer (pH 6)
and boiled for 5 min in a pressure cooker. Following incubation
with 3% hydrogen peroxide for 15 min at room temperature to
eliminate endogenous peroxidase activity, the slide was incubated
with normal goat serum (dilution 1:10; cat. no. C-0005; Jiangxi
Haoran Bio-Pharma Co., Ltd., Shanghai, China) for 20 min at 37°C to
reduce non-specific staining. The slide was then incubated with the
monoclonal rabbit anti-human SR-BI antibody (dilution 1:100; cat.
no. EP1556Y; Abcam, Cambridge, UK) overnight at 4°C in a moist
chamber. The following day, the slide was washed three times in PBS
and incubated with a horseradish peroxidase-conjugated goat
anti-rabbit antibody (dilution 1:1; cat. no. SP-9001; Zhongshan
Biotechnology Co., Beijing, China) for 30 min at room temperature.
Bound antibodies were visualized using diaminobenzidine prior to
counterstaining with hematoxylin for 5 min at room temperature.
Negative control was included by replacement of the primary
antibody with phosphate-buffered saline. The specificity of the
rabbit anti-human SR-BI antibody was confirmed by Bogan et
al (17) with the synthetic
peptide used to produce the antibody as a control. The slides were
then visualized using a bright field microscope (Olympus CX31;
Olympus Corporation, Tokyo, Japan) under ×40 or ×200
magnification.
Immunohistochemical evaluation
Immunohistochemical staining for SR-BI expression
was evaluated and scored independently by two pathologists blinded
to the clinicopathological characteristics and outcomes of the
patients. The immunostaining scores were based on the staining
intensity and the proportion of stained cells. Staining intensity
was scored as follows: 0, negative; 1, weak; 2, moderate; and 3
strong. The percentage of positive-stained cells was scored as
follows: 0, 0%; 1, <10%; 2, 10–50%; 3, 51–80%; and 4, >80%.
For each case, a modified immunoreactive score was obtained by
multiplying the intensity and the percentage scores (18), with scores ranging from 0 to 12. Tumor
specimens with a final score of 0–4 were considered to exhibit low
expression and those with a final score of 6–12 were regarded to
exhibit high expression. Discrepancies between the pathologists
were resolved by consensus following discussion.
Statistical analysis
All statistical analyses were performed using SPSS
software (version 19; IBM Corp., Armonk, NY, USA). The
χ2 test or Fisher's exact test was used to analyze the
associations between SR-BI expression and clinicopathological
factors. Survival analysis was performed using the Kaplan-Meier
method, with the log-rank test used to compare between different
patient groups. Multivariate analysis was performed using a Cox
proportional hazard model to evaluate the effect of
clinicopathological variables and SR-BI expression on OS rate
[hazard ratios (HRs) and 95% confidence intervals (CIs) were
calculated]. All statistical tests were two-sided; P<0.05 was
considered to indicate a statistically significant difference.
Results
Clinicopathological features of the
specimens
Tumor size was determined on the basis of the
maximum diameter of the primary lesions. There were 22 cases with
tumors <4 cm and 62 with tumors ≥4 cm. A total of 65 patients
had poorly differentiated tumors and 19 patients had moderately or
well differentiated tumors. The samples included 48 intestinal-type
carcinomas and 36 diffuse-type carcinomas, according to Lauren's
classification. The tumor location distribution was 11% cardia and
89% non-cardia. Lymphatic invasion was found in 23 cases. According
to the pTNM classification system, 8 cases were categorized as
stage I, 25 cases as stage II, 47 cases as stage III, and 4 cases
as stage IV. A total of 62 patients had lymph node metastasis and 4
patients had distant metastasis.
Associations between SR-BI expression
and clinicopathological parameters
In gastric non-cancerous tissue, strong SR-BI
staining was observed in the cells of the fundic glands, but was
barely detectable in the surface epithelial cells adjacent to the
gastric lumen (Fig. 1A and B).
Expression of SR-BI was observed at membranous and cytoplasmic
localizations. Among 84 cases of gastric adenocarcinoma, expression
of SR-BI was observed in 58 (69%) cases. More specifically, 24
(28.6%) samples exhibited high SR-BI expression (range 6–10,
average 8.2), whereas the remaining 60 cases (71.4%) exhibited low
SR-BI expression (range 0–4, average 1.8). No staining of SR-BI was
observed in the negative control slide. The association between
SR-BI expression and clinicopathological features of gastric
adenocarcinomas was analyzed. As summarized in Table II, the proportion of carcinomas
exhibiting high SR-BI protein expression of was 10/19 (52.6%) in
well-and moderately-differentiated cancer samples and 14/65 (21.5%)
in poorly-differentiated carcinoma samples, and a significant
inverse association was found between SR-BI expression and
histological grade (P=0.008). Similarly, high SR-BI expression was
observed more frequently in the Lauren intestinal type (Fig. 1C and D) than in the diffuse type of
gastric carcinomas (Fig. 1E and F)
according to the Lauren classification (P<0.001). In addition,
SR-BI expression was significantly associated with T stage
(P<0.001) and N stage (P=0.010). However, no significant
association was observed between SR-BI expression and age, gender,
tumor size, tumor location, lymphatic invasion, distant metastasis
or pTNM stage.
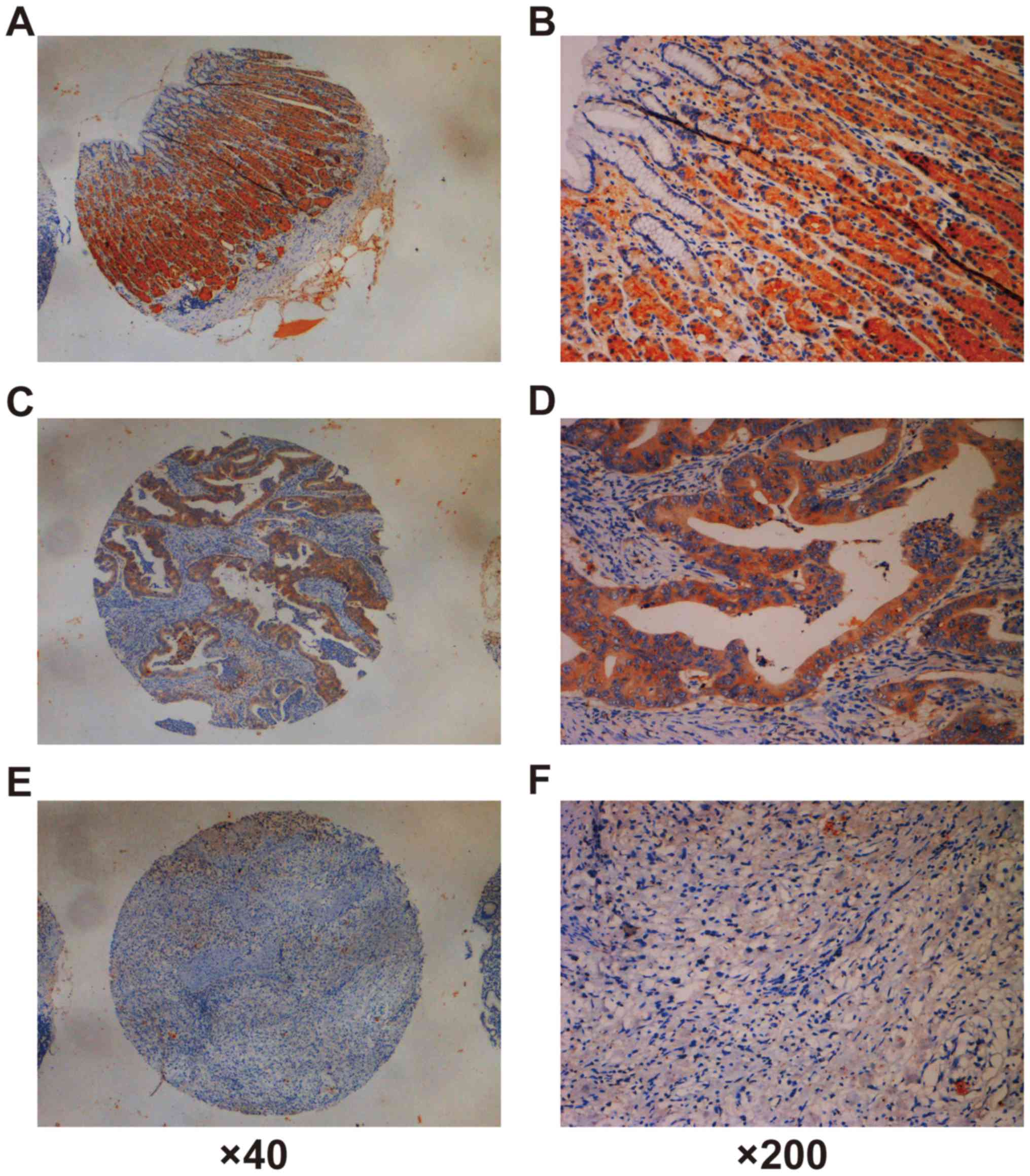 | Figure 1.Immunohistochemical staining of SR-BI
in adjacent non-cancerous tissue and gastric adenocarcinoma
tissues. (A and B) In gastric non-cancerous tissue, high SR-BI
staining was observed in the cells of the fundic glands, but was
barely detecTable in the surface epithelial cells adjacent to the
gastric lumen. (C and D) Representative images show that high SR-BI
expression was detected in a Lauren intestinal type carcinoma case
(intensity score, 2; final score, 8). (E and F) Representative
images depicting that low SR-BI expression was detected in a Lauren
diffuse type of gastric carcinoma case (intensity score, 0; final
score, 0). (A, C and E, ×40 magnification; B, D and F, ×200
magnification). SR-BI, Scavenger receptor class B type I. |
 | Table II.Association of SR-BI protein
expression with clinicopathological characteristics in patients
with gastric cancer (n=84). |
Table II.
Association of SR-BI protein
expression with clinicopathological characteristics in patients
with gastric cancer (n=84).
|
|
| SR-BI expression, %
(n) |
|
|---|
|
|
|
|
|
|---|
| Variable | Patients, n | Low | High | P-value |
|---|
| Total | 84 | 71 (60) | 29 (24) |
|
| Age, years |
|
|
| 0.118 |
|
<60 | 32 | 43 (26) | 25 (6) |
|
|
≥60 | 52 | 57 (34) | 75 (18) |
|
| Gender |
|
|
| 0.527 |
|
Female | 34 | 38 (23) | 46 (11) |
|
|
Male | 50 | 62 (37) | 54 (13) |
|
| Tumor size, cm |
|
|
| 0.346 |
|
<4 | 22 | 23 (14) | 33 (8) |
|
| ≥4 | 62 | 77 (46) | 67 (16) |
|
| Grade |
|
|
| 0.008 |
|
Well/moderate | 19 | 15 (9) | 42 (10) |
|
|
Poor | 65 | 85 (51) | 58 (14) |
|
| Lauren type |
|
|
| <0.001 |
|
Intestinal type | 48 | 40 (24) | 100 (24) |
|
| Diffuse
type | 36 | 6 (36) | 0 (0) |
|
| Tumor location |
|
|
| 0.435 |
|
Cardia | 9 | 13 (8) | 4 (1) |
|
|
Body/antrum | 75 | 87 (52) | 96 (23) |
|
| Lymphatic
invasion |
|
|
| 0.757 |
|
Negative | 61 | 72 (43) | 75 (18) |
|
|
Positive | 23 | 28 (17) | 25 (6) |
|
| pTNM stage |
|
|
| 0.203 |
|
I–II | 33 | 35 (21) | 50 (12) |
|
|
III–IV | 51 | 65 (39) | 50 (12) |
|
| T
classification |
|
|
| <0.001 |
|
T1-T2 | 12 | 5 (3) | 37 (9) |
|
|
T3-T4 | 72 | 95 (57) | 62 (15) |
|
| N
classification |
|
|
| 0.010 |
| N0 | 22 | 18 (11) | 46 (11) |
|
|
N1-N3 | 62 | 82 (49) | 54 (13) |
|
| Distant
metastasis |
|
|
| 1.000 |
| M0 | 80 | 9% (57) | 96 (23) |
|
| M1 | 4 | 5 (3) | 4 (1) |
|
SR-BI expression and patient
outcome
The association between SR-BI expression and overall
survival was assessed in gastric adenocarcinoma patients using
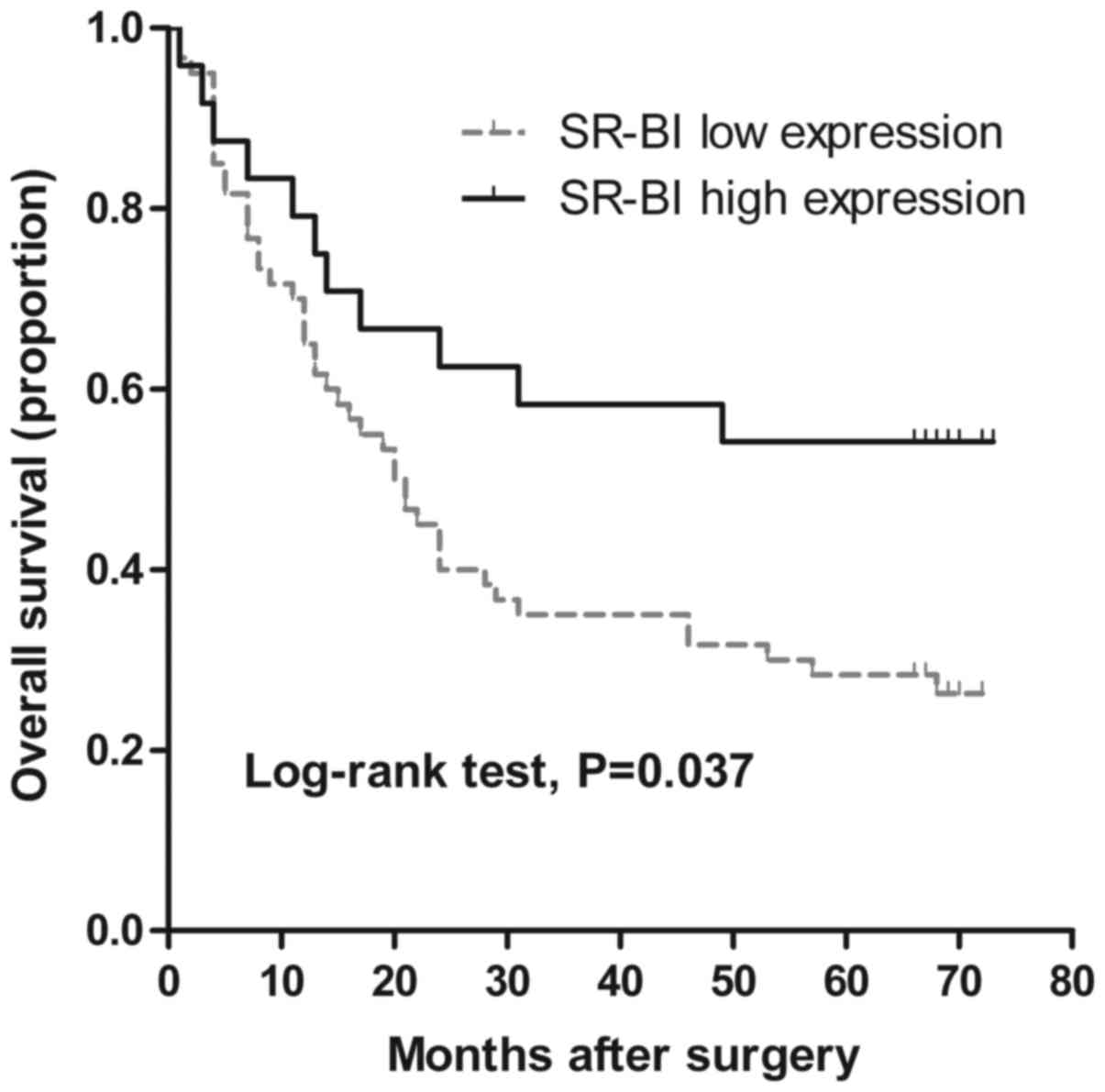
univariate analysis. As shown in Fig.
2, Kaplan-Meier survival curves indicated that patients with
low SR-BI expression had a significantly shorter OS time compared
with patients with high SR-BI expression (P=0.037 by log-rank
test). The 5-year survival rate for gastric adenocarcinoma patients
with low SR-BI expression was 28%, compared with 54% for the group
with SR-BI high expression. Additionally, tumor size, grade, Lauren
type, pTNM stage, T stage, N stage, distant metastasis and
lymphatic invasion were also associated with the risk of death
(Table III). No prognostic
associations were observed with age, gender, or tumor location.
Furthermore, multivariate analysis using the Cox regression model
revealed that T stage, N stage and lymphatic invasion were
independently and significantly associated with survival (P=0.035,
P=0.002 and P=0.013, respectively; Table
IV). However, SR-BI expression was not an independent
prognostic factor.
 | Table III.Univariate survival analysis of 84
gastric cancer patients. |
Table III.
Univariate survival analysis of 84
gastric cancer patients.
| Variable | Estimated 5-year OS
rate, % | P-value |
|---|
| Age, years |
| 0.167 |
|
<60 | 25 |
|
|
≥60 | 42 |
|
| Gender |
| 0.171 |
|
Female | 29 |
|
|
Male | 40 |
|
| Tumor size, cm |
| 0.028 |
|
<4 | 55 |
|
| ≥4 | 29 |
|
| Grade |
| 0.021 |
|
Well/moderate | 63 |
|
|
Poor | 28 |
|
| Lauren type |
| 0.027 |
|
Intestinal type | 46 |
|
| Diffuse
type | 22 |
|
| Tumor location |
| 0.546 |
|
Cardia | 22 |
|
|
Body/antrum | 37 |
|
| Lymphatic
invasion |
| <0.001 |
|
Negative | 48 |
|
|
Positive | 4 |
|
| pTNM stage |
| <0.001 |
|
I–II | 82 |
|
|
III–IV | 6 |
|
| T stage |
| 0.001 |
|
T1-T2 | 83 |
|
|
T3-T4 | 28 |
|
| N stage |
| <0.001 |
| N0 | 86 |
|
|
N1-N3 | 18 |
|
| Distant
metastasis |
| 0.009 |
| M0 | 38 |
|
| M1 | 0 |
|
| SR-BI
expression |
| 0.037 |
|
Low | 28 |
|
|
High | 54 |
|
 | Table IV.Multivariate survival results. |
Table IV.
Multivariate survival results.
|
| Overall
survival |
|---|
|
|
|
|---|
| Variable | HR | 95% CI | P-value |
|---|
| T stage | 4.688 | 1.115–19.710 | 0.035 |
| N stage | 5.400 | 1.869–15.605 | 0.002 |
| Lymphatic
invasion | 2.080 | 1.165–3.711 | 0.013 |
Discussion
Multiple studies have found that SR-BI is implicated
in the regulation of diverse tumor cell biology, including tumor
cell proliferation, apoptosis, migration and invasion (7,8). The
present study analyzed the expression of SR-BI in multiple gastric
adenocarcinoma specimens and found that SR-BI immunoreactivity was
present in 69% of cases. Specifically, high SR-BI expression was
detected in 28.6% of patients, all of whom had intestinal type
disease. In addition, low SR-BI expression was significantly
associated with clinicopathological parameters indicative of a more
aggressive tumor type, including poor histological grade, higher T
stage, higher N stage and diffuse type carcinoma. Patients with low
SR-BI expression had poorer prognoses in the patient cohort of the
present study.
The data from the normal gastric tissue were in line
with the previous paper by Lobo et al (13), which demonstrated that SR-BI was
predominantly detected in the majority of cells in the fundic
gland, but not in the normal gastric mucosa. As SR-BI was not
detected in epithelial, parietal, mucous or endocrinal cells
(13), we hypothesized that SR-BI
might be mainly located in chief cells. Further immunofluorescence
staining in future studies is warranted to determine the exact
cellular location of SR-BI.
Gastric adenocarcinoma is a heterogeneous disease
that can be classified into two major histological subtypes based
on Lauren's criteria: Intestinal type and diffuse type
adenocarcinomas. The intestinal type gastric adenocarcinoma is
possibly preceded by precancerous changes in the gastric mucosa,
including chronic atrophic gastritis, intestinal metaplasia and
dysplasia (14,19). The progression of gastric fundic gland
polyps and the transdifferentiation of chief cells was reported to
be involved in the initiation of intestinal metaplasia (19); however, diffuse-type gastric carcinoma
seems to be less associated with environmental influences and
precancerous changes (19), and it is
believed to originate from mucous neck cells or stem cells
(20). Evidence indicates that the
molecular profiles of these subtypes are distinct (21–24). In
the present study, marked differences in SR-BI expression between
the intestinal type and diffuse type supported the notion that
distinct precursors and pathways led to the oncogenesis of the two
types of gastric adenocarcinoma.
Epidemiological studies have provided evidence
indicating the presence of strong associations between serum lipid
profiles and gastric adenocarcinoma (25,26).
Considerable alterations have been documented regarding serum total
cholesterol, triglyceride, HDL cholesterol and low-density
lipoprotein cholesterol levels in patients with gastric
adenocarcinoma compared with those in non-cancer subjects (25). In particular, low serum
HDL-cholesterol was reported to be a negative prognostic factor for
gastric adenocarcinoma patients (26). Low-density lipoprotein receptor (LDLR)
levels in diffuse type gastric adenocarcinoma were significantly
lower than those in normal mucosa, but there was no significant
difference between intestinal-type gastric adenocarcinoma and
normal mucosa (27). Caveolin-1 is a
scaffolding protein that binds to cholesterol and is involved in
intracellular cholesterol trafficking. Barresi et al
(28) reported that caveolin-1
expression was significantly higher in intestinal-type gastric
adenocarcinoma than in the diffuse type. Similar to LDLR and
caveolin-1, SR-BI expression in intestinal-type cancer was also
higher than that in diffuse-type cancer in the present study. The
differences in the aforementioned cholesterol-trafficking proteins
between the two histological types indicated the presence of
distinct cholesterol metabolism in these adenocarcinomas, which
warrants further investigation.
Previous studies have shown that certain
HDL-cholesterol-elevating drugs may regulate SR-BI protein
expression. Statins increase SR-BI expression in several cell
types, including macrophages (29),
adipocytes (30), keratinocytes
(31), and endothelial cells
(32). Fibrates can affect HDL
metabolism in mice by downregulating hepatic SR-BI protein levels
(33,34). The commercial tissue microarray did
not provide detailed information regarding the plasma lipoprotein
profiles or drug treatments (e.g. statins and fibrates) of
patients, meaning that the possible influence of these factors on
SR-BI expression in gastric tissues could not be evaluated. Further
study is therefore required to answer these questions.
Several studies have reported the role of SR-BI in
diverse solid tumors (7,35,36). For
instance, SR-BI was shown to mediate the proliferative and
anti-apoptotic features of HDL in MCF-7 breast cancer cells through
modulation of cholesterol metabolism (7,8). Twiddy
et al (36) found that SR-BI
downregulation caused a significant reduction in prostate-specific
antigen production and decreased the viability of prostate cancer
cell lines in vitro. Previous studies revealed that SR-BI
was associated with more aggressive cancer phenotypes and could
serve a tumor-promoting function in breast and prostate cancer
(35,37). However, in gastric adenocarcinoma, the
role of SR-BI appeared to be different. In contrast to the findings
of those prior studies, low SR-BI expression was associated with
the more aggressive phenotype contrarily. Therefore, the role of
SR-BI may be complex and tissue-dependent, and it may have
versatile functions in different types of cancer. In gastric fundic
gland cells, SR-BI may be involved in the active exocytosis of
pepsinogen, as a high degree of membrane cholesterol metabolism is
required in this process (38),
whereas in gastric adenocarcinoma, the pepsin-secreting function of
cells was diminished (38). We
hypothesized that this may be the reason for the downregulation of
SR-BI in gastric adenocarcinoma.
Despite the strong association between SR-BI
expression and multiple types of cancer, reports linking SR-BI to
the clinical outcome of cancer patients are limited. The results of
a previous study revealed that high SR-BI expression was an
independent factor predictive of poor prognosis for patients with
breast cancer (35). Schörghofer
et al (37) reported that high
SR-BI expression was associated with shorter disease-free survival
times in prostate cancer. On the contrary, in gastric
adenocarcinoma, patients with low SR-BI expression had
significantly poorer prognosis than patients with high SR-BI
expression, indicating that different mechanisms were involved.
In summary, the results of the present study
demonstrated that low SR-BI expression was associated with
malignancy and unfavorable prognosis in patients with gastric
adenocarcinoma. Further research is therefore warranted to clarify
the role of SR-BI in gastric adenocarcinoma.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (nos. 81201865, 81202307 and
81271966), and Shandong Science and Technology Development Planning
(nos. 2015GSF118169 and 2012G0021822) and Shandong Natural Science
Foundation (no. ZR2017QH004).
References
|
1
|
McLean MH and El-Omar EM: Genetics of
gastric cancer. Nat Rev Gastroenterol Hepatol. 11:664–674. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rahman R, Asombang AW and Ibdah JA:
Characteristics of gastric cancer in Asia. World J Gastroenterol.
20:4483–4490. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Acton S, Rigotti A, Landschulz KT, Xu S,
Hobbs HH and Krieger M: Identification of scavenger receptor SR-BI
as a high density lipoprotein receptor. Science. 271:518–520. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Guo L, Song Z, Li M, Wu Q, Wang D, Feng H,
Bernard P, Daugherty A, Huang B and Li XA: Scavenger receptor BI
protects against septic death through its role in modulating
inflammatory response. J Biol Chem. 284:19826–19834. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li Y, Kakinami C, Li Q, Yang B and Li H:
Human apolipoprotein A-I is associated with dengue virus and
enhances virus infection through SR-BI. PLoS One. 8:e703902013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Feng H, Guo L, Wang D, Gao H, Hou G, Zheng
Z, Ai J, Foreman O, Daugherty A and Li XA: Deficiency of scavenger
receptor BI leads to impaired lymphocyte homeostasis and autoimmune
disorders in mice. Arterioscler Thromb Vasc Biol. 31:2543–2551.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cao WM, Murao K, Imachi H, Yu X, Abe H,
Yamauchi A, Niimi M, Miyauchi A, Wong NC and Ishida T: A mutant
high-density lipoprotein receptor inhibits proliferation of human
breast cancer cells. Cancer Res. 64:1515–1521. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Danilo C, Gutierrez-Pajares JL, Mainieri
MA, Mercier I, Lisanti MP and Frank PG: Scavenger receptor class B
type I regulates cellular cholesterol metabolism and cell signaling
associated with breast cancer development. Breast Cancer Res.
15:R872013. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wadsack C, Hirschmugl B, Hammer A,
Levak-Frank S, Kozarsky KF, Sattler W and Malle E: Scavenger
receptor class B, type I on non-malignant and malignant human
epithelial cells mediates cholesteryl ester-uptake from high
density lipoproteins. Int J Biochem Cell Biol. 35:441–454. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Leon CG, Locke JA, Adomat HH, Etinger SL,
Twiddy AL, Neumann RD, Nelson CC, Guns ES and Wasan KM: Alterations
in cholesterol regulation contribute to the production of
intratumoral androgens during progression to castration-resistant
prostate cancer in a mouse xenograft model. Prostate. 70:390–400.
2010.PubMed/NCBI
|
|
11
|
Shahzad MM, Mangala LS, Han HD, Lu C,
Bottsford-Miller J, Nishimura M, Mora EM, Lee JW, Stone RL, Pecot
CV, et al: Targeted delivery of small interfering RNA using
reconstituted high-density lipoprotein nanoparticles. Neoplasia.
13:309–319. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zheng Y, Liu Y, Jin H, Pan S, Qian Y,
Huang C, Zeng Y, Luo Q, Zeng M and Zhang Z: Scavenger receptor B1
is a potential biomarker of human nasopharyngeal carcinoma and its
growth is inhibited by HDL-mimetic nanoparticles. Theranostics.
3:477–486. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lobo MV, Huerta L, Ruiz-Velasco N,
Teixeiro E, de la Cueva P, Celdrán A, Martín-Hidalgo A, Vega MA and
Bragado R: Localization of the lipid receptors CD36 and CLA-1/SR-BI
in the human gastrointestinal tract: Towards the identification of
receptors mediating the intestinal absorption of dietary lipids. J
Histochem Cytochem. 49:1253–1260. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Berlth F, Bollschweiler E, Drebber U,
Hoelscher AH and Moenig S: Pathohistological classification systems
in gastric cancer: Diagnostic relevance and prognostic value. World
J Gastroenterol. 20:5679–5684. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yu H and Xin Y: Down-regulated expressions
of PPARγ and its coactivator PGC-1 are related to gastric
carcinogenesis and Lauren's classification in gastric carcinoma.
Chin J Cancer Res. 25:704–714. 2013.PubMed/NCBI
|
|
16
|
Kwon OK, Kim SW, Chae HD, Ryu SW, Chung
HY, Kim SW, Lee WK and Yu W: Validation of the 7th AJCC/UICC
staging system for gastric cancer and a proposal for a new TNM
system based on a prognostic score: A retrospective multicenter
study. Ann Surg Treat Res. 91:295–302. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bogan RL and Hennebold JD: The reverse
cholesterol transport system as a potential mediator of luteolysis
in the primate corpus luteum. Reproduction. 139:163–176. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Remmele W and Stegner HE: Recommendation
for uniform definition of an immunoreactive score (IRS) for
immunohistochemical estrogen receptor detection (ER-ICA) in breast
cancer tissue. Pathologe. 8:138–140. 1987.(In German). PubMed/NCBI
|
|
19
|
Hu B, El Hajj N, Sittler S, Lammert N,
Barnes R and Meloni-Ehrig A: Gastric cancer: Classification,
histology and application of molecular pathology. J Gastrointest
Oncol. 3:251–261. 2012.PubMed/NCBI
|
|
20
|
Ghandur-Mnaymneh L, Paz J, Roldan E and
Cassady J: Dysplasia of nonmetaplastic gastric mucosa. A proposal
for its classification and its possible relationship to
diffuse-type gastric carcinoma. Am J Surg Pathol. 12:96–114. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cristescu R, Lee J, Nebozhyn M, Kim KM,
Ting JC, Wong SS, Liu J, Yue YG, Wang J, Yu K, et al: Molecular
analysis of gastric cancer identifies subtypes associated with
distinct clinical outcomes. Nat Med. 21:449–456. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cancer Genome Atlas Research Network, .
Comprehensive molecular characterization of gastric adenocarcinoma.
Nature. 513:202–209. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shah MA, Khanin R, Tang L, Janjigian YY,
Klimstra DS, Gerdes H and Kelsen DP: Molecular classification of
gastric cancer: A new paradigm. Clin Cancer Res. 17:2693–2701.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shen H, Newmann AS, Hu Z, Zhang Z, Xu Y,
Wang L, Hu X, Guo J, Wang X and Wei Q: Methylenetetrahydrofolate
reductase polymorphisms/haplotypes and risk of gastric cancer: A
case-control analysis in China. Oncol Rep. 13:355–360.
2005.PubMed/NCBI
|
|
25
|
Guo E, Chen L, Xie Q, Chen J, Tang Z and
Wu Y: Serum HDL-C as a potential biomarker for nodal stages in
gastric cancer. Ann Surg Oncol. 14:2528–2534. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tamura T, Inagawa S, Hisakura K, Enomoto T
and Ohkohchi N: Evaluation of serum high-density lipoprotein
cholesterol levels as a prognostic factor in gastric cancer
patients. J Gastroenterol Hepatol. 27:1635–1640. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Caruso MG, Notarnicola M, Cavallini A and
Di Leo A: 3-Hydroxy-3-methylglutaryl coenzyme A reductase activity
and low-density lipoprotein receptor expression in diffuse-type and
intestinal-type human gastric cancer. J Gastroenterol. 37:504–508.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Barresi V, Giuffre G, Vitarelli E, Todaro
P and Tuccari G: Caveolin-1 immuno-expression in human gastric
cancer: Histopathogenetic hypotheses. Virchows Arch. 453:571–578.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Han J, Parsons M, Zhou X, Nicholson AC,
Gotto AM Jr and Hajjar DP: Functional interplay between the
macrophage scavenger receptor class B type I and pitavastatin
(NK-104). Circulation. 110:3472–3479. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhao SP, Wu ZH, Hong SC, Ye HJ and Wu J:
Effect of atorvastatin on SR-BI expression and HDL-induced
cholesterol efflux in adipocytes of hypercholesterolemic rabbits.
Clin Chim Acta. 365:119–124. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tsuruoka H, Khovidhunkit W, Brown BE,
Fluhr JW, Elias PM and Feingold KR: Scavenger receptor class B type
I is expressed in cultured keratinocytes and epidermis. Regulation
in response to changes in cholesterol homeostasis and barrier
requirements. J Biol Chem. 277:2916–2922. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kimura T, Mogi C, Tomura H, Kuwabara A, Im
DS, Sato K, Kurose H, Murakami M and Okajima F: Induction of
scavenger receptor class B type I is critical for simvastatin
enhancement of high-density lipoprotein-induced anti-inflammatory
actions in endothelial cells. J Immunol. 181:7332–7340. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mardones P, Pilon A, Bouly M, Duran D,
Nishimoto T, Arai H, Kozarsky KF, Altayo M, Miquel JF, Luc G, et
al: Fibrates down-regulate hepatic scavenger receptor class B type
I protein expression in mice. J Biol Chem. 278:7884–7890. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lan D and Silver DL: Fenofibrate induces a
novel degradation pathway for scavenger receptor B-I independent of
PDZK1. J Biol Chem. 280:23390–23396. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yuan B, Wu C, Wang X, Wang D, Liu H, Guo
L, Li XA, Han J and Feng H: High scavenger receptor class B type I
expression is related to tumor aggressiveness and poor prognosis in
breast cancer. Tumour Biol. 37:3581–3588. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Twiddy AL, Cox ME and Wasan KM: Knockdown
of scavenger receptor class B type I reduces prostate specific
antigen secretion and viability of prostate cancer cells. Prostate.
72:955–965. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Schörghofer D, Kinslechner K, Preitschopf
A, Schütz B, Röhrl C, Hengstschläger M, Stangl H and Mikula M: The
HDL receptor SR-BI is associated with human prostate cancer
progression and plays a possible role in establishing androgen
independence. Reprod Biol Endocrinol. 13:882015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kitadai Y, Sasaki A, Ito M, Tanaka S, Oue
N, Yasui W, Aihara M, Imagawa K, Haruma K and Chayama K:
Helicobacter pylori infection influences expression of genes
related to angiogenesis and invasion in human gastric carcinoma
cells. Biochem Biophys Res Commun. 311:809–814. 2003. View Article : Google Scholar : PubMed/NCBI
|