Introduction
Lung cancer is one of the five leading causes of
cancer death worldwide. The incidence of pulmonary cancer has been
increasing steadily in both men and women in the developing
countries, including China, by the year. Non-small cell lung cancer
(NSCLC), which is aggressive and has a poor prognosis, accounts for
approximately 75–85% of lung cancers (1). The proportion of lung adenocarcinoma
(LAC), a common form of NSCLC, is also increasing. Therefore,
in-depth research of the pathogenesis of LAC would be of
significance.
Tumor angiogenesis is one of the most important
hallmarks in the development of lung cancer. An extensive
microvascular system is closely related to the malignant behaviors
of LAC, and is also an important target in LAC treatment (2). p73, a homologue of the tumor suppressor
p53, regulates vascular development and cell proliferation,
migration, and differentiation. There are two isoforms of p73:
TAp73, which contains full-length N-terminal transactivating
domains; it is also termed TAp73α. The other is the ΔNp73 isoform,
which has a truncated N-terminal and promotes cancer gene programs
(3). P73-knockout (TAp73-/-) mice
develop gastrointestinal and cranial hemorrhage (4), indicating vascular fragility. In
addition, TAp73 regulates GATA-1 (5),
which is critical for endothelial and hematopoietic cell
differentiation. Accordingly, we conjectured that p73 might be
related to vasculogenesis/angiogenesis. However, the function of
TAp73 in regulating angiogenesis in cancer is hotly debated
(6,7).
Some suggest that TAp73 suppresses angiogenesis (8,9).
Conversely, others suggest that both the TAp73 and ΔNp73 forms are
proangiogenic (10–12). Interestingly, p53 status might be a
determinant of the effect of TAp73 on angiogenesis. TAp73 regulated
vascular endothelial growth factor A (VEGFA), where wild-type p53
downregulated angiogenesis and mutant p53 promoted it (13). Altogether, whether TAp73 acts as a
positive or negative regulator of angiogenesis is unclear.
The tumor suppressor gene TP53 has been
described as a critical determinant of the angiogenic potential of
tumor cells. Indeed, the TP53 gene is mutated in many human
tumors. Mutated TP53 genes promote the invasion, metastasis,
proliferation, and angiogenesis of malignant cells (14). In addition, mutant p53 protein
upregulates VEGF and promotes angiogenesis (15). In contrast to p53, analysis of TAp73
in different human tumors did not detect any significant mutation.
Rather, wild-type TAp73 is overexpressed in many human tumors,
suggesting that TAp73, as well as p53, is involved in regulating
tumor angiogenesis. However, whether TAp73 and P53 are coexpressed
in lung cancer, especially LAC, has not been reported.
Numerous endogenous regulators participate in cancer
angiogenesis. Vasohibin-1 (VASH1) is a VASH family member that has
been identified as a novel negative feedback regulator of
angiogenesis (16). It is expressed
and secreted by the termination zone of endothelial cells.
Consequently, VASH1 halts angiogenesis and exerts some
anti-angiogenic activity in endothelial cells (17). High VASH1 expression has also been
detected in some tumor tissues (colorectal cancer, NSCLC) and
correlates positively with clinicopathological features, including
lymph node status and tumor-node-metastasis (TNM) stage, and is an
independent prognostic factor (18).
It is well-established that VASH1 acts as an anti-angiogenic
factor. Its overexpression in several tumors is due to VEGF
induction. Reduced VASH1 expression has been reported in renal cell
carcinoma (RCC) (19). Lu et
al also reported that EZH2 silenced VASH1 expression in ovarian
cancer, worsening prognosis (20).
Delivery recombinant adenovirus encoding VASH1 prevents tumor
angiogenesis and tumor growth (21).
Although TAp73, p53, and VASH1 have been studied individually in
tumors, little is known about their coexpression status in lung
cancer. In the present study, we profile the association between
TAp73, p53, and VASH1 expression in LAC by immunohistochemical
(IHC) staining and statistical analysis. The study will be of
benefit in that it provides clues to the function of TAp73 in
regulating angiogenesis in LAC.
Materials and methods
Patients and tissue microarray
Microarray sections of human LAC tissues and
adjacent normal tissues with pathological information were
purchased from Shanghai Biochip (OD-CT-RsLug04-003; Shanghai,
China). Table I lists the
clinicopathological characteristics of the patients. TAp73, p53,
and VASH1 protein expression was analyzed in the 53 LAC tissue
samples and the paired adjacent normal tissues.
 | Table I.General patient characteristics
(n=106). |
Table I.
General patient characteristics
(n=106).
| Feature | % (n) |
|---|
| Mean age | 64.0 (9.9) |
| Median age
(p25-p75) | 65 (56–72) |
| Median age
(years) |
|
| ≤60 | 35.8 (38/106) |
|
>60 | 64.2 (68/106) |
| Sex |
|
| Male | 52.8 (56/106) |
|
Female | 47.2 (50/106) |
| Smoking exposure |
|
|
Non-smoker | 56.6 (60/106) |
|
Smoker | 43.4 (46/106) |
| Histological
type |
|
|
Adenocarcinoma | 50.0 (53/106) |
|
Paraneoplastic | 50.0 (53/106) |
| Disease stage |
|
| I | 1.9 (1/53) |
| I–II | 1.9 (1/53) |
| II | 75.5 (40/53) |
|
II–III | 13.2 (7/53) |
| III | 7.5 (4/53) |
Immunohistochemistry
Tissue sections (4-µm thick) were cut from each
tissue microarray block and the tissue microarrays were performed
according to the streptavidin-peroxidase IHC method. The tissue
sections were conventionally dewaxed, and antigen retrieval was
performed by submerging the slides in sodium citrate buffer (pH
6.0) under high-pressure and high-temperature conditions, 3-minute
boiling, followed by 30-min cooling at room temperature. Then, the
tissue sections were incubated in hydrogen peroxide (3%) in
phosphate-buffered saline containing 1% Triton X-100 (PBS-T) for 10
min to block endogenous peroxidase activity. The sections were
washed repeatedly in 5% bovine serum albumin in PBS-T and incubated
with mouse polyclonal anti-VASH1 antibody (1:100; ab67423; Abcam,
Hong Kong, China), rabbit polyclonal anti-P73 antibody (1:1,000;
ab14430; Abcam), and monoclonal anti-p53 antibody (1:1,000; p5813;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) in 5% bovine serum
albumin at 4°C overnight. The next day, the sites of
immunoreactivity were detected using an EnVision Detection kit/DAB
staining kit (GK500705; Dako A/S, Glostrup, Denmark) according to
the manufacturer's protocol. The slides were counterstained with
hematoxylin (ZLI-9609; ZSGB-Bio, Beijing, China) for 1 min at room
temperature, and then dehydrated and sealed using crystal mounting
solution (ZLI-9516; ZSGB-Bio).
Image analysis and scoring
Two pathologists evaluated the immunostaining
independently using a double-blind method. Staining intensity was
evaluated according to the following scale: 0 points, unstained; 1
point, faint yellow; 2 points, brownish yellow; 3 points, brown.
Staining was also assessed according to the percentage of positive
cells (0–100%). In the tissue slides, negative and positive
expression was determined by the percentage of positively stained
cells and the staining intensity; score ≤3 was defined as negative
expression; score >3 was defined as positive expression
(Table II). By multiplying the
positively stained cells by the staining intensity, the protein
expression intensity was classified on a subjective spectrum as
follows: -, 0–1 points; +, 2–4 points; ++, 5–8 points; +++, >9
points (Table III).
 | Table II.Clinical patient characteristics
according to positive/negative p53, TAp73 and VASH1 expression. |
Table II.
Clinical patient characteristics
according to positive/negative p53, TAp73 and VASH1 expression.
|
| p53 | TAp73 | VASH1 |
|---|
|
|
|
|
|
|---|
| Feature | Negative N=79%
(n) | Positive N=27%
(n) | P-value | Negative N=62%
(n) | Positive N=44%
(n) | P-value | Negative
N=60% (n) | Positive
N=46% (n) | P-value |
|---|
| Mean (±SD) | 64.8 (9.1) | 61.6 (11.2) | 0.140b | 63.5 (9.6) | 64.0 (10.0) | 0.594b | 65.1 (9.4) | 62.4 (10.3) | 0.161b |
| Median age |
|
|
|
|
|
|
|
|
|
|
≤60 | 32.9 (26/79) | 44.4 (12/27) |
| 37.1 (23/62) | 34.1 (15/44) |
| 30.0 (18/60) | 43.5 (20/46) |
|
|
>60 | 67.1 (53/79) | 55.6 (15/27) | 0.281a | 62.9 (39/62) | 65.9 (29/44) | 0.751a | 70.0 (42/60) | 56.5 (26/46) | 0.152a |
| Sex |
|
|
|
|
|
|
|
|
|
|
Male | 50.6 (40/79) | 59.3 (16/27) |
| 51.6 (32/62) | 54.5 (24/44) |
| 51.7 (31/60) | 54.3 (25/46) |
|
|
Female | 49.4 (39/79) | 40.7 (11/27) | 0.438a | 48.4 (30/62) | 45.5 (20/44) | 0.766a | 48.3 (29/60) | 45.7 (21/46) | 0.784a |
| Smoking |
|
|
|
|
|
|
|
|
|
|
Non-smoker | 53.2 (42/79) | 66.7 (18/27) |
| 56.5 (35/62) | 56.8 (25/44) |
| 61.7 (37/60) | 50.0 (23/46) |
|
|
Smoker | 46.8 (37/79) | 33.3 (9/27) | 0.222a | 43.5 (27/62) | 43.2 (19/44) | 0.970a | 38.3 (23/60) | 50.0 (23/46) | 0.230a |
| Histological
type |
|
|
|
|
|
|
|
|
|
|
Adenocarcinoma | 35.4 (28/79) | 92.6 (25/27) |
| 16.1 (10/62) | 97.7 (43/44) |
| 36.7 (22/60) | 67.4 (31/46) |
|
|
Paraneoplastic | 64.6 (51/79) | 7.4 (2/27) |
<0.001a | 83.9 (52/62) | 2.3 (1/44) |
<0.001a | 63.3 (38/60) | 32.6 (15/46) | 0.002a |
| Disease stage |
|
|
|
|
|
|
|
|
|
| I | 0 (0/28) | 4.0 (1/25) | 0.339a | 0 (0/10) | 2.3 (1/43) | 0.431a | 0 (0/22) | 3.2 (1/31) | 0.695a |
|
I–II | 3.6 (1/28) | 0 (0/25) |
| 0 (0/10) | 2.3 (1/43) |
| 0 (0/22) | 3.2 (1/31) |
|
| II | 78.6 (22/28) | 72.8 (18/25) |
| 6.0 (6/10) | 79.1 (34/43) |
| 81.8 (18/22) | 71.0 (22/31) |
|
|
II–III | 7.1 (2/28) | 20.0 (5/25) |
| 2.0 (2/10) | 11.6 (5/43) |
| 9.1 (2/22) | 16.1 (5/31) |
|
|
III | 10.7 (3/28) | 4.0 (1/25) |
| 2.0 (2/10) | 4.7 (2/43) |
| 9.1 (2/22) | 6.5 (2/31) |
|
 | Table III.Expression intensity of p53, TAp73
and VASH1 (n=106). |
Table III.
Expression intensity of p53, TAp73
and VASH1 (n=106).
|
| p53 | TAp73 | VASH1 |
|---|
|
|
|
|
|
|---|
|
| LAC | Paraneoplastic | Total | LAC | Paraneoplastic | Total | LAC | Paraneoplastic | Total |
|---|
| − | 21 | 49 | 70 | 7 | 43 | 50 | 9 | 19 | 28 |
| + | 12 | 4 | 16 | 22 | 10 | 32 | 26 | 30 | 56 |
| ++ | 14 | 0 | 14 | 21 | 0 | 21 | 13 | 4 | 17 |
| +++ | 6 | 0 | 6 | 3 | 0 | 3 | 5 | 0 | 5 |
| Total | 53 | 53 | 106 | 53 | 53 | 106 | 53 | 53 | 106 |
Statistical analysis
All statistical analyses were performed using SPSS
(version 21.0; SPSS, Inc., Chicago, IL, USA). The clinical
characteristics of the patients according to negative and positive
expression of p53, TAp73, and VASH1 were assessed using the
chi-square test and Student's t-test. Spearman's correlation was
used to determine the relationship between positive and negative
protein expression and with expression intensity. P<0.05 was
considered to indicate a statistically significant difference.
Results
Study population
Table I summarizes the
demographic and clinicopathological features of the samples, which
included 53 LAC tissue samples and 53 paraneoplastic tissue
samples. The mean patient age was 64.0 years, and 52.8% of the
patients were male. Approximately 43.4% of the patients were
smokers. Most patients had stage II LAC (75.5%); the remaining
patients had stage I (1.9%), stage I–II (1.9%), stage II–III
(13.2%), and stage III disease (7.5%).
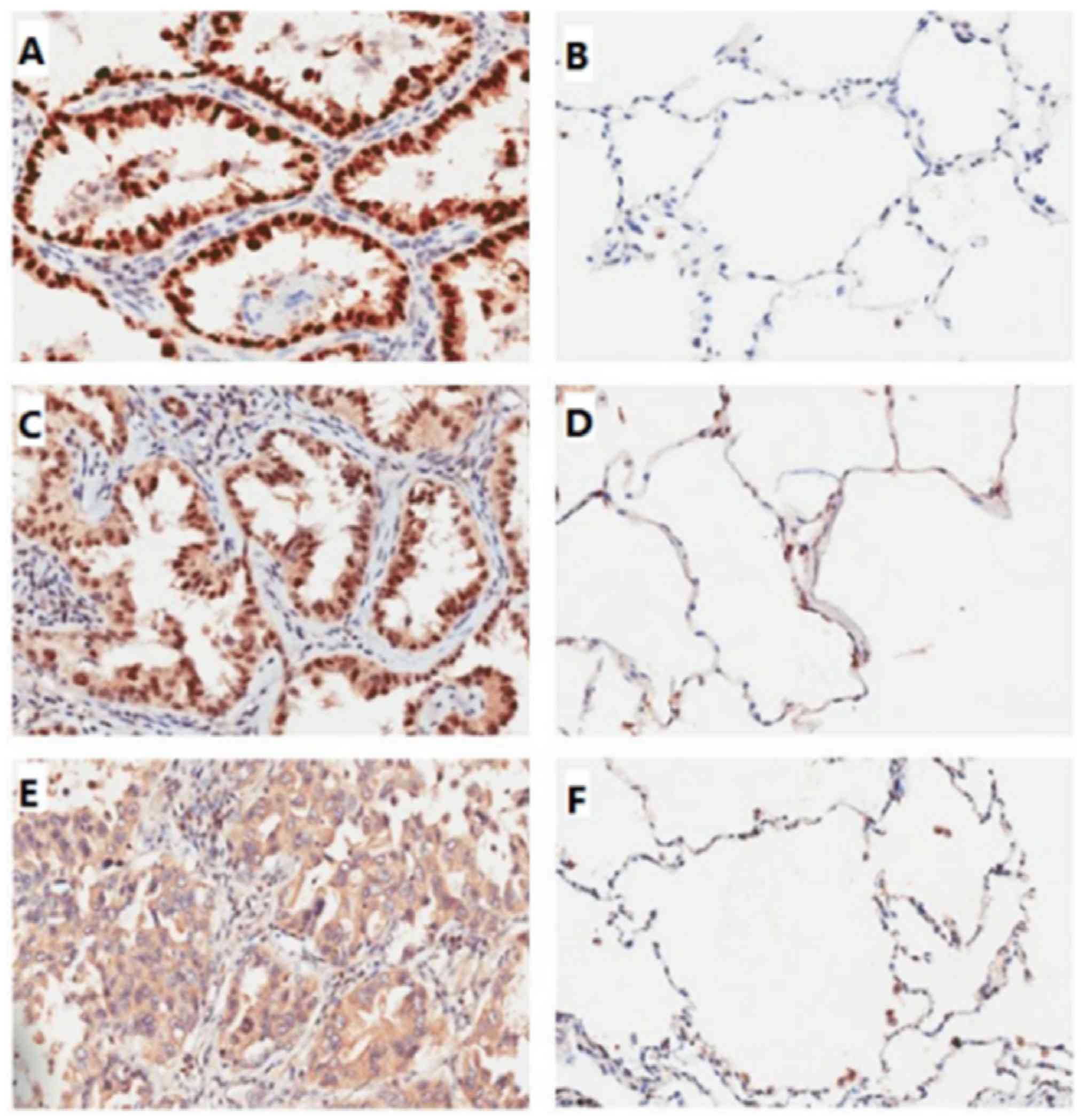
P53, TAp73, and VASH1 expression in
LAC
P53, TAp73, and VASH1 expression in the LAC and
paraneoplastic lung tissue was analyzed by immunohistochemistry.
The expression of the proteins was classified into two groups:
Negative and positive expression. P53 and TAp73 were mainly
localized to the pulmonary alveoli nuclei and expressed in LAC.
VASH1 staining was observed mainly in the cytoplasm of LAC cells
and in the alveolar epithelium. Different staining intensities were
observed for the three proteins in the LAC or paraneoplastic lung
tissues (Fig. 1). The positive
expression rates of p53, TAp73, and VASH1 were significantly higher
in LAC tissue (92.6, 97.7 and 67.4%, respectively) than in
paraneoplastic lung tissue (7.4, 2.3 and 32.6%, respectively,
P<0.01) (Tables II and III). There was a clear trend for
upregulated p53, TAp73, and VASH1 expression in LAC tissue as
compared to paraneoplastic lung tissue. However, there was no
significant difference between p53, TAp73, and VASH1 expression and
age, sex, smoking exposure, or disease stage (P>0.05) (Table II).
Correlation between positive rate of
p53, TAp73, and VASH1 expression in LAC
Immunochemistry analysis showed that the positive
rate and intensity of p53, TAp73, and VASH1 expression were higher
in LAC tissue than in paraneoplastic lung tissue. We evaluated the
correlation between the three using Pearson's correlation
coefficient. There was extremely significant positive correlation
for the rate of positive expression between p53 and TAp73 (r=0.474,
P<0.01) and between TAp73 and VASH1 (r=0.367, P<0.01;
Table IV). There was a marginal
significant correlation for the rate of positive expression between
p53 and VASH1 (r=0.187, P=0.055).
 | Table IV.Correlation between the positive
expression rate of p53, TAp73 and VASH1 (n=106). |
Table IV.
Correlation between the positive
expression rate of p53, TAp73 and VASH1 (n=106).
|
| p53 | TAp73 | VASH1 |
|---|
| p53 |
|
|
|
| r | 1.000 | 0.474 | 0.187 |
|
P-value | − | <0.001 | 0.050 |
| TAp73 |
|
|
|
| r | 0.474 | 1.000 | 0.367 |
|
P-value | <0.001 | − | 0.006 |
| VASH1 |
|
|
|
| r | 0.187 | 0.367 | 1.000 |
|
P-value | 0.050 | 0.006 | − |
Correlation between p53, TAp73, and
VASH1 expression intensity in LAC
To further estimate the correlation between p53,
TAp73, and VASH1 expression in LAC, we analyzed their expression
intensity in LAC and paraneoplastic lung tissues. Similar to the
rate of positive expression, p53 expression intensity had extremely
significant positive correlation with TAp73 (r=0.517, P<0.01)
and with VASH1 (r=0.277, P<0.01). There was also extremely
significant positive correlation the TAp73 and VASH1 expression
intensities (r=0.351, P<0.01; Table
V). Therefore, the statistical analysis suggested a positive
association between p53, TAp73, and VASH1 expression abundance in
LAC.
 | Table V.Correlation between p53, TAp73α, and
VASH1 expression intensity (n=106). |
Table V.
Correlation between p53, TAp73α, and
VASH1 expression intensity (n=106).
|
| p53 | TAp73α | VASH1 |
|---|
| p53 |
|
|
|
| r | 1.000 | 0.517 | 0.277 |
|
P-value | − | 0.004 | <0.001 |
| TAp73α |
|
|
|
| r | 0.517 | 1.000 | 0.351 |
|
P-value | 0.004 | − | <0.001 |
| VASH1 |
|
|
|
| r | 0.277 | 0.351 | 1.000 |
|
P-value | <0.001 | <0.001 | − |
Discussion
Approximately 140 million people die from lung
cancer, particularly NSCLC, each year, and lung cancer is the most
common cause of cancer-related death around the world. The
molecular basis of lung cancer is complex and warrants further
investigation.
The members of the p53 family include p53, p73, and
p63. Wild-type p53 suppresses tumor formation. The normal protein
has a very short half-life and is present in only minute amounts in
normal tissues and cells. However, TP53 is the most
frequently mutated gene in numerous human cancers, as recent
whole-exome sequencing studies have shown (22). TP53 mutations gain new activity
to promote tumor progression and angiogenesis (23). In contrast to the wild type, mutant
p53 protein produced by malignant cells is usually a product of a
point mutation in the TP53 gene, leading to the substitution
of a single amino acid that significantly prolongs the half-life of
the protein. The accumulation of high levels of p53 is a potential
novel marker of malignancy. As the mutation is found in more than
60% of NSCLC cases, and the mutant protein has a longer half-life
than the wild-type protein in the nucleus, the nuclear p53 protein
accumulation in LAC tissues we detected by IHC is likely the mutant
type. Ultimately, direct sequencing of the LAC samples would
confirm the TP53 mutations.
Another member of the p53 family is TAp73. TAp73 has
two main isoforms (24). One is the
full-length TAp73 yielded by the P1 promoter, and contains the
N-terminal transactivation domain. Cell-based assays have shown
that it is functionally analogous to p53. The other isoform is
ΔNp73 which lacks the N-terminal transactivation domain and acts as
a repressor of p53 and TAp73 (25).
Recently, it was shown that TAp73 as well as p53 are involved in
regulating tumor angiogenesis. However, the role of TAp73 in
vasculogenesis remains contradictory and unclear. Some reports have
suggested that TAp73 has a suppressive effect on angiogenesis
(8,9).
Conversely, other reports suggest that TAp73 is a proangiogenic
factor. Some researchers have proposed the explicit possibility
that TAp73 both promotes and inhibits angiogenesis in cancer
depending on the cellular context. Transient overexpression of
TAp73 leads to pro-angiogenesis. In contrast, long-term induction
of TAp73 leads to angiogenic suppression (26). Similarly, we also found TAp73 could
inhibit the ability of tube formation in human umbilical vein
endothelial cells (HUVEC) when HUVEC co-cultured with H1299 LAC
cells with TAp73 stable overexpressed not for transient
transfection (data not shown). Based on this point of view, we
speculate that TAp73 suppresses angiogenesis in the long-term
development of cancer.
In addition, a different p53 status might affect the
role of TAp73 in angiogenesis (6).
Therefore, profiling the coexpression status of TAp73 and p53 in
tumor cells is crucial. We observed significantly higher expression
of TAp73 and mutant p53 in LAC tissues compared to paraneoplastic
lung tissue. Moreover, both proteins exhibited extremely
significant positive correlation expression trends. In addition to
hypoxia, DNA damage and factors such as p53 status can be
p73-mediated angiogenesis response modulators. The mechanism may be
related to TAp73 regulation of VEGF in the presence of wild-type
p53 conditions, where TAp73 can upregulate VEGF expression
(6), thereby affecting angiogenesis;
however, the specific mechanism still requires further study. Our
results imply that TAp73 might function as an anti-angiogenic in
LAC with mutant p53 expression. Our assumption remains to be
verified in future studies.
How does TAp73 inhibit angiogenesis in LAC? It has
been reported that VASH1 is a negative regulator of angiogenesis in
normal tissue differentiation or tumor development. A secretory
protein, VASH1 can be induced as a negative feedback regulator in
endothelial cells by stimulators of angiogenesis such as VEGF and
fibroblast growth factor 2 (FGF-2) (27). Many reports have indicated that VASH1
expression is high in malignant solid tumors, and that its
expression is closely related to tumor angiogenesis, such as in
breast (28), liver (29), renal (19), ovarian (30), colorectal (18), and lung cancer (31). Similar to these reports, we found the
higher VASH1 expression in LAC tissue than in the adjacent normal
lung tissues. More interestingly, there was a significant positive
correlation between VASH1 and TAp73 expression levels similar to
that between VASH1 and mutant p53. To our knowledge, there are few
reports on the mechanism of TAp73 regulation of VASH1, until now.
Considering TAp73 functions as a transcription factor, we used the
MatInspector Database to analyze the promoter/enhancer region of
VASH1, and found several potential p73 binding sites (data not
shown). Whether TAp73 regulates VASH1 expression remains to be
elucidated.
Collectively, our results show that TAp73, mutant
p53, and VASH1 expression is significantly higher in LAC tissue
compared to paraneoplastic lung tissue. Moreover, the expression
trends of TAp73, mutant p53, and VASH1 are significantly positively
correlated. The results imply the function of TAp73 in regulating
tumor angiogenesis.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant nos. 81071675 and 81472022), the
Natural Science Foundation of Beijing (grant no. 5122021) and the
Key Research Project of Medical Science of Hebei Province (grant
no. 20170185). We thank Dr. Fei Pei and Dr. Min Li at the
Department of Pathology, School of Basic Medical Sciences, Peking
University for their help in image analysis and scoring of tissue
microarray.
References
|
1
|
He YY, Zhang CX, Yang YY, Niu FY, Zeng Z,
Yan HH, Xu CR, Guan JL, Zhong WZ, Yang LL, et al: Prognostic
significance of genotype and number of metastatic sites in advanced
non-small-cell lung cancer. Clin Lung Cancer. 15:441–447. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yazdani S, Miki Y, Tamaki K, Ono K,
Iwabuchi E, Abe K, Suzuki T, Sato Y, Kondo T and Sasano H:
Proliferation and maturation of intratumoral blood vessels in
non-small cell lung cancer. Hum Pathol. 44:1586–1596. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Deyoung MP and Ellisen LW: P63 and p73 in
human cancer: Defining the network. Oncogene. 26:5169–5183. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yang A, Walker N, Bronson R, Kaghad M,
Oosterwegel M, Bonnin J, Vagner C, Bonnet H, Dikkes P, Sharpe A, et
al: p73-deficient mice have neurological, pheromonal and
inflammatory defects but lack spontaneous tumors. Nature.
404:99–103. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Marqués-Garcia F, Ferrandiz N,
Fernández-Alonso R, González-Cano L, Herreros-Villanueva M,
Rosa-Garrido M, Fernández-García B, Vaque JP, Marqués MM, Alonso
ME, et al: p73 plays a role in erythroid differentiation through
GATA1 induction. J Biol Chem. 284:21139–21156. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Vikhanskaya F, Bani MR, Borsotti P,
Ghilardi C, Ceruti R, Ghisleni G, Marabese M, Giavazzi R, Broggini
M and Taraboletti G: p73 overexpression increases VEGF and reduces
thrombospondin-1 production: Implications for tumor angiogenesis.
Oncogene. 20:7293–7300. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Guan M, Peng HX, Yu B and Lu Y: p73
Overexpression and angiogenesis in human colorectal carcinoma. Jpn
J Clin Oncol. 33:215–220. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Petrova V, Mancini M, Agostini M, Knight
RA, Annicchiarico-Petruzzelli M, Barlev NA, Melino G and Amelio I:
TAp73 transcriptionally represses BNIP3 expression. Cell Cycle.
14:2484–2493. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Stantic M, Sakil HA, Zirath H, Fang T,
Sanz G, Fernandez-Woodbridge A, Marin A, Susanto E, Mak TW,
Arsenian Henriksson M and Wilhelm MT: TAp73 suppresses tumor
angiogenesis through repression of proangiogenic cytokines and
HIF-1α activity. Proc Natl Acad Sci USA. 112:pp. 220–225. 2015;
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dulloo I, Phang BH, Othman R, Tan SY,
Vijayaraghavan A, Goh LK, Martin-Lopez M, Marques MM, Li CW, Wang
de Y, et al: Hypoxia-inducible TAp73 supports tumorigenesis by
regulating the angiogenic transcriptome. Nat Cell Biol. 17:511–523.
2015. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dulloo I, Hooi PB and Sabapathy K:
Hypoxia-induced DNp73 stabilization regulates Vegf-A expression and
tumor angiogenesis similar to TAp73. Cell Cycle. 14:3533–3539.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fernandez-Alonso R, Martin-Lopez M,
Gonzalez-Cano L, Garcia S, Castrillo F, Diez-Prieto I,
Fernandez-Corona A, Lorenzo-Marcos ME, Li X, Claesson-Welsh L, et
al: p73 is required for endothelial cell differentiation, migration
and the formation of vascular networks regulating VEGF and TGFβ
signaling. Cell Death Differ. 22:1287–1299. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Salimath B, Marmé D and Finkenzeller G:
Expression of the vascular endothelial growth factor gene is
inhibited by p73. Oncogene. 19:3470–3476. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nigro JM, Baker SJ, Preisinger AC, Jessup
JM, Hostetter R, Cleary K, Bigner SH, Davidson N, Baylin S, Devilee
P, et al: Mutations in the p53 gene occur in diverse human tumour
types. Nature. 342:705–708. 1989. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zietz C, Rössle M, Haa C, Sendelhofer A,
Hirschmann A, Stürzl M and Löhrs U: MDM-2 oncoprotein
overexpression, p53 gene mutation, and VEGF up-regulation in
angiosarcomas. Am J Pathol. 153:1425–1433. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kerbel RS: Vasohibin-1: The feedback on a
new inhibitor of angiogenesis. J Clin Invest. 114:884–886. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kern JM, Bauer K, Rychli J, Wojta J,
Ritsch A, Gastl G, Gunsilius E and Untergasser G: Alternative
splicing of vasohibin-1 generates an inhibitor of endothelial cell
proliferation, migration, and capillary tube formation.
Arterioscler Thromb Vasc Biol. 28:478–484. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yan Y, Shen Z, Ye Y, Jiang K, Zhang H,
Shen C, Mustonen H, Puolakkainen P and Wang S: A novel molecular
marker of prognosis in colorectal cancer: Vasohibin-1. Med Oncol.
31:8162014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kanomata N, Sato Y, Miyaji Y, Nagai A and
Moriya T: Vasohibin-1 is a new predictor of disease-free survival
in operated patients with renal cell carcinoma. J Clin Pathol.
66:613–619. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lu C, Han HD, Mangala LS, Ali-Fehmi R,
Newton CS, Ozbun L, Armaiz-Pena GN, Hu W, Stone RL, Munkarah A, et
al: Regulation of tumor angiogenesis by EZH2. Cancer Cell.
18:185–197. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li DJ, Zhou K, Wang SG, Shi Z and Yang Z:
Recombinant adenovirus encoding vasohibin prevents tumor
angiogenesis and inhibits tumor growth. Cancer Sci. 101:448–452.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kandoth C, McLellan MD, Vandin F, Ye K,
Niu B, Lu C, Xie M, Zhang Q, McMichael JF, Wyczalkowski MA, et al:
Mutational landscape and significance across 12 major cancer types.
Nature. 502:333–339. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Oren M and Rotter V: Mutant p53
gain-of-function in cancer. Cold Spring Harb Perspect Biol.
2:a0011072010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Melino G, Laurenzi VD and Vousden KH: p73:
Friend or foe in tumorigenesis. Nat Rev Cancer. 2:605–615. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Levrero M, Laurenzi DV, Costanzo A, Gong
J, Wang JY and Melino G: The p53/p63/p73 family of transcription
factors: Overlapping and distinct functions. J Cell Sci.
113:1661–1670. 2000.PubMed/NCBI
|
|
26
|
Amelio I, Inoue S, Markert EK, Levine AJ,
Knight RA, Mak TW and Melino G: TAp73 opposes tumor angiogenesis by
promoting hypoxia-inducible factor 1α degradation. Proc Natl Acad
Sci USA. 112:pp. 226–231. 2015; View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yoshinaga K, Ito K, Moriya T, Nagase S,
Takano T, Niikura H, Sasano H, Yaegashi N and Sato Y: Roles of
intrinsic angiogenesis inhibitor, vasohibin, in cervical
carcinomas. Cancer Sci. 102:446–451. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tamaki K, Sasano H, Maruo Y, Takahashi Y,
Miyashita M, Moriya T, Sato Y, Hirakawa H, Tamaki N, Watanabe M, et
al: Vasohibin-1 as a potential predictor of aggressive behavior of
ductal carcinoma in situ of the breast. Cancer Sci. 101:1051–1058.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang Q, Tian X, Zhang C and Wang Q:
Upregulation of vasohibin-1 expression with angiogenesis and poor
prognosis of hepatocellular carcinoma after curative surgery. Med
Oncol. 29:2727–2736. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Takahashi Y, Koyanagi T, Suzuki Y, Saga Y,
Kanomata N, Moriya T, Suzuki M and Sato Y: Vasohibin-2 expressed in
human serous ovarian adenocarcinoma accelerates tumor growth by
promoting angiogenesis. Mol Cancer Res. 10:1135–1146. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang T, Yu TT, Zhang DM, Hou XM, Liu XJ,
Zhao D and Shan L: Vasohibin-1 expression detected by
immunohistochemistry correlates with prognosis in non-small cell
lung cancer. Med Oncol. 31:9632014. View Article : Google Scholar : PubMed/NCBI
|