Introduction
Papillary thyroid carcinoma (PTC) represents ~90% of
differentiated thyroid carcinomas in Japan (2004), which are the
most common malignant tumors of the endocrine system (1). Papillary thyroid microcarcinoma (PTMC)
is defined as PTCs with a diameter of <10 mm, and account for
39.5% of all PTCs in Italy (between 1993 and 2002) (2). Certain factors including radiation
exposure, iodine deficiency and a family history of PTCs are
associated with tumorigenesis and prognosis; however, the
expression status of specific biomarkers, including galectin-3 and
cytokeratin 19 (CK19) may also be used to predict tumor progression
(3,4).
PTMC grows slowly without obvious symptoms, however
due to the increased ultrasound detection rate, the incidence rate
of PTMC has been rising gradually in recent years (5). The majority of PTMCs are identified by
accident or are combined with other thyroid diseases (6). PTMCs may be standardly diagnosed by
removing thyroid lesions and observing their pathological features
and by immunohistochemistry staining of biomarkers (for example
galectin-3, CK-19 and TTF-1) (7–9). Surgical
treatment may lead to a favorable prognosis for PTMC; however, due
to the low risk of papillary thyroid cancer, various postoperative
treatments have little effect on prognostic outcome, and to the
best of our knowledge no previous studies have identified which
therapeutic method is the best option (10). Certain PTMCs are more malignant
compared with others and have a poor prognosis with early-stage
lymphocytic metastasis (11,12). These highly malignant tumors may be
associated with multilocus-tumors, tumor size and dilation of a
thyroid cyst (2,13–15).
Interstitial fibrosis (IF), which is composed of
fibroblasts and a variable number of collagen fibers, has been
observed in numerous malignant cancers (16–20). This
suggests that IF may be a factor associated with tumor prognosis.
In previous studies (21–23), fibrosis appears to be associated with
an increased cancer recurrence rate and mortality. Previous
observations have suggested that dense fibrosis may be another
vital indicator for the diagnosis of PTC (24), however, few studies have focused on
the associations between IF and PTC. Therefore, the aim of the
present study is to perform a retrospective analysis of the
clinical parameters and biomarkers of PTC and to determine the
association and interactions between IF and PTMC.
Materials and methods
Patients
A total of 511 patients were recruited into the
present study from the First Hospital of China Medical University
(Shenyang, China), between January 2009 and December 2013; 340
patients were diagnosed with PTMC with IF and 171 patients were
diagnosed with PTMC without IF. In total, 78.86% (304) of patients
were female, 21.14% were male. The age range was 20–75 years, with
an mean age of 46.83±10.69. All patients underwent a thyroidectomy
and met the following criteria: i) Diagnosed with PTMC according to
pathological standards (25); ii) did
not receive steroids or drugs that may induce fibrosis; and iii)
did not present with any other disease than PTMC. Patients who had
PTMC combined with thyroid inflammation or systemic diseases were
excluded from the study. The present study was approved by the
Institutional Review Board of China Medical University and by the
First Hospital of China Medical University Medical Research Ethics
Committee (Shenyang, China). Written informed consent was obtained
from all patients prior to their inclusion within the present
study.
All patients were divided into 2 groups. The
experimental group was composed of patients with IF (n=340) and the
control group was composed of patients without IF (n=171). Patients
with IF were defined as having a fibrotic area composed of
fibroblasts and collagen fibers within the tumor, which occupied
the central part of the tumor and formed a radially expanding
fibrosclerotic core. The patient's baseline characteristics,
including age, sex, calcification, serum thyroid-stimulating
hormone (TSH) level, tumor size, tumor node and metastasis stage,
tumor multiplicity, bilaterality, extrathyroidal extension (ETE),
subtype of papillary microcarcinoma and lymphocytic metastasis,
were evaluated. The thyroid tumor stage was classified in
accordance with the American Joint Committee on Cancer (26). Postoperative follow-up ended on 1st
September 2016.
Histopathology
Each specimen slice was stained with hematoxylin and
eosin for observing the pathological form of the cancer tissue.
Freshly resected specimens, ~1 cm in thickness, were fixed in 10%
neutral formaldehyde for 12 h and then dehydrated using graded
series of analytical pure ethanol at room temperature as follows:
60% (1 h), 75% (30 min), 80% (15 min), 95% (10 min), 100% (5 min),
then the specimens were placed in xylene at room temperature (10
min × 2), and immersed in paraffin (efficient slice paraffin,
melting point 56–58°C) at 59°C for 1 h, embedded in paraffin and
cooled at room temperature. Paraffin specimens were then cut into 4
µm slices, dewaxed using xylene (15 min × 2) and hydrated using
graded series of analytical pure ethanol at room temperature as
follows: 100% (5 min × 2), 95, 85 and 75% ethanol (3 min each).
Specimens were then stained with hematoxylin for 8 min at room
temperature, followed by washing with 1% hydrochloric acid alcohol
for a few seconds and then washed with tap water for 10 min.
Specimens were then stained with eosin for 5 min at room
temperature, and then specimens were dehydrated using a graded
series of ethanol at room temperature as follows: 75, 85 and 95% (2
min each), 100% (1 min × 2); transparent in xylene at room
temperature (5 min × 2); neutral gum (cat no., G8590; Solarbio,
Beijing, China) sealed slice. Light microscopy was used to observe
specimens (magnification, ×100). The immunohistochemistry of all
slices was performed by a minimum of 2 pathologists from the
Pathology Department of the First Hospital of China Medical
University and each experiment was repeated 3 times to confirm
results. All cases were classified according to multiple clinical
biomarkers, including cellular tumor antigen p53, proliferation
marker protein Ki-67 (Ki-67), CK19, thyroglobulin (Tg), thyroid
transcription factor I (TTF-1) and galectin-3. These biomarkers are
used for the common diagnosis of PTMC, p53 and Ki67 positive
samples may exhibit the proliferation of tumor cells, while CK19,
TTF-1, Tg and galectin-3 positive samples may predict that the
origin of the malignant cells is the thyroid epithelium.
Immunohistochemistry
Paraffin embedded sections (4 µm-thick) were dewaxed
and hydrated as aforementioned. Specimens were then washed with
phosphate-buffered saline (PBS), citric acid heat antigen repair
for 2 min, incubated with 3% hydrogen peroxide for blocking
endogenous peroxidase for 10 min at room temperature, washed with
PBS, then incubated with primary antibodies at room temperature for
60 min. Negative control specimens were incubated with PBS instead
of the primary antibody. All antibodies were produced by Fuzhou
Maixin Biotech Co., Ltd. (Fujian, China), and were supplied at
ready-to-use dilutions. The primary antibodies (100 µl) used were
as follows: Galectin-3 (cat. no. MAB-0572), CK19 (cat. no.
Kit-0030), TTF-1 (cat. no. MAB-0599), Ki-67 (cat. no. Kit-0005),
p53 (cat. no. Kit-0010) and Tg (cat. no. MAB-0161). Sections were
then washed with PBS 3 times and incubated with enzyme-labeled goat
anti-mouse/rabbit immunoglobulin G polymer at room temperature
(cat. no. KIT-5030) for 15 min, and then washed with PBS. Following
this, DAB (cat. no. DAB-1031; Fuzhou Maixin Biotech Co., Ltd.) was
added for 3–5 min at room temperature for color development to
assist in light microscopy analysis (magnification, ×100), and then
washed with tap water for 10 min. Finally, specimens were stained
with hematoxylin for 8 min at room temperature, washed with tap
water, and the procedure of dehydration and transparency was the
same as aforementioned. Neutral gum was used to seal the slice.
Specimens were observed using light microscopy. IF was determined
using 2 pathologists who were blinded to the study aims. Each
experiment was repeated three times.
Statistical analysis
Data analysis was performed using SPSS (version 20;
IBM Corp., Armonk, NY, USA). Continuous data were presented as the
mean ± standard deviation. Dichotomous variables were compared
using the χ2 test or Fisher's exact test, while the
Student's t-test was used to compare continuous variables. The
survival data were analyzed using Kaplan-Meier curves and the
log-rank test and Cox proportional hazard models. P<0.05 was
considered to indicate a statistically significant difference.
Results
Differences between PTMC with IF and
without IF
There were 511 cases of PTMC examined in the present
study. Patients with IF accounted for 66.54% (340 cases), while
patients without IF accounted for 33.46% (171 cases) of the cases.
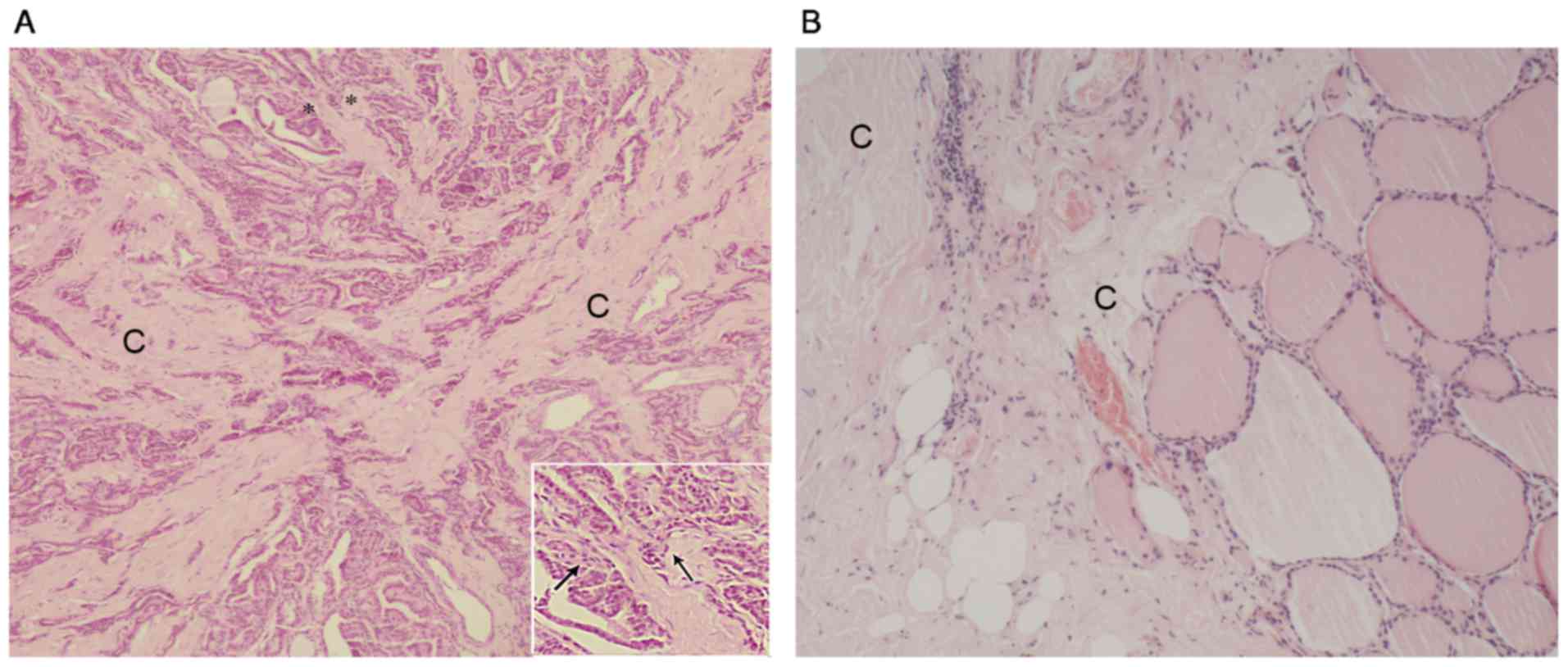
The differences between PTMC and normal thyroid tissue in a
fibrotic formation may be observed in Fig. 1. In Fig.
1A, the normal follicular structure interrupted by squashed
cancer cells, accompanied by the proliferation of fibroblasts and
collagen fibers. Fig. 1B demonstrates
the complete thyroid follicular structure in the nodular goiter
tissue with IF, collagen fibers are positioned next to the
follicles. Diffuse collagen fibers appear next to the nodular
goiter and there is almost no fibroblast proliferation.
Association of IF with clinical
parameters
The details and clinical characteristics of all
patients included in the present study are presented in Table I. Older patients (≥45 years) had a
significantly increased incidence of IF (P=0.012) compared with
patients <45 years. In addition, significantly more females
presented with IF (P=0.024) compared with males that presented with
IF. The median tumor size was 5.0 mm (range, 1.0–10 mm). Using the
median size as the cut-off point, a tumor ≥5.0 mm was revealed to
be associated with a significantly increased possibility of
fibrotic formation compared with PTMC without IF (P=0.017).
Fibrosis was revealed to be significantly associated with lymph
node metastasis (P=0.0003). However, there was no significant
association between calcification, serum TSH levels, tumor
multiplicity, bilaterality, ETE or subtype of PTMC and IF.
 | Table I.Characteristics of patients with PTMC
with or without IF. |
Table I.
Characteristics of patients with PTMC
with or without IF.
| Characteristic | PTMC with IF (%) | PTMC without IF
(%) | Total (%) | P-value |
|---|
| Age (years) |
|
|
| 0.0331 |
| Mean ±
SD | 47.81±10.44 | 44.88±10.94 | 46.83±10.69 |
|
| Range | 21–75 | 20–68 | 20–75 |
|
|
<45 | 120 (35.29) | 80 (46.78) | 200 (39.13) |
|
| ≥45 | 220 (64.71) | 91 (53.22) | 311 (60.87) | 0.012 |
| Sex |
|
|
|
|
|
Female | 278 (81.77) | 125 (73.10) | 403 (78.86) | 0.0236 |
| Male | 62 (18.23) | 46 (26.9) | 108 (21.14) |
|
| Calcification |
|
| 511 (80.44) | 0.3889 |
|
Present | 261 (76.76) | 137 (80.12) | 398 (77.89) |
|
|
Absent | 79 (23.24) | 34 (19.88) | 113 (22.11) |
|
| TSH (mmol/l) |
|
|
| 0.863 |
| Mean ±
SD | 1.76±1.49 | 1.78±1.343 | 1.77±1.44 |
|
|
Range | 0.002–10.43 | 0.0020–7.61 | 0.002–10.44 |
|
| Tumor size
(mm) |
|
|
| 0.0136 |
| Mean ±
SD | 4.93±2.46 | 4.21±2.27 | 4.69±2.42 |
|
|
Range | 0.5–10 | 0.6–9 | 0.5–10 |
|
| Diameter ≥5 mm | 201 (59.12) | 82 (47.95) | 283 (55.38) | 0.0166 |
| Diameter <5
mm | 139 (40.88) | 89 (52.05) | 228 (44.62) |
|
| Multiplicity |
|
|
| 0.2595 |
|
Absent | 262 (77.06) | 124 (72.51) | 386 (75.54) |
|
|
Present | 78 (22.94) | 47
(27.49) | 125 (24.46) |
|
| Bilaterality |
|
|
| 0.6463 |
|
Absent | 301 (88.53) | 149 (87.13) | 450 (88.06) |
|
|
Present | 39 (11.47) | 22 (12.87) | 61 (11.94) |
|
| ETE |
|
|
| 0.1322 |
|
Absent | 290 (85.29) | 154 (90.06) | 444 (86.89) |
|
|
Present | 50 (14.71) | 17 (9.94) | 67
(39.18) |
|
| Subtype |
|
|
| 0.7377 |
| classic
papillary | 326 (95.88) | 165 (96.49) | 491 (96.07) |
|
|
follicular | 14 (4.12) | 6 (3.51) | 20 (3.93) |
|
| Lymph
metastasis |
|
|
| 0.0003 |
| LM
present | 116 (34.18) | 32 (18.71) | 148 (28.96) |
|
| LM
absent | 224 (65.72) | 139 (81.29) | 363 (65.17) |
|
| Lymph node
status |
|
|
| 0.0058 |
| N0 | 226 (66.47) | 137 (80.12) | 363 (71.04) |
|
|
N1a | 74 (21.76) | 22 (12.87) | 96 (18.78) |
|
|
N1b | 40 (11.77) | 12 (7.02) | 52 (30.41) |
|
| TNM stage |
|
|
| 0.0067 |
| I | 278 (81.76) | 159 (92.98) | 437 (85.52) |
|
|
III | 37 (10.88) | 8
(4.68) | 45 (8.81) |
|
| IV | 21 (3.53) | 4 (2.34) | 25 (4.89) |
|
Association of IF with pathological
biomarkers
Common biomarkers were observed and analyzed to
determine whether they were associated with the presence of IF in
tumors. Galectin-3 and CK19 revealed a significant association with
IF (P=0.022 and P=0.008, respectively; Table II). There were no other significant
associations observed between the presence of Tg, TTF-1, p53or Ki67
and the cases of IF.
 | Table II.Association between interstitial
fibrosis and common biomarkers using the χ2 text. |
Table II.
Association between interstitial
fibrosis and common biomarkers using the χ2 text.
| Biomarkers | Fibrosis-present
(%) | Fibrosis-absent
(%) | Total | P-value |
|---|
| p53 |
|
|
|
|
|
Positive | 161 (63.39) | 105 (63.64) | 266 | 0.9587 |
|
Negative | 93 (36.61) | 60 (36.36) | 153 |
|
|
Total | 254 | 165 | 419 |
|
| Ki67 |
|
|
|
|
|
Positive | 184 (54.12) | 107 (62.57) | 291 | 0.0685 |
|
Negative | 156 (45.88) | 64
(37.43) | 220 |
|
|
Total | 340 | 171 | 511 |
|
| CK19 |
|
|
|
|
|
Positive | 316 (93.49) | 145 (86.31) | 461 | 0.0075 |
|
Negative | 22 (6.51) | 23 (13.69) | 45 |
|
|
Total | 338 | 168 | 506 |
|
| Tg |
|
|
|
|
|
Positive | 268 (82.72) | 133 (82.10) | 401 | 0.8658 |
|
Negative | 56 (17.28) | 29 (17.90) | 85 |
|
|
Total | 324 | 162 | 486 |
|
| TTF-1 |
|
|
|
|
|
Positive | 296 (88.36) | 137 (83.54) | 433 | 0.8659 |
|
Negative | 39 (11.44) | 27 (16.59) | 66 |
|
|
Total | 335 | 164 | 499 |
|
| Galectin-3 |
|
|
|
|
|
Positive | 293 (88.25) | 159 (94.08) | 452 | 0.0220 |
|
Negative | 39 (11.75) | 9 (5.33) | 48 |
|
|
Total | 332 | 168 | 500 |
|
Results of survival data
Due to the decreased mortality rate of patients with
PTMC, and since only 4 patients suffered mortality during the
follow-up period, recurrence-free survival was used as the outcome
of interest. A lack of recurrence outcome due to a lack of
follow-up, loss to the follow-up period and non-thyroid-cancer
mortality was defined as censored data. The censoring rate was 93.0
and 81.2% in the IF and no IF groups, respectively. The number of
loss to follow-up period patients was 33 in the IF group and 52 in
the no IF group. Each group had 1 patient who suffered from
non-thyroid-cancer mortality. The median follow-up time was 46
months (range, 5.0–90 months) and the total recurrence rate was
14.87% (76 cases). The fibrosis group demonstrated an 18.82% (64
cases) recurrence rate and the no-fibrosis group had a 7.02% (12
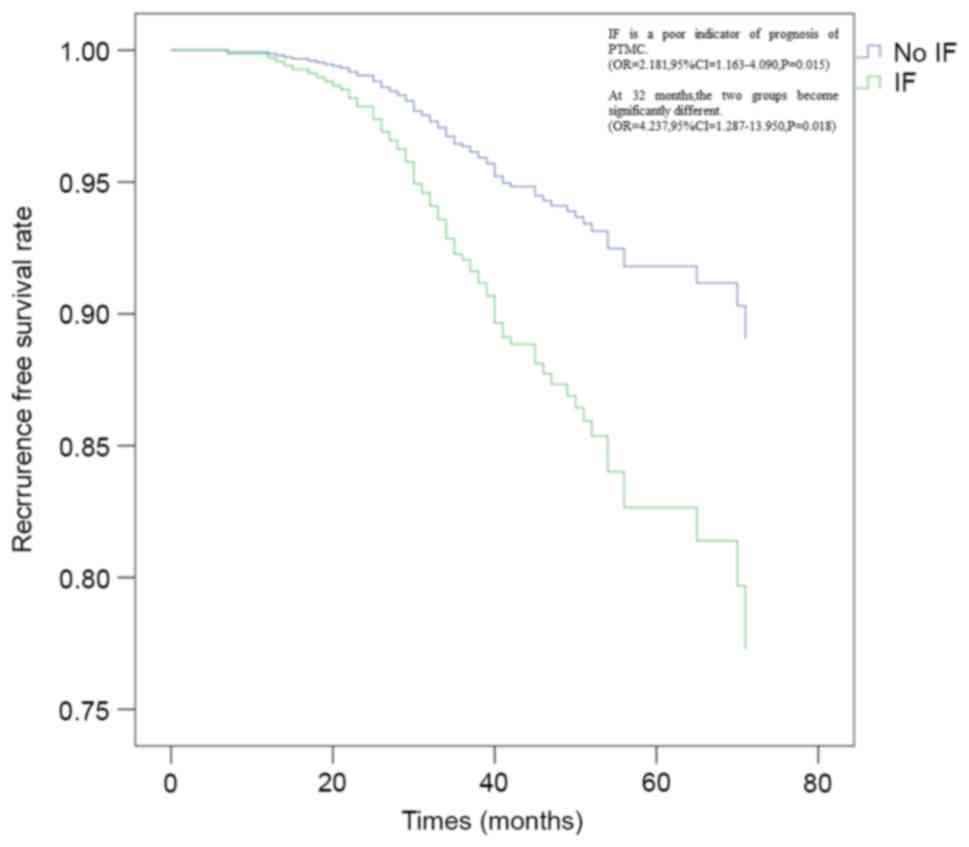
cases) recurrence rate. Multivariate analysis by Cox's proportional
hazard model revealed that the presence of IF (hazard ratio =
2.18195%; confidence interval = 1.163–4.090; P=0.015) was an
independent factor for the prediction of a poor prognosis in
patients with PTMC (Fig. 2).
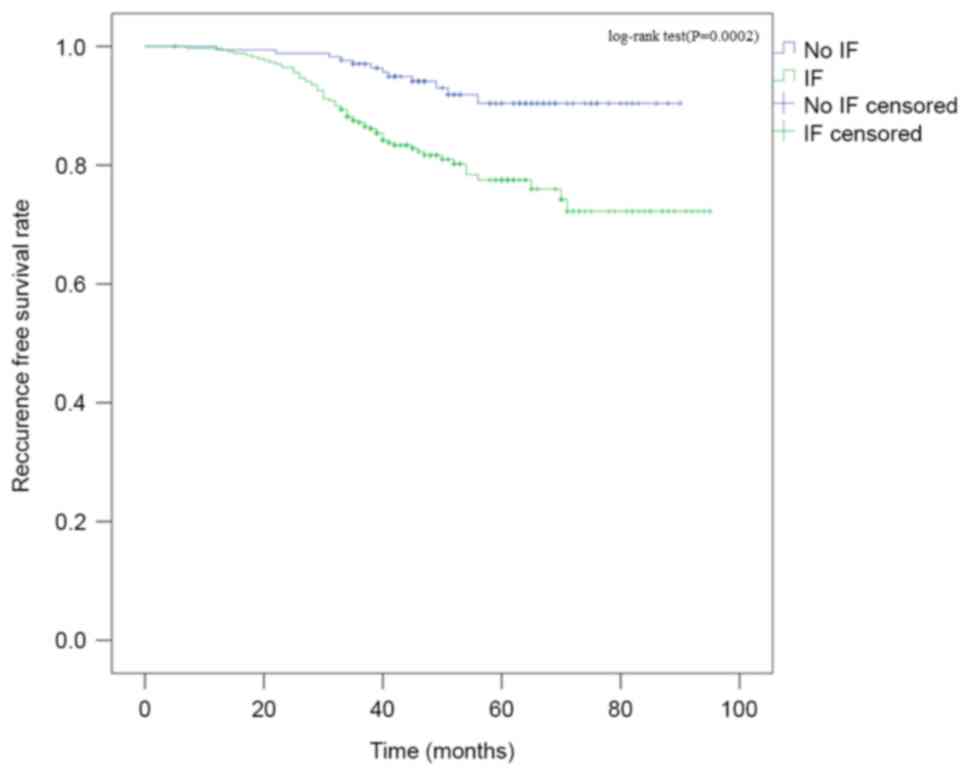
Kaplan-Meier survival curves revealed the same
outcome (log rank, P=0.002) (Fig. 3).
At 32 months, the two groups become significantly different as
analyzed by Cox's proportional hazard model (hazard ratio =
4.23795%; confidence interval = 1.287–13.950; P=0.018). Other poor
prognostic indicators as presented in Table III were age (>45 years; P=0.49),
extrathyroidal extension (P<0.001) and lymphocytic metastasis
(P<0.001). Therefore, IF is a poor indicator of prognosis of
PTMC.
 | Table III.Risk factors of PTMC recurrence by
Cox's proportional hazard model. |
Table III.
Risk factors of PTMC recurrence by
Cox's proportional hazard model.
| Variable | Odds ratio | 95% confidence
interval | P-value |
|---|
| Sex | 1.656 | 1.001–2.739 | 0.049 |
| Age (>45
years) | 0.483 | 0.198–1.180 | 0.110 |
| Multiplicity | 1.758 | 0.628–4.922 | 0.283 |
| Bilaterality | 3.735 | 2.261–6.169 | <0.001 |
| ETE | 1.289 | 0.793–2.094 | 0.305 |
| IF | 2.227 | 1.186–4.181 | 0.013 |
| Lymph
metastasis | 3.560 | 2.164–5.858 | <0.001 |
Discussion
Cancer-associated fibroblasts have been described in
multiple types of cancer; they appear primarily as abnormal IF and
have been extensively studied (27–30).
Fibrotic density, when there are no superior clear parameters, may
be considered as a vital index of prognosis (24), however, few previous studies have
focused on the correlation between IF and PTMC (31) and to the best of our knowledge the
association between fibrosis and specific biomarkers has not been
previously reported.
IF has not been clearly defined within the medical
community. In general, IF is considered to occupy the central
section of the tumor and often consists of radiating and expanding
fibrous bands (32). Rebecchini et
al (33) revealed that a
characteristic feature of PTC was that interstitial fibers occupied
40–60% of the central section of the tumors. In a study performed
by Isarangkul (24), 33 of 37 PTC
specimens presented interstitial changes consisting of dense
collagen and fibroblasts. It was suggested that PTC was
significantly associated with fibrosis when compared with the
follicular subtype (odds ratio = 37.95; P<0.01). It is clear
that there are correlations between tumorigenesis and fibrosis in
many tumors, including breast, ovarian, esophageal, colorectal,
pancreatic and prostate cancers (16–20).
Interstitial changes influence tumor infiltration, metastasis,
prognosis and neovascularization (34). In the present study observations under
a light microscope demonstrated that the majority of IF cases were
accompanied by an incomplete fibrotic boundary of the tumor, as
with the fibrosis from the central section of the tumor, which
disturbs the formation of the fiber encapsulation on the tumor. It
was also revealed that IF is a poor indicator of prognosis;
therefore, the formation of fibrotic lesions may be referred to as
a more aggressive behavior of the tumor.
In the present study, tumors with fibrosis were
significantly associated with a patient's age (P=0.0331) and tumor
size (P=0.0166), as previously reported (35). Female patients were more likely to
have fibrotic changes compared with male patients. Whether an
estrogen receptor (ER) wound contributed to this change requires
further study. It has been previously reported that fibrotic focus
has an association with ERs in breast cancer (34).
Although certain previous studies have considered
that the grade of IF may predict tumor recurrence and metastasis
(34), there is little evidence for
this in thyroid carcinoma. However, De Matos et al (36) detected the expression of galectin-3 in
three types of thyroid tissue, the results of which revealed that
the expression ratios in 84 cases of PTC and 38 follicular
carcinoma cases were 72.6 and 21.0%, respectively, whereas it was
nearly zero in non-tumor cases. Additionally, galection-3 is
considered as serving a key role in cellular adhesion and
interactions between cells and their matrix, which may reflect
tumor metastasis conditions (37).
Certain previous studies have reported that galectin-3 may be
viewed as a potential tumor marker of metastasis and poor prognosis
(3). In the present study, it was
revealed that a higher expression level of galectin-3 was
significantly associated with fibrosis formation. Therefore,
patients who are galectin-3 positive with PTMC with IF may be
considered as evidence that the formation of IF predicts prognosis,
alternatively IF formation may be as a result of the expression of
galectin-3. Given the difficulties of diagnosing thyroid tumors,
specimens stained to determine IF and the expression of galectin-3
may increase the sensitivity and specificity of a diagnosis and the
prediction of a prognosis, particularly when PTMC is
determined.
It was previously reported that the expression of
CK19 was decreased in normal thyroid tissue; however, CK19 may be
viewed as a vital biomarker for cancer when carcinoma cells of PTMC
are positive, compared with normal thyroid tissue (38). Although there has been no evidence
that CK-19 is a predictor of prognosis, the association between IF
and CK19 is positive in the present study, it also can be
speculated that the IF has an effect on the PTMC, additional
studies are required to determine the association between IF and
CK19.
To conclude, it was revealed that there is an
association between IF and PTMC, how the underlying mechanism
behind this association remains unclear. The present study
demonstrated that IF combined with specific biomarkers may be an
important diagnostic tool for PTMC. Additional studies are required
to determine the course of the development of IF and whether it is
associated with tumorigenesis and prognosis.
Acknowledgements
The authors would like to thank the staff,
particularly Professor Yuchen Han at the Institute of Pathology of
China Medical University for advice and support.
References
|
1
|
Kammori M, Fukumori T, Sugishita Y, Hoshi
M and Yamada T: Therapeutic strategy for low-risk thyroid cancer in
Kanaji Thyroid Hospital. Endocr J. 61:1–12. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Roti E, Rossi R, Trasforini G, Bertelli F,
Ambrosio MR, Busutti L, Pearce EN, Braverman LE and Degli Uberti
EC: Clinical and histological characteristics of papillary thyroid
microcarcinoma: Results of a retrospective study in 243 patients. J
Clin Endocrinol Metab. 91:2171–2178. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tang W, Huang C, Tang C, Xu J and Wang H:
Galectin-3 may serve as a potential marker for diagnosis and
prognosis in papillary thyroid carcinoma: A meta-analysis. Onco
Targets Ther. 9:455–460. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Isic Dencic T, Cvejic D, Paunovic I, Tatic
S, Havelka M and Savin S: Cytokeratin19 expression discriminates
papillary thyroid carcinoma from other thyroid lesions and predicts
its aggressive behavior. Med Oncol. 30:3622013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Haugen BR, Alexander EK, Bible KC, Doherty
GM, Mandel SJ, Nikiforov YE, Pacini F, Randolph GW, Sawka AM,
Schlumberger M, et al: 2015 American thyroid association management
guidelines for adult patients with thyroid nodules and
differentiated thyroid cancer: The American Thyroid association
guidelines task force on thyroid nodules and differentiated thyroid
cancer. Thyroid. 26:1–133. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pacini F: Thyroid microcarcinoma. Best
Pract Res Clin Endocrinol Metab. 26:421–429. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Giannini R, Faviana P, Cavinato T, Elisei
R, Pacini F, Berti P, Fontanini G, Ugolini C, Camacci T, De Ieso K,
et al: Galectin-3 and oncofetal-fibronectin expression in thyroid
neoplasia as assessed by reverse ranscription-polymerase chain
reaction and immunochemistry in cytologic and pathologic specimens.
Thyroid. 13:765–770. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cheung CC, Ezzat S, Freeman JL, Rosen IB
and Asa SL: Immunohistochemical diagnosis of papillary thyroid
carcinoma. Mod Pathol. 14:338–342. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kreft A, Hansen T and Kirkpatrick CJ:
Thyroid transcription factor 1 expression in cystic lesions of the
neck: An immunohistochemical investigation of thyroglossal duct
cysts, branchial cleft cysts and metastatic papillary thyroid
cancer. Virchows Arch. 447:9–11. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
McLeod DS, Sawka AM and Cooper DS:
Controversies in primary treatment of low-risk papillary thyroid
cancer. Lancet. 381:1046–1057. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Patron V, Bedfert C, Le Clech G, Aubry K
and Jegoux F: Pattern of lateral neck metastases in N0 papillary
thyroid carcinoma. BMC Cancer. 11:82011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hartl DM, Leboulleux S, Al Ghuzlan A,
Baudin E, Chami L, Schlumberger M and Travagli JP: Optimization of
staging of the neck with prophylactic central and lateral neck
dissection for papillary thyroid carcinoma. Ann Surg. 255:777–783.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pazaitou-Panayiotou K, Capezzone M and
Pacini F: Clinical features and therapeutic implication of
papillary thyroid microcarcinoma. Thyroid. 17:1085–1092. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chow SM, Law SC, Chan JK, Au SK, Yau S and
Lau WH: Papillary microcarcinoma of the thyroid-prognostic
significance of lymph node met astasis and multifocality. Cancer.
98:31–40. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim TY, Hong SJ, Kim JM, Kim WG, Gong G,
Ryu JS, Kim WB, Yun SC and Shong YK: Prognostic parameters for
recurrence of papillary thyroid microcarcinoma. BMC Cancer.
8:2962008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fisher ER, Palekar AS, Sass R and Fisher
B: Scar cancers: Pathologic findings from the national surgical
adjuvant breast project (protocol no. 4)-IX. Breast Cancer Res
Treat. 3:39–59. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang C, Fu L, Fu J, Hu L, Yang H, Rong
TH, Li Y, Liu H, Fu SB, Zeng YX and Guan XY: Fibroblast growth
factor receptor 2-positive fibroblasts provide a suitable
microenvironment for tumor development and progression in
esophageal carcinoma. Clin Cancer Res. 15:4017–4027. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hwang RF, Moore T, Arumugam T,
Ramachandran V, Amos KD, Rivera A, Ji B, Evans DB and Logsdon CD:
Cancer-associated stromal fibroblasts promote pancreatic tumor
progression. Cancer Res. 68:918–926. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kozel PJ, Friedman RA, Erway LC, Yamoah
EN, Liu LH, Riddle T, Duffy JJ, Doetschman T, Miller ML, Cardell EL
and Shull GE: Balance and hearing deficits in mice with a null
mutation in the gene encoding plasma membrane
Ca2+-ATPase isoform. J Bio Chem. 273:18693–18696. 1998.
View Article : Google Scholar
|
|
20
|
Nazareth MR, Broderick L, Simpson-Abelson
MR, Kelleher RJ Jr, Yokota SJ and Bankert RB: Characterization of
human lung tumor-associated fibroblasts and their ability to
modulate the activation of tumor-associated T cells. J Immunol.
178:5552–5562. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kondo T, Okabayashi K, Hasegawa H, Tsuruta
M, Shigeta K and Kitagawa Y: The impact of hepatic fibrosis on the
incidence of liver metastasis from colorectal cancer. Br J Cancer.
115:34–39. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen X, Xiao W, Chen W, Liu X, Wu M, Bo Q,
Luo Y, Ye S, Cao Y and Liu Y: MicroRNA-26a and −26b inhibit lens
fibrosis and cataract by negatively regulating Jagged-1/Notch
signaling pathway. Cell Death Differ. 24:1431–1442. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Miura K, Hamanaka K, Koizumi T, Kitaguchi
Y, Terada Y, Nakamura D, Kumeda H, Agatsuma H, Hyogotani A,
Kawakami S, et al: Clinical significance of preoperative serum
albumin level for prognosis in surgically resected patients with
non-small cell lung cancer: Comparative study of normal lung,
emphysema, and pulmonary fibrosis. Lung Cancer. 111:88–95. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Isarangkul W: Dense fibrosis. Another
diagnostic criterion for papillary thyroid carcinoma. Arch Pathol
Lab Med. 117:645–666. 1993.PubMed/NCBI
|
|
25
|
Livolsi VA and Saavedra JA: Papillary
carcinoma. DeLellis RA, Lloyd RV, Heitz PU and Eng C: World Health
Organization Classification of Tumours: Pathology and Genetics of
Tumours of Endocrine Organs. 8. IARC Press; Lyon, France: pp.
57–66. 2004
|
|
26
|
Edge SB and Compton CC: The American Joint
Committee on Cancer: The 7th edition of the AJCC cancer staging
manual andthe future of TNM. Ann Surg Oncol. 17:1471–1474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kumar V, Donthireddy L, Marvel D,
Condamine T, Wang F, Lavilla-Alonso S, Hashimoto A, Vonteddu P,
Behera R, Goins MA, et al: Cancer-associated fibroblasts neutralize
the anti-tumor effect of CSF1 receptor blockade by inducing
PMN-MDSC infiltration of tumors. Cancer Cell. 32:654–668.e5. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sewell-Loftin MK, Bayer SVH, Crist E,
Hughes T, Joison SM, Longmore GD and George SC: Cancer-associated
fibroblasts support vascular growth through mechanical force. Sci
Rep. 7:125742017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pang T, Wang X, Gao J, Chen W, Shen XJ,
Nie MM, Luo T, Yin K, Fang G, Wang KX and Xue XC: Fiber-modified
hexon-chimeric oncolytic adenovirus targeting cancer associated
fibroblasts inhibits tumor growth in gastric carcinoma. Oncotarget.
8:76468–76478. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tao L, Huang G, Song H, Chen Y and Chen L:
Cancer associated fibroblasts: An essential role in the tumor
microenvironment. Oncol Lett. 14:2611–2620. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang J, Wang Y, Li D and Jing S: Notch
and TGF-β/Smad3 pathways are involved in the interaction between
cancer cells and cancer-associated fibroblasts in papillary thyroid
carcinoma. Tumour Biol. 35:379–385. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Van den Eynden GG, Colpaert CG, Couvelard
A, Pezzella F, Dirix LY, Vermeulen PB, Van Marck EA and Hasebe T: A
fibrotic focus is a prognostic factor and a surrogate marker for
hypoxia and (lymph)angiogenesis in breast cancer: Review of the
literature and proposal on the criteria of evaluation.
Histopathology. 51:440–451. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Rebecchini C, Nobile A, Piana S, Sarro R,
Bisig B, Gerasimos SP, Saglietti C, Matter M, Marino L and
Bongiovanni M: Papillary thyroid carcinoma with fibromatosis-like
stroma: Are port of two cases. Endocr Pathol. 13:219–221. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mujtaba SS, Ni YB, Tsang JY, Chan SK,
Yamaguchi R, Tanaka M, Tan PH and Tse GM: Fibrotic focus in breast
carcinomas: Relationship with prognostic parameters and biomarkers.
Ann Surg Oncol. 20:2842–2849. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhou H, Kimura K, Orita T, Nishida T and
Sonoda KH: Inhibition by female sex hormones of collagen
degradation by corneal fibroblasts. Mol Vis. 17:3415–422.
2011.PubMed/NCBI
|
|
36
|
De Matos PS, Ferreira AP, de Oliveira
Facuri F, Assumpção LV, Metze K and Ward LS: Usefulness of HBME-1,
cytokeratin 19 and galectin-3 immunostainingin the diagnosis of
thyroid malignancy. Histopathology. 47:391–401. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Htwe TT, Karim N, Wong J, Jahanfar S and
Mansur MA: Differential expression of galectin-3 in advancing
thyroid cancer cells: A clue toward understanding tumour
progression and metastasis. Singapore Med J. 51:856–859.
2010.PubMed/NCBI
|
|
38
|
Erkılıç S, Aydın A and Koçer NE:
Diagnostic utility of cytokeratin 19 expression in multinodular
goiter with papillary areas and papillary carcinoma of thyroid.
Endocr Pathol. 13:207–211. 2002. View Article : Google Scholar : PubMed/NCBI
|