Introduction
Mucin 5AC oligomeric muscus/gel-forming (MUC5AC) is
a principal component of the gastric mucosa (1,2) and
constitutes an important part of the ecological niche, where the
stomach bacterium Helicobacter pylori is located (3). Adhesion to the gastric mucosa is
important in the life cycle of H. pylori (4). As MUC5AC is an important receptor for
gastric mucosa adhesion (5–7), efficient colonization of the stomach
requires high expression of MUC5AC by gastric epithelial cells
(8,9).
Previous studies have demonstrated that the stomach epithelium
reacts to H. pylori infection by activating pro-inflammatory
signaling pathways (10), thereby
activating innate defense mechanisms against H. pylori,
which may include the downregulation of MUC5AC expression in the
human gastric mucosa (2,6,11,12). Downregulation of MUC5AC expression in
the gastric mucosa is evident following H. pylori-associated
transformation of the gastric epithelium into cancer (13–15). As
MUC5AC expression is key for the ecological niche required for
H. pylori (6,7), it is to be expected that, in response to
evolutionary pressure, the bacterium may have evolved compensatory
mechanisms to combat the downregulation of MUC5AC. However, to
date, such mechanisms have been incompletely characterized.
The association between H. pylori infection
and gastric epithelial MUC5AC expression is well-recognized
(6–9,16), there
are a limited number of studies regarding the molecular mechanisms
that mediate H. pylori-dependent compensatory responses,
with respect to host downregulation of MUC5AC mucin gene
expression. The downregulation of MUC5AC expression appears to be
derived from an epithelial reaction towards the urease virulence
factor of H. pylori (17).
Therefore, it may be hypothesized that other virulence factors may
mediate compensatory responses. In view of the importance of H.
pylori for gastric oncological transformation and its
involvement in a number of types of non-malignant disease (18), the identification of virulence factors
potentially involved is required. Of these virulence factors,
cytotoxin-associated gene A (CagA) has been associated with the
interaction and modification of cellular phenotype and
intracellular signaling pathways e.g., CagA induction of tumor
suppressor gene hypermethylation via AKT-NFκB pathway in gastric
cancer development, and is likely to mediate these effects
(19–21).
The aforementioned considerations prompted the
present study to investigate the interaction between H.
pylori-derived CagA and MUC5AC expression in the gastric
epithelium. It has already been established that upon CagA
injection into the gastric epithelial cytosol by the bacteria, a
number of cellular responses facilitating bacterial propagation and
pathological responses are initiated (22–24).
Therefore, CagA expression may affect MUC5A expression. It has been
well established that CagA is injected directly from the bacteria
into the gastric epithelial cytosol through its needle-like
structure (23). In the present
study, to isolate the effects of CagA from other H.
pylori-induced molecules, in particular the urease virulence
factor, a CagA expression construct was created that allows the
cell-autonomous introduction of the bacterial protein into gastric
epithelial cells. The results of the present study indicated that
CagA is able to drive MUC5AC expression in gastric epithelial
cells. Therefore, CagA insertion into host cells by H.
pylori may constitute a bacterial defense mechanism against
host downregulation of MUC5AC.
Materials and methods
Cell lines and cell culture
The AGS gastric adenocarcinoma cell line was
purchased from the Shanghai Cell Bank (Shanghai, China). AGS cells
were cultured in Ham's F-12 medium supplemented with 10% fetal
bovine serum and were maintained at 37°C in an atmosphere
containing 5% CO2 (all Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) (25).
Transfection of the CagA gene
Control group (CK), empty-plasmid group (pCDNA3.1)
and overexpression CagA group (pCDNA3.1-CagA) were set up. The
vector used for expressing CagA protein was the pcDNA 3.1 plasmid
from Life Technologies (Thermo Fisher Scientific, Inc.). The
CagA-coding insert was established by total gene synthesis. For
transfection, 4 µg CagA plasmid and 6 µl Lipofectamine®
2000 (Invitrogen; Thermo Fisher Scientific, Inc.) were added to 200
µl F12 medium. Following mixing and incubated at room temperature
for 20 min, the mixture was added to AGS cells that had been plated
and had reached 75% cell confluence.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) for CagA, GAPDH and MUC5AC
expression in the AGS model of the gastric epithelium
The AGS cells were harvested and RNA was extracted
with RNaEXTM Total RNA Isolation Solution (Generay Biotech Co.,
Ltd., Shanghai, China). Total RNA (1 µg) was mixed with 2 µl
reagent 5X Prime Script RT Master mix (Vazyme Biotech Co., Ltd.,
Nanjing, China) and RNase Free dH2O up to 10 µl, prior
to being reverse transcribed in a one-step process at 50°C for 15
min and the introduction of inactivated enzymes at 85°C for 5 sec,
each for once cycle according to the manufacturer's protocols. The
cDNA product is finally stored at −80°C. The mRNA levels of MUC5AC
of all groups were assessed using qPCR. The forward and reverse
primer sequences used for determining H. pylori-CagA
expression were 5′-ATTCACAATAACGCTCTG-3′ and
5′-ACCACCTGCTATGACTAA-3′, respectively. The forward and reverse
primer sequences for establishing Homo GAPDH (Gene ID, 2597;
product size, 258 bp) expression were 5′-AGAAGGCTGGGGCTCATTTG-3′
and 5′-AGGGGCCATCCACAGTCTTC-3′, respectively. The forward and
reverse primer sequences for determining Homo MUC5AC (Gene ID,
4586; product size, 281 bp) expression were
5′-ACGGGAAGCAATACACGG-3′ and 5′-GGTCTGGGCGATGATGAA-3′,
respectively. For the qPCR amplification reaction, 10 µl IQ
SYBR-Green Supermix (Tiangen Biotech Co., Ltd., Beijing, China) was
used in conjunction with 1 µl cDNA product, 1 µl forward primer (10
µM), 1 µl reverse primer (10 µM) and 8 µl water. The thermocycling
conditions used were as follows: 50.0°C for 3 min, 95.0°C for 15
min, followed by 40 cycled of 95.0°C for 10 sec, 59°C for 25 sec
and 72°C for 25 sec. Quantification was performed using the
2−∆∆Cq method (26).
Verification of CagA expression at the
protein level
The protein expression of the CagA transgene in AGS
cells was validated using western blot analysis. Total protein was
extracted from non-transfected AGS cells, vector
control-transfected AGS cells and AGS cells transfected with
CagA-encoding plasmid. The cells were lysed by protein extraction
buffer (Beyotime Institute of Biotechnology, Haimen, China) and the
protein concentration was determined by the BCA method. The CagA
levels were assessed by adding 30 µg of protein per lane,
electrophoresis using a 10% polyacrylamide gel, followed by
transfer to a polyvinylidene difluoride (PVDF) membrane. The PVDF
membrane was subsequently blocked with a solution containing 5%
skimmed milk powder and 5% bovine serum albumin (BSA (Shanghai
Biyuntian Bio-Technology Co., Ltd.) for 1 h at room temperature,
followed by washing and the addition of the CagA antibody (catalog
no. sc-25766; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) at
4°C overnight. Following washing three times with Tris-buffered
saline containing Tween-20 for 15 min, the PVDF membrane was
exposed to the goat anti-rabbit IgG (H+L) Highly Cross-Adsorbed
Secondary Antibody (catalog no. A16113; Invitrogen; Thermo Fisher
Scientific, Inc.) and incubated for 1 h at room temperature,
followed by repeated washing. Subsequently, the membrane was
visualized using BeyoECL Plus (Shanghai Biyuntian Bio-Technology
Co., Ltd.), according to the manufacturer's protocol and routine
procedures using a gel imaging analyzer (Chemidoc XRS+; Bio-Rad
Laboratories, Inc., Hercules, CA, USA).
Immunofluorescent analysis of MUC5AC
expression
The protein levels of MUC5AC of all groups were
assessed using fluorescence. Cells (3×104 per well)
exposed to the appropriate transfections and conditions were grown
for 24 h in 24-well plates under routine conditions, washed three
times with PBS and fixed with a 4% paraformaldehyde solution for 15
min at 37°C. Subsequently, the samples were washed with PBS twice
for 5 min and permeabilized using a 10-min incubation with 0.1%
Triton X-100 in PBS. Following two washes for 5 min with PBS,
non-specific immunoreactivity was blocked via a 90-min incubation
with 1% BSA in 0.3 M glycine at 37°C (plates were sealed).
Subsequently, MUC5AC antibody (catalog no. sc-398985, dilution
1:100; Santa Cruz Biotechnology, Inc.) was added and the samples
were incubated overnight at 4°C. The following day, the
fluorescence-labeled (Alexa Fluor® 555) Alexa
Fluor® 555 goat anti-mouse IgG (H+L) antibody (catalog
no. A21424; dilution 1:800; Invitrogen; Thermo Fisher Scientific,
Inc.) was added, and the experimental samples were incubated under
humidified conditions for 1 h at 37°C. For staining of nuclei,
4′,6-diamidino-2-phenylindole was added, and the samples were
incubated for 10 min. Subsequently, the samples were washed three
times with PBS for 5 min and mounted on glass slides and analyzed
using magnification, ×200 fluorescence microscopy (IX73; Olympus
Corporation, Tokyo, Japan), to assess the subcellular distribution
of MUCAC immunoreactivity.
Statistical analysis
The RT-qPCR results of CagA and MUC5AC expression in
AGS cells were statistically analyzed and calculated using the CFX
Manager PCR analysis software (version 3.0; Bio-Rad Laboratories,
Inc.). Following densitometric analysis using Image J software
(National Institutes of Health, Bethesda, MD, USA), the CagA
protein levels, determined using western blot analysis, were
established. The data were subsequently analyzed using the
Student's t-test or by one-way analysis of variance test for
selected data pairs, following by the Tukey post-hoc test.
P<0.05 was considered to indicate a statistically significant
difference. The experiments were repeated 3 times.
Results
Exogenous expression of CagA in an
experimental model of gastric epithelium
To investigate the effects of exogenous CagA
expression in the gastric epithelium, AGS cells were transduced
with a CagA-expressing plasmid, and the results were compared with
cells transduced with an empty vector or non-transduced cells. This
strategy successfully established heterologous CagA expression at
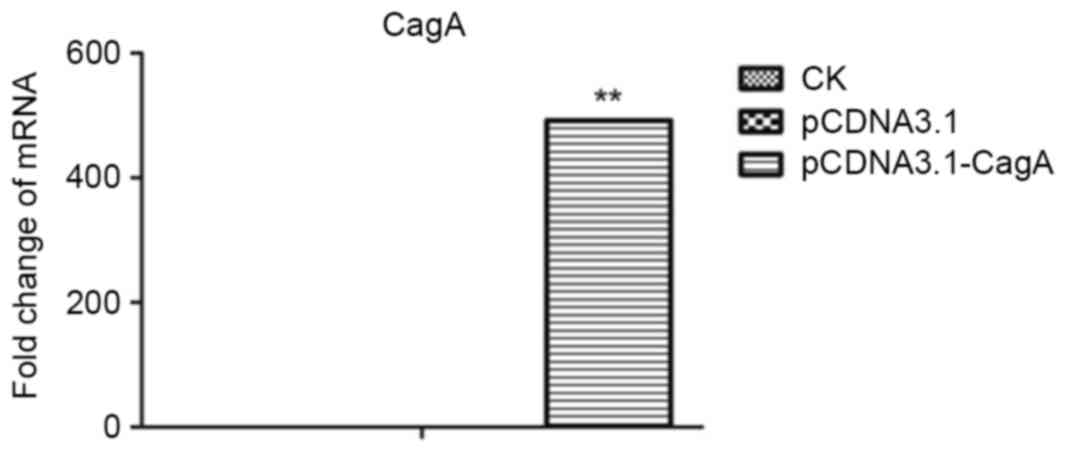
the RNA level. The level of CagA expression in all groups as
detected by RT-qPCR are shown in Table
I and Fig. 1. The level of CagA
expression in the pCDNA3.1-CagA group was significantly increased
compared with the pCDNA3.1 group and the untreated AGS cells group
(P<0.01, P<0.01; Fig. 1). In
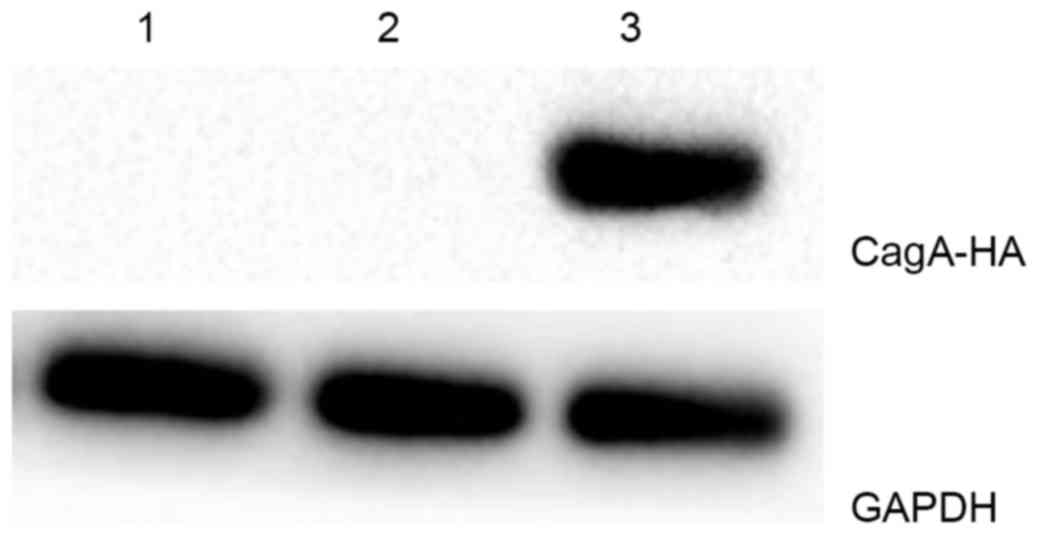
addition, at the protein level, the induction of exogenous CagA
expression was successful. Western blots of all experimental groups
are shown in Fig. 2, and CagA protein
was only detected in the pCDNA3.1-CagA group. Therefore, this
experimental set-up enabled the present study to analyze the effect
of exogenous CagA expression on the levels of MUC5AC in AGS
cells.
 | Table I.CagA mRNA levels in CK, pCDNA3.1 and
pCDNA3.1-CagA groups. |
Table I.
CagA mRNA levels in CK, pCDNA3.1 and
pCDNA3.1-CagA groups.
| Group | mRNA expression
level (mean ± standard deviation; n=3) |
|---|
| CK | 0.00±0.00 |
| pCDNA3.1 | 0.00±0.00 |
| pCDNA3.1-CagA | 491.11±0.41 |
CagA expression upregulates
MUC5AC
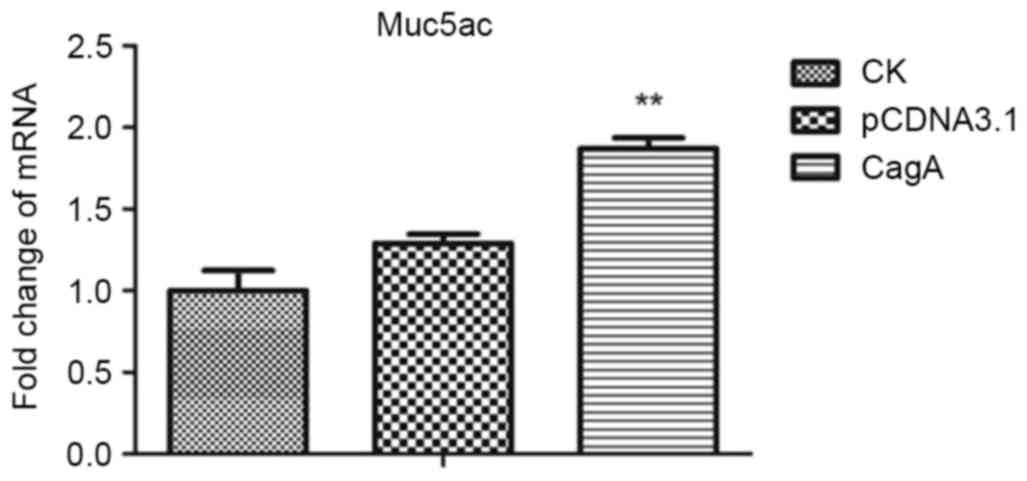
AGS cultures transduced with CagA were analyzed for
MUC5AC expression using RT-qPCR and compared with the appropriate
controls. As evident from the results presented in Table II and Fig.
3, heterologous CagA expression increased MUC5AC expression at
the RNA level, compared with the control group. The level of MUC5AC
expression in the pCDNA3.1-CagA group was significantly increased
compared with that of the pCDNA3.1 and CK groups (P<0.01,
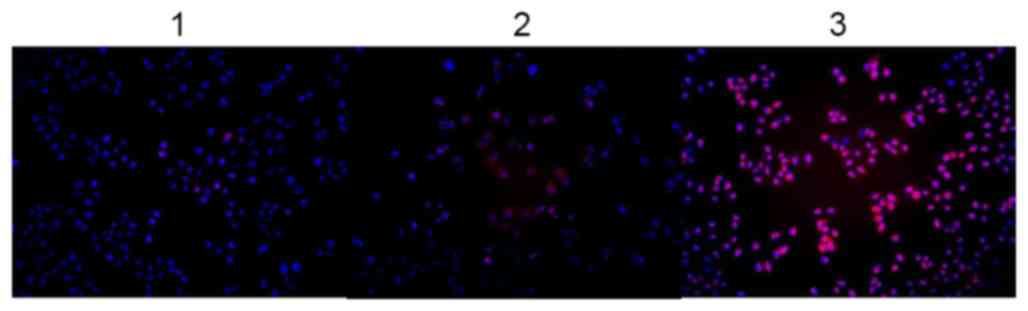
P<0.01, respectively). As presented in Fig. 4, this result was validated using
immunofluorescence assays, demonstrating substantial upregulation
of MUC5AC, following the transfection of AGS cells with the CagA
expression plasmid, compared with the control. Therefore, the
results of the present study demonstrated that CagA was able to
increase MUC5AC expression.
 | Table II.MUC5AC mRNA levels in CK, pCDNA3.1
and pCDNA3.1-CagA groups. |
Table II.
MUC5AC mRNA levels in CK, pCDNA3.1
and pCDNA3.1-CagA groups.
| Group | mRNA expression
level (mean ± standard deviation; n=3) |
|---|
| CK | 1.00±0.12 |
| pCDNA3.1 | 1.29±0.06 |
| pCDNA3.1-CagA | 1.87±0.07 |
Discussion
At present, it is typically accepted that the
gastric epithelium reacts to H. pylori infection by
downregulating MUC5AC (2,6,11–15). This may be a protective response
towards H. pylori, which enables the removal of this
organism from its ecological niche. However, the precise
interaction between H. pylori and MUC5AC remains unclear.
The gastric epithelial downregulation of MUC5AC may involve
bacterial urease, as evident from experiments involving AGS gastric
cancer cells that were infected with the UreB-isogenic mutant of
H. pylori (17). It is
speculated that evolutionary pressure has favored the emergence of
H. pylori variants that may counteract this effect. In the
present study, it was demonstrated that the CagA virulence factor
may exhibit a function in this process. The results of the present
study revealed that the expression of MUC5AC, determined using
RT-qPCR and immunofluorescence assays, was significantly
upregulated upon the introduction of cellular CagA. Therefore, the
injection of CagA into the gastric epithelial cytosol by H.
pylori represents a countermeasure of H. pylori against
gastric epithelial defensive downregulation of the MUC5AC
glycoprotein in response to bacterial infection.
MUC5AC has been demonstrated to co-localize with
H. pylori and functions as an important H. pylori
receptor, which tethers H. pylori to the gastric mucosa
(5,6).
Therefore, it may be hypothesized that H. pylori aims at
creating a favorable environment by stimulating gastric epithelial
MUC5AC production, which facilitates bacterial adherence. In this
sense, injection of CagA into gastric epithelial cells may
stimulate these cells to produce MUC5AC early in the infection
process, causing a transient increase in MUC5AC expression that
facilitates additional colonization (27). A subsequent interaction between the
bacterium and gastric cells leads to morphological alterations in
the cell and the injury of host-cell epithelial barrier that is
associated with H. pylori infection (28). MUC5AC is only produced by the stomach
surface epithelium (29), which is
consistent with this cell type being the important target for H.
pylori during the infection process (30). These findings indicated that CagA has
a role as a MUC5AC-targeting bacterial effector. The molecular
mechanisms underlying how the virulence factor may result in
transactivation of the MUC5AC promotor remain unknown. However,
mucin production is typically a protective response against
bacterial infection, and therefore, it may be exploited by the CagA
protein (31–33). MUC5AC, as a principal component of the
mucosal layer, protects the stomach surface from chemical,
enzymatic, mechanical and microbial challenge, and its upregulation
constitutes an intuitive response of the epithelium to infection
(1,2,7,11,29,34–36).
Additional studies are required to delineate the molecular
mechanisms, which mediate the effects of CagA on MUC5AC
transcription. However, the results of the present study have
revealed that CagA is sufficient for cell-autonomous upregulation
of MUC5AC and have therefore, to the best of our knowledge,
revealed a novel mechanism employed by this bacterium for its
colonization of the stomach.
Glossary
Abbreviations
Abbreviations:
|
PVDF
|
polyvinylidene difluoride
|
|
CK
|
control group
|
|
pCDNA3.1-CagA
|
CagA overexpression group
|
|
pCDNA3.1
|
empty plasmid group
|
References
|
1
|
Ho SB, Takamura K, Anway R, Shekels LL,
Toribara NW and Ota H: The adherent gastric mucous layer is
composed of alternating layers of MUC5AC and MUC6 mucin proteins.
Dig Dis Sci. 49:1598–1606. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Byrd JC, Yunker CK, Xu QS, Sternberg LR
and Bresalier RS: Inhibition of gastric mucin synthesis by
Helicobacter pylori. Gastroenterology. 118:1072–1079. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hunt RH, Camilleri M, Crowe SE, El-Omar
EM, Fox JG, Kuipers EJ, Malfertheiner P, McColl KE, Pritchard DM,
Rugge M, et al: The stomach in health and disease. Gut.
64:1650–1668. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Allen A, Newton J, Oliver L, Jordan N,
Strugala V, Pearson JP and Dettmar PW: Mucus and H. pylori. J
Physiol Pharmacol. 48:297–305. 1997.PubMed/NCBI
|
|
5
|
van den Brink GR, Hardwick JC, Tytgat GN,
Brink MA, Ten Kate FJ, Van Deventer SJ and Peppelenbosch MP: Sonic
hedgehog regulates gastric gland morphogenesis in man and mouse.
Gastroenterology. 121:317–328. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Van de Bovenkamp JH, Mahdavi J,
Korteland-Van Male AM, Büller HA, Einerhand AW, Borén T and Dekker
J: The MUC5AC glycoprotein is the primary receptor for Helicobacter
pylori in the human stomach. Helicobacter. 8:521–532. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kocer B, Ulas M, Ustundag Y, Erdogan S,
Karabeyoglu M, Yldrm O, Unal B, Cengiz O and Soran A: A
confirmatory report for the close interaction of Helicobacter
pylori with gastric epithelial MUC5AC expression. J Clin
Gastroenterol. 38:496–502. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lindén SK, Wickström C, Lindell G,
Gilshenan K and Carlstedt I: Four modes of adhesion are used during
Helicobacter pylori binding to human mucins in the oral and gastric
niches. Helicobacter. 13:81–93. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Magalhães A and Reis CA: Helicobacter
pylori adhesion to gastric epithelial cells is mediated by glycan
receptors. Braz J Med Biol Res. 43:611–618. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
van Den Brink GR, ten Kate FJ, Ponsioen
CY, Rive MM, Tytgat GN, van Deventer SJ and Peppelenbosch MP:
Expression and activation of NF-kappa B in the antrum of the human
stomach. J Immunol. 164:3353–3359. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kang HM, Kim N, Park YS, Hwang JH, Kim JW,
Jeong SH, Lee DH, Lee HS, Jung HC and Song IS: Effects of
Helicobacter pylori Infection on gastric mucin expression. J Clin
Gastroenterol. 42:29–35. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shi D, Qiu XM and Bao YF: Effects of
Helicobacter pylori infection on MUC5AC protein expression in
gastric cancer. Future Oncol. 9:115–120. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shi D, Qiu XM and Yan XJ: The changes in
MUC5AC expression in gastric cancer before and after Helicobacter
pylori eradication. Clin Res Hepatol Gastroenterol. 38:235–240.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang RQ and Fang DC: Effects of
Helicobacter pylori infection on mucin expression in gastric
carcinoma and pericancerous tissues. J Gastroenterol Hepatol.
21:425–431. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Matsuda K, Yamauchi K, Matsumoto T, Sano
K, Yamaoka Y and Ota H: Quantitative analysis of the effect of
Helicobacter pylori on the expressions of SOX2, CDX2, MUC2, MUC5AC,
MUC6, TFF1, TFF2 and TFF3 mRNAs in human gastric carcinoma cells.
Scand J Gastroenterol. 43:25–33. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Morgenstern S, Koren R, Moss SF, Fraser G,
Okon E and Niv Y: Does Helicobacter pylori affect gastric mucin
expression? Relationship between gastric antral mucin expression
and H. pylori colonization. Eur J Gastroenterol Hepatol. 13:19–23.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Perrais M, Rousseaux C, Ducourouble MP,
Courcol R, Vincent P, Jonckheere N and Van Seuningen I:
Helicobacter pylori urease and flagellin alter mucin gene
expression in human gastric cancer cells. Gastric Cancer.
17:235–246. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
McLean MH and El-Omar EM: Genetics of
gastric cancer. Nat Rev Gastroenterol Hepatol. 11:664–674. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang BG, Hu L, Zang MD, Wang HX, Zhao W,
Li JF, Su LP, Shao Z, Zhao X, Zhu ZG, et al: Helicobacter pylori
CagA induces tumor suppressor gene hypermethylation by upregulating
DNMT1 via AKT-NFκB pathway in gastric cancer development.
Oncotarget. 7:9788–9800. 2016.PubMed/NCBI
|
|
20
|
Figura N, Marano L, Moretti E and Ponzetto
A: Helicobacter pylori infection and gastric carcinoma: Not all the
strains and patients are alike. World J Gastrointest Oncol.
8:40–54. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tohidpour A: CagA-mediated pathogenesis of
Helicobacter pylori. Microb Pathog. 93:44–55. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Handa O, Naito Y and Yoshikawa T: CagA
protein of Helicobacter pylori: A hijacker of gastric epithelial
cell signaling. Biochem Pharmacol. 73:1697–1702. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tanaka H, Yoshida M and Azuma T: The role
of CagA in H. pylori infection. Nihon Rinsho. 67:2245–2249.
2009.(In Japanese).
|
|
24
|
Stein M, Ruggiero P, Rappuoli R and
Bagnoli F: Helicobacter pylori CagA: From pathogenic mechanisms to
its use as an anti-cancer vaccine. Front Immunol. 4:3282013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Perrais M, Pigny P, Buisine MP, Porchet N,
Aubert JP and Van Seuningen-Lempire I: Aberrant expression of human
mucin gene MUC5B in gastric carcinoma and cancer cells.
Identification and regulation of a distal promoter. J Biol Chem.
276:15386–15396. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hayashi T, Senda M, Morohashi H, Higashi
H, Horio M, Kashiba Y, Nagase L, Sasaya D, Shimizu T, Venugopalan
N, et al: Tertiary structure-function analysis reveals the
pathogenic signaling potentiation mechanism of Helicobacter pylori
oncogenic effector CagA. Cell Host Microbe. 12:20–33. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wu J, Xu S and Zhu Y: Helicobacter pylori
CagA: A critical destroyer of the gastric epithelial barrier. Dig
Dis Sci. 58:1830–1837. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nordman H, Davies JR, Lindell G, de Bolós
C, Real F and Carlstedt I: Gastric MUC5AC and MUC6 are large
oligomeric mucins that differ in size, glycosylation and tissue
distribution. Biochem J. 364:191–200. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hatakeyama M: Oncogenic mechanisms of the
Helicobacter pylori CagA protein. Nat Rev Cancer. 4:688–694. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fukuda M, Kawakubo M, Ito Y, Kobayashi M,
Lee H and Nakayama J: Assay of human gastric mucin as a natural
antibiotic against Helicobacter pylori. Methods Enzymol.
415:164–179. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kawakubo M, Ito Y, Okimura Y, Kobayashi M,
Sakura K, Kasama S, Fukuda MN, Fukuda M and Katsuyama T: Natural
antibiotic function of a human gastric mucin against Helicobacter
pylori infection. Science. 305:1003–1006. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kobayashi M, Lee H, Nakayama J and Fukuda
M: Roles of gastric mucin-type O-glycans in the pathogenesis of
Helicobacter pylori infection. Glycobiology. 19:453–461. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ota H, Nakayama J, Shimizu T, Nakayama J,
Graham DY and Katsuyama T: Relation of H pyroli to gastric mucins
and gastric surface mucous gel layer. Gut. 48:869–871. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tanaka S, Mizuno M, Maga T, Yoshinaga F,
Tomoda J, Nasu J, Okada H, Yokota K, Oguma K, Shiratori Y and Tsuji
T: H. pylori decreases gastric mucin synthesis via inhibition of
galactosyltransferase. Hepatogastroenterology. 50:1739–1742.
2003.PubMed/NCBI
|
|
36
|
Jia Y, Persson C, Hou L, Zheng Z, Yeager
M, Lissowska J, Chanock SJ, Chow WH and Ye W: A comprehensive
analysis of common genetic variation in MUC1, MUC5AC, MUC6 genes
and risk of stomach cancer. Cancer Causes Control. 21:313–321.
2010. View Article : Google Scholar : PubMed/NCBI
|