Introduction
Approximately 640,000 patients with oral squamous
cell carcinoma (OSCC) are diagnosed each year worldwide, with a
rising incidence in many countries (1). Despite evolution in its management, the
overall survival (OS) rate has not improved significantly during
the past 20 years, with 5-year survival rates between 45 and 50%
(2). Pathological lymph node (LN)
metastases (pN+) are recognized as an adverse prognostic
factor in OSCC (3). LN-associated
factors, such as the number of positive nodes, the presence of
extracapsular spread (ECS), the number of nodes with ECS, the
presence of contralateral neck metastases (pN2c), the presence of
lower neck metastases (level IV/V), the number of dissected LNs and
the LN density may influence survival rates (4–6). In this
context, the number and location of all retrieved LNs from the neck
dissection (ND) specimen is of critical importance to the diagnosis
of the number, ratio, and location of positive LNs.
At present, to retrieve LNs from an ND sample,
surgeons can only rely on their sense of touch or the color
difference between superficial LNs and the surrounding tissue.
However, such techniques can easily overlook small and/or deep LNs.
Another problem is that once the specimen is removed from the
patient, it is hard to determine the location of the LNs,
especially when resected by less experienced surgeons.
Here we introduce a straightforward device, named
optical lymph nodes detection system (OLNDS), to improve LNs
location accuracy detection rate. Our experience had demonstrated
that it could be useful in identifying and locating LNs, especially
for less experienced surgeons.
Materials and methods
Patients
An ND specimen from each of 63 patients (Group 1)
who had primary surgery for OSCC with ND (radical or selective) in
the Department of Oral and Maxillofacial Surgery, Nanjing
Stomatological Hospital, Medical School of Nanjing University
between November 2014 and May 2015 were evaluated with the OLNDS in
addition to the traditional method. LNs were isolated from the ND
specimen by a qualified resident doctor or a qualified attending
doctor. The data of another 70 patients (Group 2) were retrieved
from our database. These patients went through primary surgery for
OSCC with ND (radical or selective) in the same department between
January 2011 and December 2012. Their ND specimens were subjected
only to the traditional LN location method by the same attending
doctor who participated in the study. Ethical approval had
previously been obtained from the Nanjing Stomatological Hospital
Research Ethics Committee. Written informed consents were obtained
from participants before delivery.
Patient information is summarized in Table I. In brief, among patients in Group 1,
37 (57.8%) were male and 26 (42.2%) female. Patient ages ranged
between 29 and 89 years (mean ± SEM, 59.52±1.418). In Group 2, 30
(42.6%) were male and 40 (57.4%) were female. Patient ages ranged
between 35 and 81 years (mean ± SEM, 59.06±0.349).
 | Table I.Clinical pathologic characteristics of
patients with oral squamous cell carcinoma. |
Table I.
Clinical pathologic characteristics of
patients with oral squamous cell carcinoma.
|
| Status of the lymph
nodes |
|---|
|
|
|
|---|
| Clinical
variable | Group 1 | Group 2 |
|---|
| Total | 63 | 70 |
| Sex |
|
|
| Male | 37 | 40 |
|
Female | 26 | 30 |
| Age (years) |
|
|
|
<60 | 27 | 33 |
| ≥60 | 36 | 37 |
| Type of neck
dissection |
|
|
|
Radical | 45 | 56 |
|
Selected | 18 | 14 |
| Positive lymph
nodes |
|
|
| Yes | 19 | 25 |
| No | 44 | 45 |
| TNM stage |
|
|
| Early
stage | 36 | 24 |
| Late
stage | 27 | 46 |
| Histopathology
grading |
|
|
| I | 23 | 33 |
|
II–III | 40 | 34 |
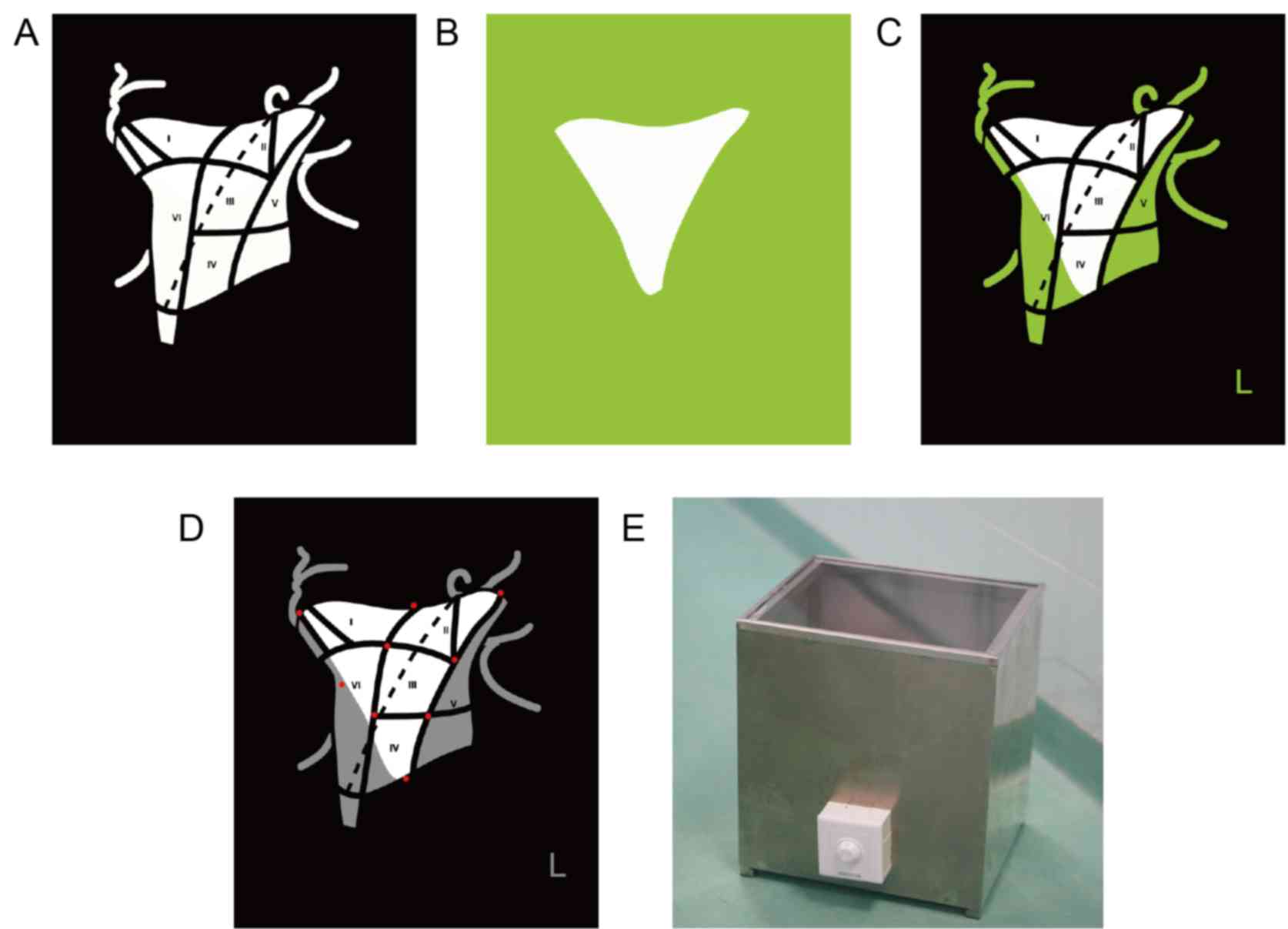
The OLNDS consists of two parts: the LN localization
board and the LN detection light box.
The LN localization board
The LN localization board is a transparent acrylic
board printed with black neck division guidance. The white area in
Fig. 1A is transparent, while the
black is printed as black to review neck division and block out
extraneous light. It can be used together with a light blockage
board (Fig. 1B) to suit different ND
tissue requirements. The board can also be printed as in Fig. 1C, using a different color to block
extraneous light. Magnets are inlayed into the board (as indicated
by red spots in Fig. 1D) to stretch
(make it thinner) and to fix the ND specimen. The LN localization
board can be sterilized and be used during the operation, so that
the chief surgeon can put the ND specimen onto the board directly
to ensure the accuracy of its location.
The LN detection light box
A lamp is installed on the base of the LN detection
light box and a piece of highly transparent glass is used to place
the positioning plate. A switch in front of the box allows for the
brightness of the lamp to be adjusted (Fig. 1E). During the operation, the chief
surgeon placed the ND specimen directly onto the board with
accurately located specimen. The ND specimen was then searched for
LNs without the assistance of OLNDS. When no more LNs could be
found with the traditional method, the specimen was searched for
LNs under the guidance of OLNDS. The number of LNs from each region
by different methods was recorded. The diameter of the smallest LN
found by the two different methods was measured with vernier
calipers.
Statistical analysis
The paired Student's t-test was used to
compare the difference between the number and smallest diameter of
the LNs found by different methods. The level of significance was
set at 5% (P<0.05). Statistics were analyzed with Prism 5.0.
Results
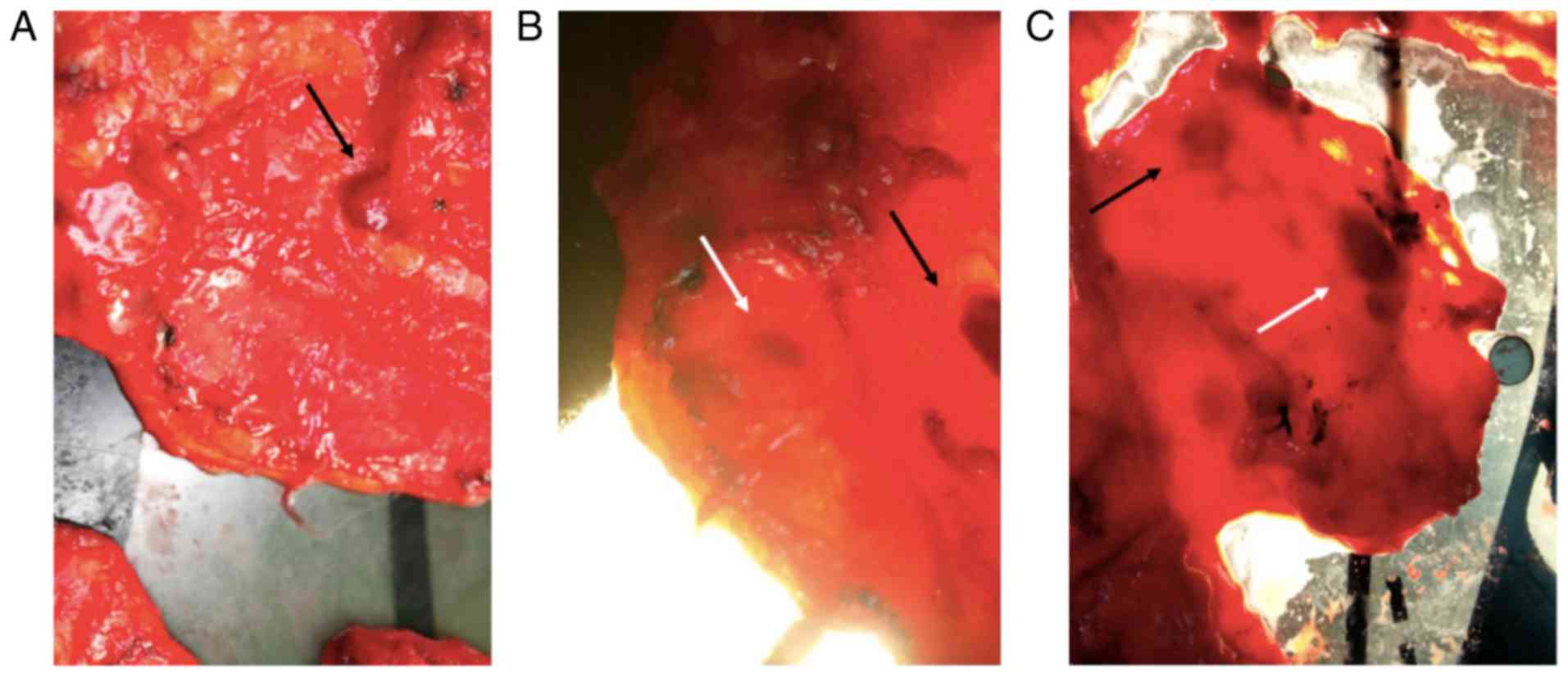
OLNDS helped to reveal LNs
When the light was off, only superficial LNs were
seen (black arrow indicating superficial LN; Fig. 2A). When the light was turned on, even
deeper LNs were clearly revealed as round dark spots (white arrow;
Fig. 2B), while superficial LNs were
clearer than when the light was off (black arrow; Fig. 2B). To distinguish LNs (white arrow;
Fig. 2C) from thick tissue (black
arrow; Fig. 2C), LNs appeared to be
round and uniform, while the thick tissue had a rough outline and
was not evenly dark inside. Moreover, the dark spot of thick tissue
would disappear when squeezed or stretched, while the LNs would
not.
As the light was strong, directly viewing it could
obscure the tissue; therefore, unnecessary light was blocked,
letting only necessary light pass the tissue. This was accomplished
with a light-blockage board (Fig. 1B)
or a light-blockage printed area (Fig.
1C). Both the light-blockage board and the light-blockage area
can be shaped to fit different ND specimens. The OLNDS was found to
be a powerful tool for locating and identifying LNs, especially for
less experienced resident doctors.
As the LN localization board can be used during the
operation and as the surgeon can place the ND specimen immediately
after dissection, the location of the tissue can be highly
accurate. In this context, even a resident doctor can locate the
LNs accurately.
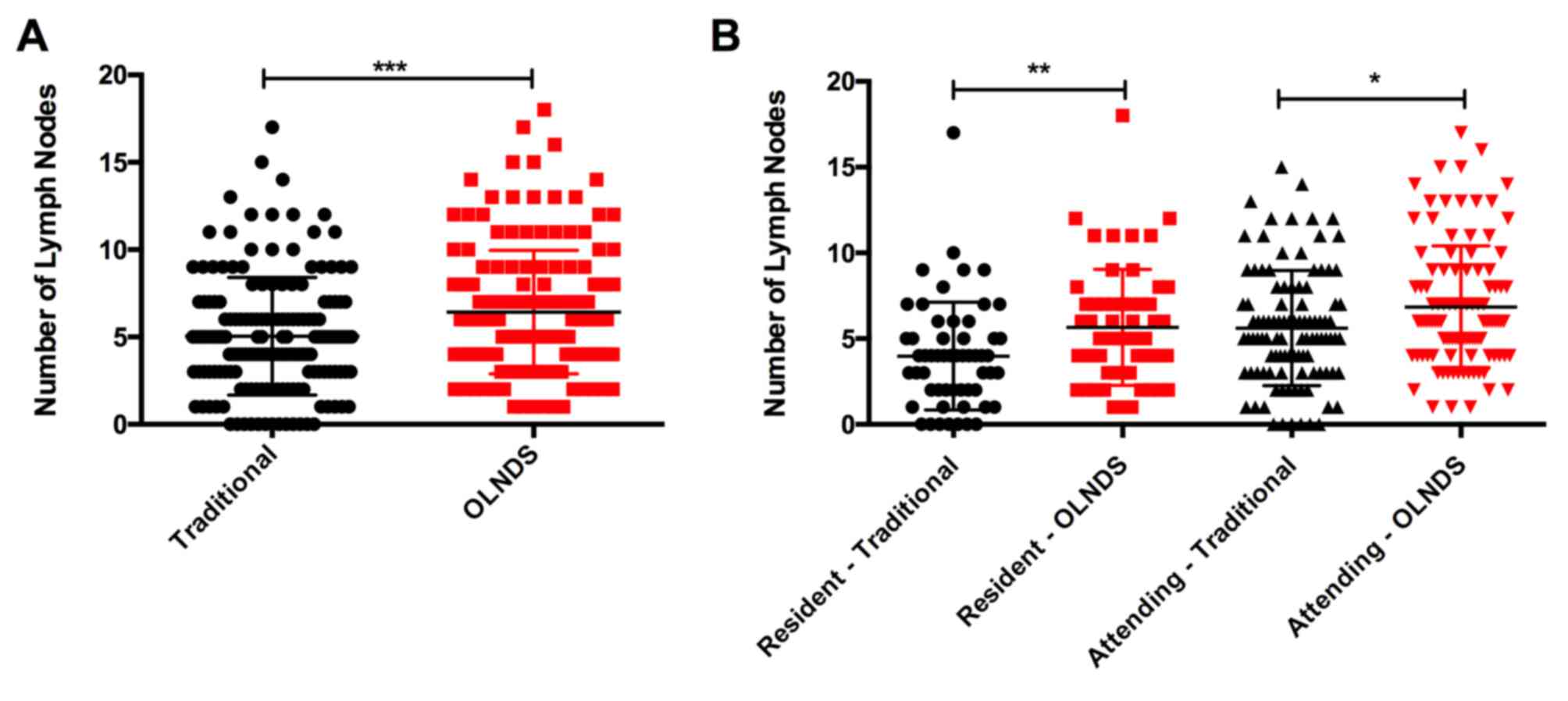
In addition to accurate location, in Group 1, OLNDS
also helped identify more LNs than the traditional method
(P=0.0006; Fig. 3). The statistical
significance was higher for the resident doctor (P=0.0042) than the
attending doctor (P=0.0206). Again, compared with data retrieved
(Group 2), OLNDS found more LNs (mean, 6.01) than with the
traditional method only (mean, 2.29) in each region.
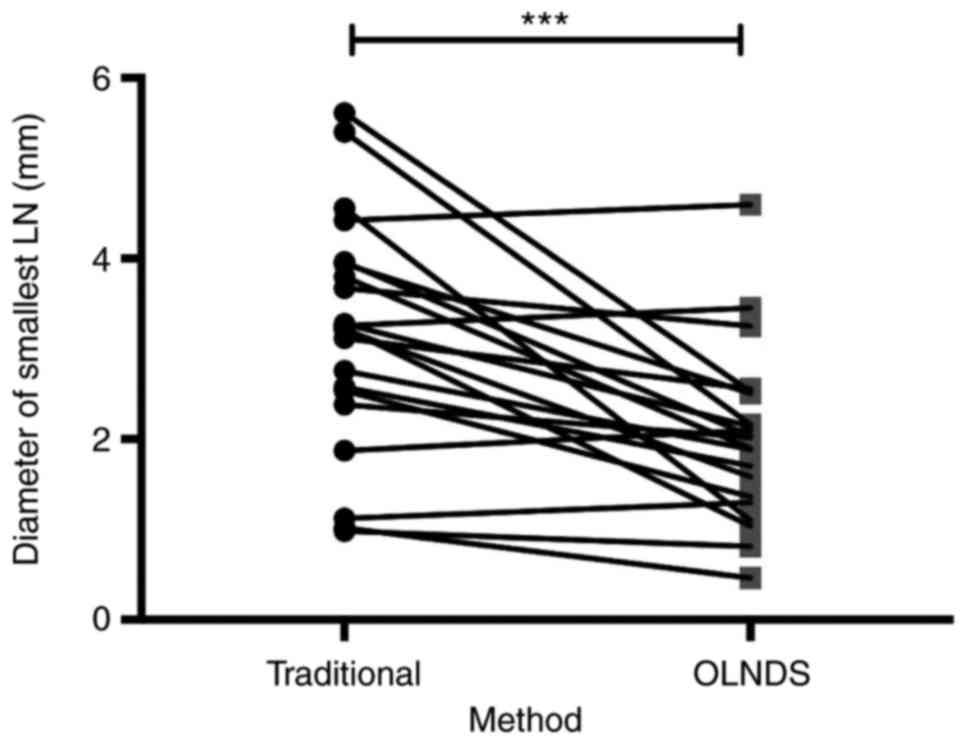
OLNDS could detect smaller LNs compared with the
conventional method. For 21 patients in Group 1, the diameter of
the smallest LNs found by different method (traditional or with
OLNDS) were measured with Vernier calipers. With the traditional
method, the average diameter of the smallest LNs detected was 3.18
mm (SEM, 0.28). With OLNDS, the average diameter of the smallest
LNs detected was 2.02 mm (SEM, 0.21). The difference between these
two groups was significant (P=0.0001; Fig. 4), indicating that smaller LNs, which
could otherwise not be discovered by the traditional method, could
be found with OLNDS.
OLNDS reduced false detection
rate
The LNs retrieved from the ND specimens of 64
patients in Group 1 were sent for pathological analysis to the
Department of Oral Pathology, Nanjing Stomatological Hospital,
Medical School of Nanjing University. Among the 1,471 LNs found, 5
were not LNs (false detection ratio, 0.0034). Comparably, among 70
patients in Group 2, 943 LNs were isolated from ND specimens by the
same attending doctor who participated in this experiment, among
which 28 were not LNs (false detection ratio, 0.0296).
Discussion
The presence of LN metastasis has long been one of
the most important prognostic factors for the survival of patients
with OSCC (7). LN associated factors,
including the number (or density) of LNs (8–10), the
number (or ratio) of positive LNs and LNs with extracapsular
metastasis (11–14), and the location of positive LNs or LNs
with extracapsular metastasis (6,13), were
reported to be related to the prognosis of OSCC patients and could
guide adjuvant therapy. According to the 8th edition of the TNM
staging system, the number of positive LNs has become a crucial
factor in determining the N category (15).
However, an accurate location and thorough
separation of the LNs is essential. Unfortunately, once an ND
specimen is removed from the patient, with the loss of anatomic
references, it is difficult to divide the specimen accurately
according to the neck division standard (16), especially for inexperienced doctors.
Moreover, the traditional method of identifying LNs relied merely
on differences of touch and color between the LNs and connective
tissues. This method could lead to the incomplete separation of LNs
and also result in false detection by mistaking adipose tissue for
LNs.
As described earlier, the OLNDS was designed to
locate LDS more accurately than the traditional method, especially
for junior doctors. Further, magnets could be used to fix and
stretch the specimen, thus making the tissue thinner for the light
to come through. Light blockage board or LN localization board
printed with light blockage area could block out unnecessary light.
As the light was quite strong, by using different light blockage
combination could ensure that light only came through the tissue,
thus making it much easier for the surgeons to look directly at the
tissue.
In our actual practice with OLNDS, the ND specimen
was first submitted to the traditional LN separation procedure.
When no more LNs could be found with the traditional procedure, the
light was turned on and the same surgeon searched for LNs again
with the help of OLNDS. In most of the patients, we found more LNs,
which would otherwise have been missed, with OLNDS. This effect was
more obvious for junior doctors, who often lack experience compared
with senior doctors.
Further, with OLNDS, surgeons could find smaller LNs
than with the traditional method. For small LNs hidden inside the
specimen, it is natural that surgeons could not see or feel them.
However, under the strong light of OLNDS, the small LNs revealed
themselves as little dark spots.
Another problem with LN separation was false
detection; in most cases, fat tissue was mistaken for LNs. With
OLNDS, the false detection rate was reduced. Some fat tissue can be
round like a LN, and some LNs can be light in color like fat
tissue. With OLNDS, the difference can be visualized as LNs
generally have a higher density. The major disadvantage of OLNDS is
that it is more time-consuming than the traditional method alone.
This is the challenge in applying the OLNDS.
The follow-up period of patients in group 1 was
between 5 and 29 months (average 23 months). During this time, no
recurrence was recorded. Seven patients died from OSCC, and 10 had
metastasis. Among the 63 patients in group 1, the 2-year OS rate
was 88.9%, the 2-year recurrence-free survival rate was 100%, and
the 2-year metastasis-free survival (MFS) rate was 84.1%. We
reviewed 680 OSCC patients whose ND specimen was searched for LNs
with the traditional method and found their 2-year OS rate was
90.9%, the 2-year recurrence-free survival rate was 89.5%, and the
2-year MFS rate was 90.0%. We attribute the seemingly different
2-year OS, RFS, and MFS rates to the comparatively small sample we
included in this study. As the OLNDS was designed to complement the
traditional method and was used only after no more LN could be
found with the traditional method, it would only improve the
separation of LNs. As we include more patients in the study and
expand the follow-up period, we believe we can better design
treatment for and improve the prognosis of OSCC patients.
In conclusion, even though OLNDS is never meant to
be an independent LN separation method, it can be a powerful
supplement to the traditional method and may significantly increase
the location accuracy separation rate of LNs. As LN metastasis is
an important prognosis-related factor not only of oral cancer but
also melanoma, breast cancer, and other solid tumors (17–19), OLNDS
can be used to deepen the study of LN metastasis of various
cancers. Its value should be further verified by linking the
accurate number and location of positive LNs with prognosis.
Acknowledgements
The optical lymph nodes detection system is
protected by patent. Patent nos. ZL201520506264.4 (the lymph node
localization board) and ZL201520506339.9 (the lymph node detection
light box). This study was supported partly by the National Key
Disciplines Constructional Project Funding, China, partly by
Nanjing Municipal Key Medical Laboratory Constructional Project
Funding (since 2012), and partly by the Center of Nanjing Clinical
Medicine Tumor Project (since 2014).
References
|
1
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bagan JV and Scully C: Recent advances in
oral oncology 2007: Epidemiology, aetiopathogenesis, diagnosis and
prognostication. Oral Oncol. 44:103–108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Woolgar JA, Triantafyllou A, Lewis JS Jr,
Hunt J, Williams MD, Takes RP, Thompson LD, Slootweg PJ, Devaney KO
and Ferlito A: Prognostic biological features in neck dissection
specimens. Eur Arch Otorhinolaryngol. 270:1581–1592. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liao CT, Hsueh C, Lee LY, Lin CY, Fan KH,
Wang HM, Huang SF, Chen IH, Kang CJ, Ng SH, et al: Neck dissection
field and lymph node density predict prognosis in patients with
oral cavity cancer and pathological node metastases treated with
adjuvant therapy. Oral Oncol. 48:329–336. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shingaki S, Takada M, Sasai K, Bibi R,
Kobayashi T, Nomura T and Saito C: Impact of lymph node metastasis
on the pattern of failure and survival in oral carcinomas. Am J
Surg. 185:278–284. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Woolgar JA: Detailed topography of
cervical lymph-note metastases from oral squamous cell carcinoma.
Int J Oral Maxillofac Surg. 26:3–9. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yuasa-Nakagawa K, Shibuya H, Yoshimura R,
Miura M, Watanabe H, Kishimoto S and Omura K: Cervical lymph node
metastasis from early-stage squamous cell carcinoma of the oral
tongue. Acta Otolaryngol. 133:544–551. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Amar A, Chedid HM, Rapoport A, Cernea CR,
Dedivitis RA, Curioni OA and Brandão LG: Prognostic significance of
the number of lymph nodes in elective neck dissection for tongue
and mouth floor cancers. Braz J Otorhinolaryngol. 78:22–26. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Patel SG, Amit M, Yen TC, Liao CT,
Chaturvedi P, Agarwal JP, Kowalski LP, Ebrahimi A, Clark JR, Cernea
CR, et al: Lymph node density in oral cavity cancer: Results of the
international consortium for outcomes research. Br J Cancer.
109:2087–2095. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ampil FL, Caldito G and Ghali GE: Can the
lymph node ratio predict outcome in head and neck cancer with
single metastasis positive-node? Oral Oncol. 50:e18–e20. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liao CT, Wang HM, Chang JT, Ng SH, Hsueh
C, Lee LY, Lin CH, Chen IH, Huang SF and Yen TC: Analysis of risk
factors for distant metastases in squamous cell carcinoma of the
oral cavity. Cancer. 110:1501–1508. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shibuya Y, Hasegawa T, Akashi M, Shigeta
T, Minamikawa T and Komori T: Oral squamous cell carcinoma with
multiple neck metastases-cases with more than ten pathologically
positive lymph nodes in the unilateral side. J Oral Maxillofac
Surg. 71:793–797. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ebrahimi A, Gil Z, Amit M, Yen TC, Liao
CT, Chaturvedi P, Agarwal JP, Kowalski LP, Kohler HF, Kreppel M, et
al: The prognosis of N2b and N2c lymph node disease in oral
squamous cell carcinoma is determined by the number of metastatic
lymph nodes rather than laterality: Evidence to support a revision
of the American Joint Committee on Cancer staging system. Cancer.
120:1968–1974. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sayed SI, Sharma S, Rane P, Vaishampayan
S, Talole S, Chaturvedi P, Chaukar D, Deshmukh A, Agarwal JP and
D'Cruz AK: Can metastatic lymph node ratio (LNR) predict survival
in oral cavity cancer patients? J Surg Oncol. 108:256–263. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Huang SH and O'Sullivan B: Overview of the
8th edition TNM classification for head and neck cancer. Curr Treat
Options Oncol. 18:402017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Robbins KT, Clayman G, Levine PA, Medina
J, Sessions R, Shaha A, Som P and Wolf GT; American Head and Neck
Society, ; American Academy of Otolaryngology-Head and Neck
Surgery, : Neck dissection classification update: Revisions
proposed by the American Head and Neck Society and the American
Academy of Otolaryngology-Head and Neck Surgery. Arch Otolaryngol
Head Neck Surg. 128:751–758. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Leong SP and Tseng WW: Micrometastatic
cancer cells in lymph nodes, bone marrow, and blood: Clinical
significance and biologic implications. CA Cancer J Clin.
64:195–206. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Balch CM, Soong SJ, Gershenwald JE,
Thompson JF, Reintgen DS, Cascinelli N, Urist M, McMasters KM, Ross
MI, Kirkwood JM, et al: Prognostic factors analysis of 17,600
melanoma patients: Validation of the american joint committee on
cancer melanoma staging system. J Clin Oncol. 19:3622–3634. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Leong SP, Nakakura EK, Pollock R, Choti
MA, Morton DL, Henner WD, Lal A, Pillai R, Clark OH and Cady B:
Unique patterns of metastases in common and rare types of
malignancy. J Surg Oncol. 103:607–614. 2011. View Article : Google Scholar : PubMed/NCBI
|