Introduction
Esophageal cancer (EC) is one of the most aggressive
types of cancers. In China, EC is the fourth leading cause of
cancer-associated mortality (1). In
Western countries, Barrett's adenocarcinoma is the most common
histological type, whereas esophageal squamous cell carcinoma is
dominant in East Asia (2,3). Among the currently available treatments,
surgical resection is considered to be a potentially curative
option for early stage EC. However, owing to the delay of
diagnosis, the majority of patients will miss the optimal window
for radical surgery. For patients with locally advanced EC,
radiotherapy combined with chemotherapy has become an important
therapeutic strategy (4,5). However, the prognosis of recurrent or
metastatic EC remains poor, despite the development of novel
chemotherapies and targeted drugs. Recently the roles of immune
checkpoints and immunotherapies have been investigated in several
types of malignancies (6,7).
Programmed death-ligand 1 (PD-L1), is a 40-kDa
transmembrane protein, and plays a major role in suppressing the
immune system. The binding of PD-L1 to its receptor, PD-1, produces
inhibitory signals, leading to evasion of the tumor from host
monitoring and induction of therapeutic resistance (8,9). PD-L1 has
been reported to be highly expressed in various types of cancers
and associated with tumor prognosis, such as in liver cancer, head
and neck cancers, and lung cancer (10–14).
Furthermore, numerous studies have found that PD-L1 overexpression
could be considered a predictive biomarker for immune checkpoint
inhibitors (15,16). With regard to EC, although a number of
studies have found that PD-L1 is overexpressed (17,18), the
predictive ability of PD-L1 remains controversial.
Increasing evidence has suggested that PD-L1 serves
an anti-immune role through the regulation of inflammatory
cytokines or signaling pathways. For example, the interleukin
6/Janus kinase/signal transducer and activator of transcription 3
(IL-6/JAK/STAT3) signaling pathway have been reported to regulate
PD-L1 expression (19). The
neutrophil-lymphocyte ratio (NLR), platelet-lymphocyte ratio (PLR)
and lymphocyte-monocyte ratio (LMR), which are considered to
reflect a systemic inflammatory response, have been reported to be
associated with poor prognosis in various types of cancers
(20–22). However, there is no consensus
regarding the significance of these inflammatory parameters in the
prognosis of EC. Furthermore, to date, no study has explored the
association between PD-L1 and these systemic inflammatory markers
in EC.
Based on these observations, a retrospective
analysis of data was performed to assess the significance of PD-L1
expression for predicting survival outcomes in Asian patients
treated with definitive CRT, and the associations between PD-L1 and
inflammatory markers in EC were further investigated to provide
novel insights into the development of immunotherapies.
Materials and methods
Patient eligibility and tissue
specimens
This study was approved by the Department of
Radiation Oncology, The First Affiliated Hospital of China Medical
University (Shenyang, China), and patients treated between January
2009 and December 2012 were included. A waiver of individual
informed consent was granted. In this study, 104 patients were
enrolled based on the following selection criteria: i) Confirmed EC
by pathological diagnosis; ii) formalin-fixed paraffin-embedded
(FFPE) specimens from pathological biopsy available; iii) all
patients were treated with radical radiotherapy or definitive CRT
initially and did not receive any prior treatments; iv) neutrophil,
lymphocyte, platelet and monocyte counts could be obtained from
medical records within a week prior to treatment. Upon application
of these criteria, patients with inflammation and any malignancy
with the exception of EC were excluded. Tumor stages were
determined according to the seventh edition of American Joint
Committee on Cancer/Union for International Cancer Control
(AJCC/UICC) (23). In addition, 10
pairs of surgically resected cancer tissues were collected along
with adjacent non-cancerous tissues as controls. Patient clinical
and pathological information was also collected from medical
records. Due to the restrictions of the selected conditions and the
lack of necessary information, pretreatment complete blood profiles
were only available for 83 (79.8%) patients. Clinical
characteristics are shown in Table I.
Descriptive data are represented as means and standard deviations.
The follow-up was conducted every 3 months for the first 2 years,
then once every 6 months via personal interview or by telephone,
the deadline was July 2016.
 | Table I.General clinical characteristics of
the 104 patients. |
Table I.
General clinical characteristics of
the 104 patients.
| Variables | Value |
|---|
| Age (years) |
|
|
≤65 | 53 (51.0) |
|
>65 | 51 (49.0) |
| Sex |
|
|
Male | 88 (84.6) |
|
Female | 16 (15.4) |
| Pathological
type |
|
|
SCC | 99 (95.2) |
|
Others | 5 (4.8) |
| Location |
|
|
Upper | 29 (27.9) |
|
Middle | 52 (50.0) |
|
Lower | 23 (22.1) |
| Length (cm) |
|
| ≤5 | 46 (44.2) |
|
>5–7 | 26 (25.0) |
|
>7 | 32 (30.8) |
|
T-classification |
|
|
T1-2 | 14 (13.5) |
|
T3-4 | 90 (86.5) |
|
N-classification |
|
| N0 | 36 (34.6) |
| N1 | 63 (60.6) |
| N2 | 5 (4.8) |
| Clinic stage |
|
| I | 5 (4.8) |
| II | 21 (20.2) |
|
III | 78 (75.0) |
| Radiotherapy dose
(Gy) |
|
| 60 | 51 (49.0) |
| 66 | 53 (51.0) |
| Therapeutic
method |
|
|
Radiotherapy alone | 53 (51.0) |
|
Concurrent chemoradiation | 41 (39.4) |
|
Sequential chemoradiation | 10 (9.6) |
| Neutrophil count
(×109/ml) | 4.73±1.89 |
| Lymphocyte count
(×109/ml) | 2.00±0.71 |
| Platelet count
(×109/ml) | 244.58±78.27 |
| Monocyte count
(×109/ml) | 0.51±0.25 |
| NLR | 2.64±1.34 |
| PLR | 138.87±64.69 |
| LMR | 4.62±2.30 |
Treatment
A non-invasive mask was used to immobilize the
patient's head and neck during treatment. The primary esophageal
gross tumor volumes (GTV-nx) together with involved metastatic
lymph nodes (GTV-nd) were determined from imaging examinations,
barium meal and endoscopic findings. Clinical target volume (CTV)
was defined as the GTV plus 3–5 mm to the anterior, posterior,
right and left directions and 2.5 cm into the superior and inferior
regions. CTV also encompassed a supraclavicular lymphonodus drawing
region (in patients with upper or middle EC) or the drainage area
of the lymph nodes around the stomach and cardia (in patients with
lower EC). A margin of 0.5 cm in all directions was added to the
CTV to generate the planning target volume. The organs at risk were
delineated, including the spinal cord, lung and heart/pericardium.
CT-based treatment planning was performed using a Pinnacle
radiation therapy planning system (version 9.0; Philips Medical
systems B.V., Eindhoven, The Netherlands). Patients were treated
with intensity-modulated radiation therapy or three-dimensional
conformal radiotherapy, which was delivered by a PRIMUS™ linear
accelerator (Siemens, AG, Munich, Germany) using 6-MV photon beams.
The radiotherapy regimens were 2.0 Gy/day, five times weekly, with
a total dose of 60 or 66 Gy. In addition, among the 104 patients
enrolled, 41 received concurrent chemotherapy with cisplatin (75
mg/m2) by intravenous injection (IV) on day 1 and
5-flurouracil (1,000 mg/m2) continuous IV on days 1–4;
starting on the first day of irradiation and repeated after 21
days, 10 received sequential chemotherapy with four cycles of
chemotherapy before radiotherapy, using the same schedule, And 53
patients received radiotherapy alone.
Immunohistochemical staining
(IHC)
FFPE tumor tissues (4-µm thick) were subjected to
IHC with a streptavidin-peroxidase (SP) method (Biotin-Streptavidin
Immunohistochemistry Kit; cat no. SP-9001; ZSGB-Bio, Beijing,
China). The sections were de-waxed in xylene and ethanol. Antigen
retrieval was performed by using a pressure cooker to heat tissue
sections in Tris-ethylene-diamine tetra-acetic acid buffer (pH 9.0)
for ~4 min. Endogenous peroxidase activity was blocked with 0.3%
H2O2 for 20 min followed by washing twice in
phosphate-buffered saline (PBS). The sections were mounted on glass
slides, pre-incubated with the blocking serum from the kit (liquid
A) and incubated at 4°C overnight with a monoclonal anti-PD-L1
antibody at a 1:300 dilution (cat. no. ab205921; Abcam, Cambridge
UK) or PBS instead of primary antibodies as a blank control.
Subsequently, incubation at 25°C for 10 min with the biotinylated
secondary antibody from the kit (liquid B), horseradish peroxidase
(25°C, 15 min, liquid C) and 3,3′-diaminobenzidine chromogen
(ZSGB-Bio) were performed sequentially. The slides were then
counterstained with hematoxylin. Following dehydration with xylene
and gradient ethanol (concentrations, 70, 75, 80, 90, 95 and 100%,
in turn) the sections were covered with neutral balsam.
Evaluation of PD-L1 expression
Immunohistochemical slides were observed under
low-power magnification (×40) to identify the extent of staining,
and immunostaining was further evaluated at high-power
magnification (×200). Two independent observers blinded to all of
the clinical data assessed PD-L1 expression semi-quantitatively.
PD-L1 expression-positive cases were determined by staining
intensity and the percentage of positive tumor cells, according to
a method described previously (24).
Staining intensity was scored on a 4-point scale: 0, no staining;
1, weak staining; 2, moderate staining; and 3, strong staining
respectively. The proportion of positive cells were scored as
follows: 0, 0% stained cells; 1, 1–30% stained cells; 2, 30–60%
stained cells; and 3, >60% stained cells. Two observers
discussed controversial cases, and a single consensus score was
established by multiplying the scores for intensity and extent of
staining. A receiver-operating characteristic (ROC) curve was
calculated and the appropriate cut-off value was selected to
distinguish between negative and positive cases. A score of ≥2 was
considered to represent positive expression of PD-L1.
Statistical analysis
All statistical analyses were conducted by SPSS 16.0
software. (SPSS, Inc., Chicago, IL, USA) The associations between
the clinicopathological factors and PD-L1 expression were explored
using a χ2 test. The values of NLR, PLR and LMR were
compared according to PD-L1 expression status (positive/negative)
using a Student's t-test. Spearman's correlation tests were used to
analyze possible associations. Cox proportional hazards models with
univariate and multivariate analyses were performed to assess the
associations of clinicopathological factors with OS. The OS rates
were calculated using the Kaplan-Meier method and were compared
using the log-rank test. All tests were two-sided and P<0.05 was
considered to indicate a statistically significant difference.
Results
PD-L1 expression in EC
The clinicopathological characteristics of the 104
patients with EC are presented in Table
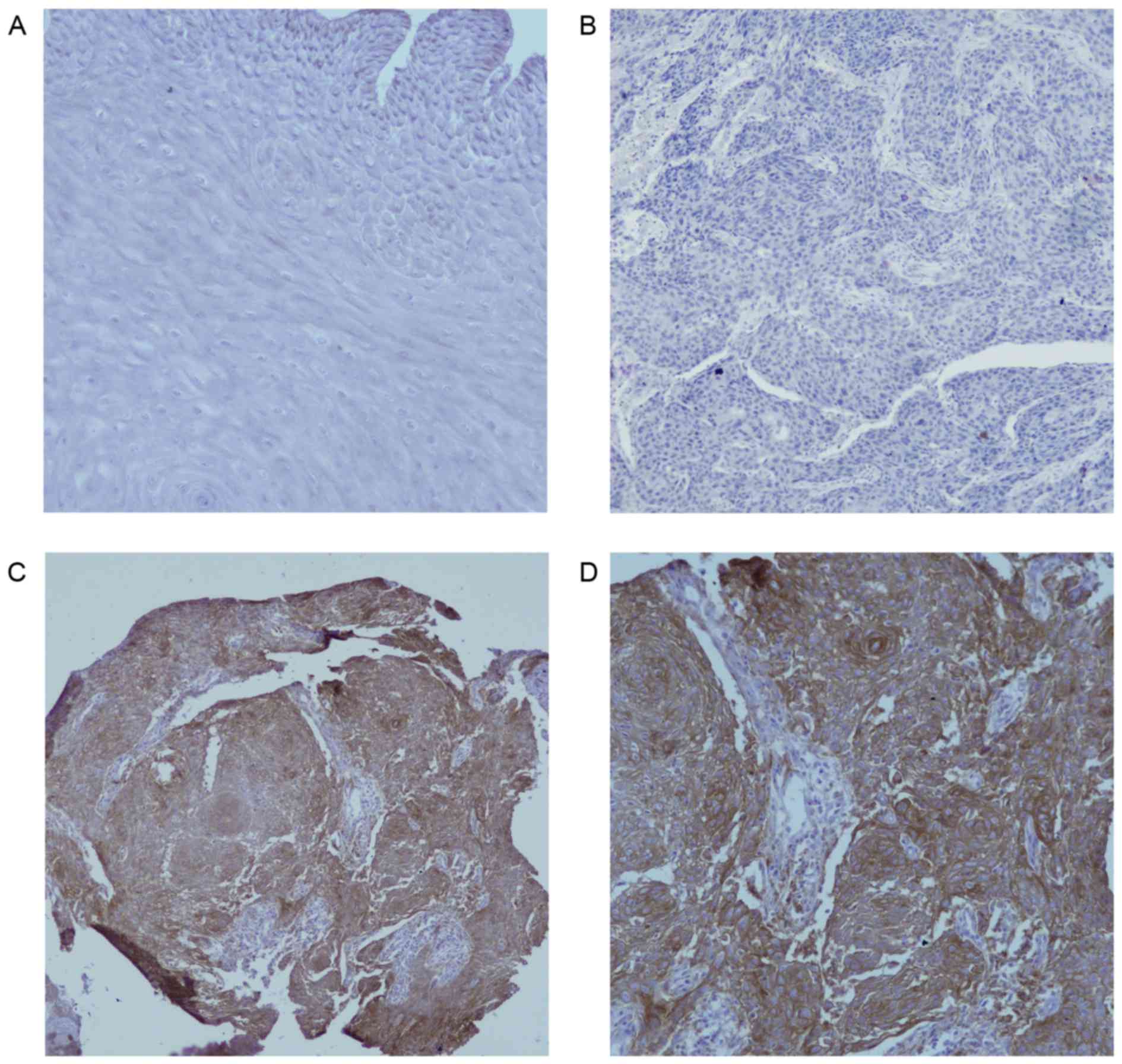
I. Fig. 1A indicated that there
was no PD-L1 protein staining in normal esophageal epithelium
(magnification ×200). In Fig. 1B, the
negative expression in EC was demonstrated (magnification ×200), as
shown in Fig. 1C and D, PD-L1 was
overexpressed on the membrane or in the cytoplasm (or both) of EC
cells under different magnifications (×100 and ×200, respectively)
in some cases, in contrast to normal esophageal epithelial tissues.
Of the 104 EC tissues, 39 (37.5%) showed positive PD-L1 staining
and focal distribution. No statistically significant differences in
clinical parameters were noted between the groups of positive and
negative PD-L1 expression (Table
II).
 | Table II.Association between PD-L1 expression
and clinical parameters. |
Table II.
Association between PD-L1 expression
and clinical parameters.
|
|
| PD-L1 expression,
n |
|
|---|
|
|
|
|
|
|---|
| Variables | Total cases, n | Negative | Positive | P-value |
|---|
| Patients | 104 | 65 | 39 |
|
| Age (years) |
|
|
| 0.447 |
|
≤65 | 53 | 35 | 18 |
|
|
>65 | 51 | 30 | 21 |
|
| Sex |
|
|
| 0.575 |
|
Male | 88 | 54 | 34 |
|
|
Female | 16 | 11 | 5 |
|
| Pathological
type |
|
|
| 0.064 |
|
SCC | 99 | 64 | 35 |
|
|
Others | 5 | 1 | 4 |
|
| Location |
|
|
| 0.417 |
|
Upper | 29 | 21 | 8 |
|
|
Middle | 52 | 31 | 21 |
|
|
Lower | 23 | 13 | 10 |
|
| Length (cm) |
|
|
| 0.273 |
| ≤5 | 46 | 25 | 21 |
|
|
>5–7 | 26 | 17 | 9 |
|
|
>7 | 32 | 23 | 9 |
|
|
T-classification |
|
|
| 0.103 |
|
T1-2 | 14 | 6 | 8 |
|
|
T3-4 | 90 | 59 | 31 |
|
|
N-classification |
|
|
| 0.966 |
| N0 | 36 | 22 | 14 |
|
| N1 | 63 | 40 | 23 |
|
| N2 | 5 | 3 | 2 |
|
| Clinical stage |
|
|
| 0.276 |
| I | 5 | 2 | 3 |
|
| II | 21 | 11 | 10 |
|
|
III | 78 | 52 | 26 |
|
Association between PD-L1 expression
and systemic inflammation biomarkers
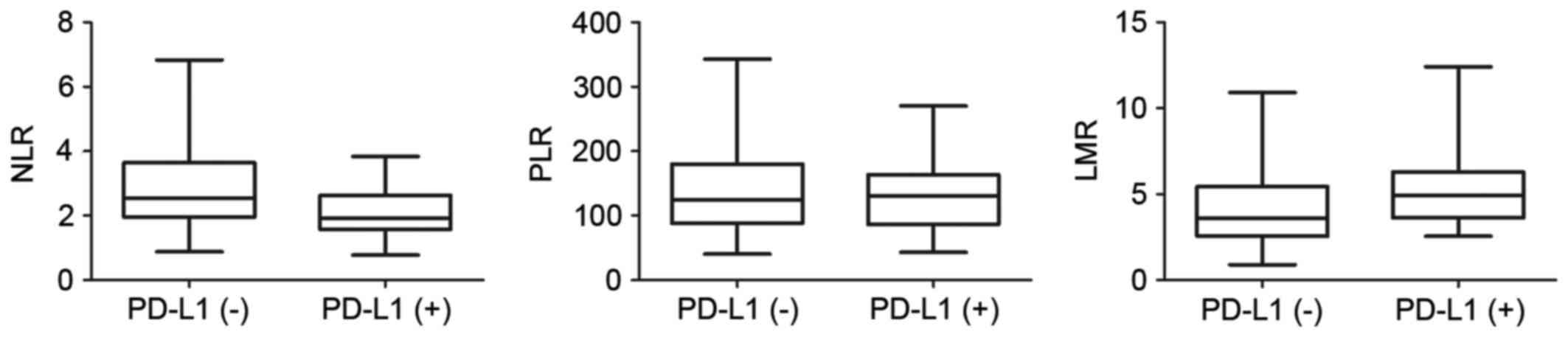
Among the 83 patients for whom the complete
pretreatment blood profiles were available, the mean values of NLR,
PLR and LMR were 2.64±1.34, 138.87±64.69 and 4.62±2.30,
respectively (Table I). To further
investigate the potential correlation of these blood biomarkers
with PD-L1 expression in EC tissues, the 83 patients were divided
into two groups according to PD-L1 expression level. The
distributions of these blood parameters are shown in the box
diagrams (Fig. 2). The only
statistically significant association identified was between NLR
and PD-L1 expression level (Student's t-test, P=0.001); patients in
the PD-L1(+) group had lower NLRs than those in the PD-L1(−) group
(Spearman correlation, r=−0.308; P=0.005).
Prognostic significance of clinical
factors and PD-L1 expression
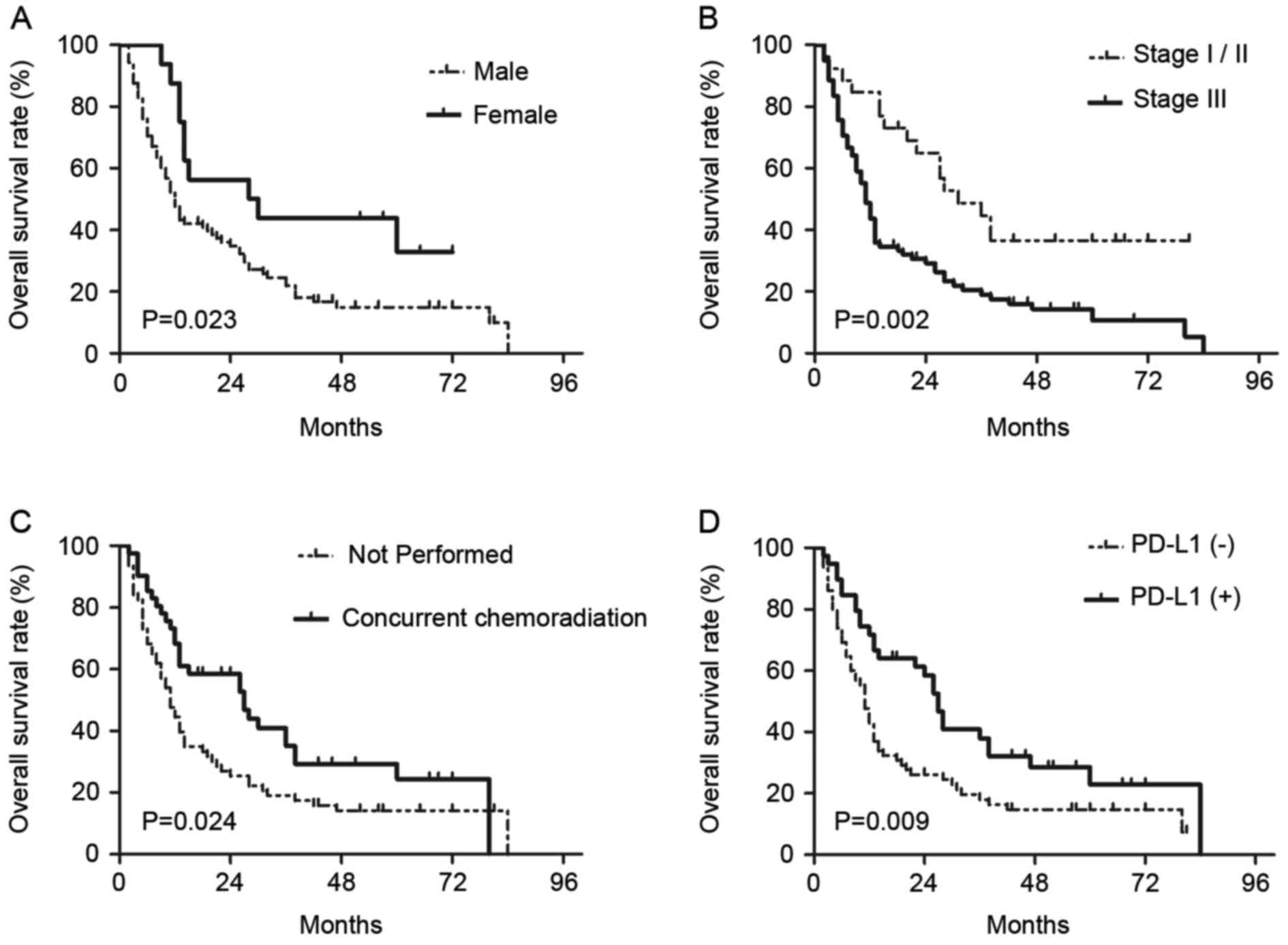
On univariate analyses, it was identified that sex
(female vs. male, P=0.029), T-classification (T3-4 vs. T1-2,
P=0.045), clinical stage (III vs. I–II, P=0.003), concurrent
chemotherapy (performed vs. not performed, P=0.028) and PD-L1
expression (positive vs. negative, P=0.012) were statistically
significantly associated with OS (Table
III). On subsequent multivariate analyses, sex (female vs.
male; HR, 0.449; 95% CI, 0.229–0.880; P=0.02), clinical stage (III
vs. I–II; HR, 2.471; 95% CI, 1.171–5.212; P=0.018), concurrent
chemotherapy (performed vs. not performed; HR, 0.590; 95% CI,
0.368–0.945; P=0.028) and PD-L1 expression (positive vs. negative;
HR, 0.600; 95% CI, 0.372–0.965; P=0.035) were independent
prognostic factors for patients with EC treated with radical CRT
(Table III). Survival curves of
these prognostic factors are shown in Fig. 3. The survival curve of PD-L1
demonstrated that patients with positive PD-L1 expression had
increased survival times compared with patients with negative PD-L1
expression, and the median OS times were 26 and 11 months,
respectively.
 | Table III.Univariate and multivariate analyses
of risk factors associated with overall survival in patients
treated with radical CRT. |
Table III.
Univariate and multivariate analyses
of risk factors associated with overall survival in patients
treated with radical CRT.
|
|
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|
|
|---|
| Variables | Test group | Reference
group | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age (years) | ≤65 | >65 | 0.992 | 0.974–1.010 | 0.375 | – | – | – |
| Sex | Female | Male | 0.478 | 0.246–0.929 | 0.029a | 0.449 | 0.229–0.880 | 0.020a |
| Pathological
type | SCC | Others | 1.824 | 0.573–5.804 | 0.309 | – | – | – |
| Tumor location | Lower | Upper/middle | 0.802 | 0.465–1.384 | 0.428 | – | – | – |
| Tumor length
(cm) | >7 | ≤5/5–7 | 1.236 | 0.771–1.982 | 0.379 | – | – | – |
|
T-classification | T3-4 | T1-2 | 2.115 | 1.015–4.405 | 0.045a | 0.833 | 0.309–2.247 | 0.718 |
|
N-classification | N+ | N0 | 1.354 | 0.852–2.152 | 0.200 | – | – | – |
| Clinical stage | III | I/II | 2.326 | 1.341–4.035 | 0.003a | 2.471 | 1.171–5.212 | 0.018a |
| Concurrent
chemoradiation | Performed | Not performed | 0.602 | 0.383–0.948 | 0.028a | 0.590 | 0.368–0.945 | 0.028a |
| PD-L1
expression | Positive | Negative | 0.552 | 0.348–0.877 | 0.012a | 0.600 | 0.372–0.965 | 0.035a |
| NLR (mean) | >2.64 | ≤2.64 | 1.310 | 0.796–2.156 | 0.288 | – | – | – |
| PLR (mean) | >138.87 | ≤138.87 | 1.066 | 0.650–1.749 | 0.800 | – | – | – |
| LMR (mean) | >4.62 | ≤4.62 | 0.687 | 0.420–1.123 | 0.134 | – | – | – |
Discussion
The present study, investigated PD-L1 expression,
the associations between PD-L1 expression and various inflammatory
markers, and the prognostic relevance of these factors in patients
with EC treated with definitive CRT. In contrast to previous
studies (9,17), this present study offered several
novel observations that should be considered. The research on PD-L1
expression in EC was limited and controversial (18,24,25). In
the present patient cohort, 37.5% of EC tissue specimens exhibited
positive staining. A review of previous studies regarding PD-L1
expression in EC was conducted (Table
IV); the electronic databases PubMed up to September 2016 were
systematically searched. The search was performed using the
following terms: ‘esophageal cancer;’ ‘programmed death ligand 1;’
(or ‘PD-L1’), ‘prognosis.’ In previous studies, the percentage of
cases exhibiting positive PD-L1 expression ranged from 18.4–82.8%.
Regarding the differences in PD-L1 expression rate, besides
differences in the antibodies used, we speculate that differing
PD-L1 evaluation criteria may be responsible for the large range.
In the majority of studies, PD-L1 expression scores were determined
according to the product of the percentage of stained tumor cells
and the intensity of staining, and an appropriate cut-off value was
selected to distinguish between negative and positive cases.
However, in several reports on PD-L1 expression, the criterion for
positive staining was 5% of tumor cells showing membrane staining.
For instance, a cut-off value of 5% was commonly used in lung
cancer, renal cell carcinoma and melanoma (26–28). We
speculate that different tumors may require different evaluation
criteria due to tissue specificity. On the other hand, this
divergence is from the PD-L1 itself. PD-L1 expression is dynamic,
susceptible to the tumor microenvironment and unevenly distributed
in tumor tissue. Thus, it is necessary to formulate a unified
standard for evaluation and to explore more accurate detection
methods in the future.
 | Table IV.Summary of studies reporting PD-L1
expression and survival in patients with esophageal cancer (ranked
according to publication time). |
Table IV.
Summary of studies reporting PD-L1
expression and survival in patients with esophageal cancer (ranked
according to publication time).
|
|
|
|
|
| Classification (no.
of patients) |
|
|
|
|
|
|---|
|
|
|
|
|
|
|
|
|
|
|
|
|---|
| First author | Sample size | Detection method of
PD-L1 | Cut-off value | PD-L1 positive rate
(%) | T | N | M | AJCC/UI CC
stages | Treatment | The effect of PD-L1
(+) on DFS | The effect of PD-L1
(+) on OS | (Refs.) |
|---|
| Ohigashi | 41 | qPCR/IHC | ≥1 (qPCR); ≥10% of
TCs (IHC) | 43.9 | T1 (7); T2 (20); T3
(14) | N0 (15); N1
(26) | M0 (33); M1
(8) | I (7); II (19); III
(7); IV(8) | Surgery | Not listed | Negative | (45) |
| Loos | 101 | IHC | ≥4 | 73.3 | T1 (39); T2 (27);
T3 (35) | N0 (52); N1 (44);
N3 (5) | M0 (91); M1
(10) | I (46); II (19);
III (27); IV(9) | Surgery | Negative | Negative | (17) |
| Chen | 99 | IHC | >0 | 82.8 | T1+T2 (35); T3+T4
(64) | N0 (65); N+
(34) | M0 (93); M1
(6) | I (5); II (57); III
(31); IV (6) | Surgery | Not listed | Negative | (46) |
| Lim | 73 | IHC | H-score ≥20 | 56.2 | Not listed | N0 (14); N+
(59) | Not listed | I (4); II (26); III
(43) | Neoadjuvant CRT or
chemotherapy prior to surgery | None | Negative | (42) |
| Leng | 106 | IHC | ≥3 | 46.2 | Not listed | Not listed | Not listed | I (17); II (61);
III (23); IV (5) | Surgery | Not listed | Negative | (47) |
| Chen | 162 | IHC | ≥2 | 45 | ≤T3 (100); T4
(62) | N0 (39); N+
(123) | M0 (94); M1
(68) | Not listed | Neoadjuvant CRT or
definitive CRT | Not listed | Negative | (9) |
| Tanaka | 180 | IHC | ≥4 | 29.4 | T1 (37); T2 (31);
T3 (94); T4 (18) | N0 (53); N+
(127) | M0 (132); M1
(48) | I (19); II (53);
III (60); IV (48) | Radical resection
with or without neoadjuvant chemotherapy | Not listed | Negative | (24) |
| Chen | 536 | IHC | 5% of TCs with at
least moderate staining | 41.4 | T1 (28); T2 (92);
T3 (405); T4 (11) | N0 (222); N1 (170);
N3 (107); N4 (37) | Not listed | I (61); II (195);
III (273); IV (7) | Surgery | Positive | None | (25) |
| Hatogai | 196 | IHC | ≥1% of TCs with
membrane staining | 18.4 | T1 (0); T2 (32); T3
(157); T4 (7) | N0 (51); N1 (59);
N3 (63); N4 (24) | M0 (179); M1
(17) | I (7); II (51); III
(121); IV (17) | Surgery | Not listed | Positive | (18) |
| Zhu | 133 | IHC | >5% of TCs | 42.1 | T3 (133) | N0 (133) | M0 (133) | Not listed | Surgery | Negative | Negative | (48) |
| Ito | 90 | IHC | ≥7 | 18.9 | T1+T2 (52); T3+T4
(38) | N0 (45); N+
(45) | M0 (80); M1
(10) | Not listed | Surgery | None | Negative | (49) |
It is known that there is a complex relationship
between inflammation and immunity, which together constitute the
tumor microenvironment, and both are associated with invasion and
recurrence in cancer patients. NLR, PLR and LMR are considered
systemic inflammatory indicators and have been investigated in
patients with various types of tumors (29–32);
however, no consensus has been reached on their association with
the prognosis of such patients. The present study explored the
hypothesis that the blood parameters of NLR, PLR and LMR are
associated with PD-L1 expression and OS. However, the results
demonstrated no correlations between OS and NLR, PLR or LMR.
Further studies utilizing a larger number of patients would be
beneficial. Notably, NLR was associated with PD-L1 expression,
whereas PLR and LMR were not. To the best of our knowledge, the
present study was the first to demonstrate the correlation between
PD-L1 and systemic inflammatory markers in EC. Previous studies on
the correlation between PD-L1 and NLR, PLR and LMR have only
involved hepatocellular carcinoma (HCC). Wang et al
(33) reported that, in hepatitis
B-associated HCC, NLR was associated with PD-L1 expression within
the center of the tumor, but NLR was not associated with PLR or
prognostic nutritional index (PNI). Furthermore, the group with an
NLR greater than the median had higher PD-L1 expression (33). In contrast to previous experiments,
the present study showed that the NLR value was negatively
correlated with PD-L1 expression.
Cancer is a systemic disease; therefore, we
considered the results that patients in the PD-L1 (+) group had
lower NLRs than those in the PD-L1(−) group may be due to the host
response to the tumor. It is reported that tumors are able to
secrete a multitude of inflammatory factors, such as
granulocyte-colony stimulating factor (G-CSF), IL-6, and
interferon-γ (IFN-γ), which could induce systemic reactions
(34), and can affect the generation
of blood cells. Furthermore, PD-L1 expression may be increased in
response to cytokine exposure (such as G-CSF and IFN-γ) (35,36). Thus,
it is possible that the systemic inflammatory markers correlate
with PD-L1 expression. In addition, T cells may act as an
intermediary between PD-L1 and inflammatory markers. Several
studies have suggested PD-L1 expression in human tumor tissue may
lead to T cell exhaustion, while the pretreatment NLR level was
found to be closely associated with T cell infiltration (37). Furthermore, the relationship between
inflammation and immunity may change with the different stages of a
tumor. Therefore, the mechanisms between PD-L1 and inflammation
require further exploration. The aforementioned blood parameters
possess the benefit of being easily obtained and low-cost. The
relationship between NLR and PD-L1 expression may provide guidance
for the development and adjustment of future immunotherapeutic
strategies.
Previous studies have investigated the prognostic
value of PD-L1 in EC (Table IV).
Numerous reports demonstrated that patients with positive PD-L1
expression had a higher risk of mortality than patients lacking
PD-L1 expression. However, in the current study, PD-L1 protein
expression was a protective factor in patients treated with
definitive CRT, consistent with the reports by Hatogai et al
(18) and Chen et al (25) in surgical patients with EC.
Furthermore, similar findings were reported in melanoma and,
nasopharyngeal carcinoma as well as lung cancer (28,38,39). It is
well-established that the balance between cell birth and cell death
is important; once this balance is disrupted, tumor progression
will occur (40,41). There may be other pathways or
receptors associated with PD-L1 that have not yet been identified,
which the effect of antitumor was stronger than the effect of PD-L1
leading to the evasion of tumor cells from host monitoring in tumor
microenvironment, when PD-L1 is highly expressed. Furthermore, in
all of the aforementioned studies, the patients received surgical
intervention, whereas only patients who underwent definitive CRT
were enrolled in the present study. This discrepancy between the
current findings and previous findings may be explained by the
different treatment strategies applied, as radiotherapy and
chemotherapy may affect the expression of PD-L1 (9,42).
Additionally, radiation is able to produce an immunogenic effect in
tumors, which may contribute to the host immune response against
tumor cells (43,44). Although a number of unanswered
questions require further investigation, the current study
suggested that the role of PD-L1 in the prognosis of patients
treated with definitive CRT had significant value.
There are certain limitations to the current study.
First, due to the retrospective nature, as well as the
single-center design, certain data regarding immune parameters
could not be obtained. Therefore, a prospective and multicenter
study will be necessary in the future to confirm these results. In
addition, the sample size was small, due to the restrictions of the
inclusion and exclusion criteria. Finally, the, potential
mechanisms linking PD-L1 expression with NLR, PLR and LMR were not
investigated. Animal experiments and a large range of clinical
studies will be conducted in due course.
In conclusion, these findings highlight that, in the
tumor microenvironment, PD-L1 expression may reflect antitumor
immunity and is a useful prognostic marker. The associations
between PD-L1 expression and inflammatory markers provide a novel
perspective for the further study of the mechanisms of inflammation
and immunity. Given the complexity of the tumor microenvironment, a
more comprehensive perspective on tumor therapeutic strategies
should be formulated in the future.
References
|
1
|
Chen W, Zheng R, Zeng H and Zhang S: The
incidence and mortality of major cancers in China, 2012. Chin J
Cancer. 35:732016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Parkin DM, Läärä E and Muir CS: Estimates
of the worldwide frequency of sixteen major cancers in 1980. Int J
Cancer. 41:184–197. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cooper JS, Guo MD, Herskovic A, Macdonald
JS, Martenson JA Jr, Al-Sarraf M, Byhardt R, Russell AH, Beitler
JJ, Spencer S, et al: Chemoradiotherapy of locally advanced
esophageal cancer: Long-term follow-up of a prospective randomized
trial (RTOG 85-01). Radiation therapy oncology group. JAMA.
281:1623–1627. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ajani JA, Winter K, Komaki R, Kelsen DP,
Minsky BD, Liao Z, Bradley J, Fromm M, Hornback D and Willett CG:
Phase II randomized trial of two nonoperative regimens of induction
chemotherapy followed by chemoradiation in patients with localized
carcinoma of the esophagus: RTOG 0113. J Clin Oncol. 26:4551–4556.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Robert C, Schachter J, Long GV, Arance A,
Grob JJ, Mortier L, Daud A, Carlino MS, McNeil C, Lotem M, et al:
Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med.
372:2521–2532. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Forde PM, Reiss KA, Zeidan AM and Brahmer
JR: What lies within: Novel strategies in immunotherapy for
non-small cell lung cancer. Oncologist. 18:1203–1213. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ritprajak P and Azuma M: Intrinsic and
extrinsic control of expression of the immunoregulatory molecule
PD-L1 in epithelial cells and squamous cell carcinoma. Oral Oncol.
51:221–228. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen MF, Chen PT, Chen WC, Lu MS, Lin PY
and Lee KD: The role of PD-L1 in the radiation response and
prognosis for esophageal squamous cell carcinoma related to IL-6
and T-cell immunosuppression. Oncotarget. 7:7913–7924.
2016.PubMed/NCBI
|
|
10
|
Brodská B, Otevřelová P and Kuželová K:
Correlation of PD-L1 surface expression on leukemia cells with the
ratio of PD-L1 mRNA variants and with electrophoretic mobility.
Cancer Immunol Res. 4:815–819. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gu X, Gao XS, Xiong W, Guo W, Han L, Bai
Y, Peng C, Cui M and Xie M: Increased programmed death ligand-1
expression predicts poor prognosis in hepatocellular carcinoma
patients. Onco Targets Ther. 9:4805–4813. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Inamura K, Yokouchi Y, Sakakibara R,
Kobayashi M, Subat S, Ninomiya H, Nagano H, Nomura K, Okumura S and
Ishikawa Y: Relationship of tumor PD-L1 expression with EGFR
wild-type status and poor prognosis in lung adenocarcinoma. Jpn J
Clin Oncol. 46:935–941. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Joneja U, Vranic S, Swensen J, Feldman R,
Chen W, Kimbrough J, Xiao N, Reddy S, Palazzo J and Gatalica Z:
Comprehensive profiling of metaplastic breast carcinomas reveals
frequent overexpression of programmed death-ligand 1. J Clin
Pathol. 70:255–259. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zou MX, Peng AB, Lv GH, Wang XB, Li J, She
XL and Jiang Y: Expression of programmed death-1 ligand (PD-L1) in
tumor-infiltrating lymphocytes is associated with favorable spinal
chordoma prognosis. Am J Transl Res. 8:3274–3287. 2016.PubMed/NCBI
|
|
15
|
Aguiar P Jr, Lopes G, Santoro I, Tadokoro
H, Barreto C and De Mello R: P2.47 (also presented as PD1.02): The
role of PD-L1 expression as a predictive biomarker in advanced
NSCLC: An update of a network meta-analysis: Track: Immunotherapy.
J Thorac Oncol. 11 Suppl:S247–S248. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen CL, Pan QZ, Zhao JJ, Wang Y, Li YQ,
Wang QJ, Pan K, Weng DS, Jiang SS, Tang Y, et al: PD-L1 expression
as a predictive biomarker for cytokine-induced killer cell
immunotherapy in patients with hepatocellular carcinoma.
Oncoimmunology. 5:e11766532016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Loos M, Langer R, Schuster T, Gertler R,
Walch A, Rauser S, Friess H and Feith M: Clinical significance of
the costimulatory molecule B7-H1 in Barrett carcinoma. Ann Thorac
Surg. 91:1025–1031. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hatogai K, Kitano S, Fujii S, Kojima T,
Daiko H, Nomura S, Yoshino T, Ohtsu A, Takiguchi Y, Doi T and
Ochiai A: Comprehensive immunohistochemical analysis of tumor
microenvironment immune status in esophageal squamous cell
carcinoma. Oncotarget. 7:47252–47264. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang N, Zeng Y, Du W, Zhu J, Shen D, Liu
Z and Huang JA: The EGFR pathway is involved in the regulation of
PD-L1 expression via the IL-6/JAK/STAT3 signaling pathway in
EGFR-mutated non-small cell lung cancer. Int J Oncol. 49:1360–1368.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen PC and Feng JF: A novel
inflammation-based stage (I Stage) in patients with resectable
esophageal squamous cell carcinoma. Mediators Inflamm.
2016:53967472016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kara M, Uysal S, Altinisik U, Cevizci S,
Güçlü O and Dereköy FS: The pre-treatment neutrophil-to-lymphocyte
ratio, platelet-to-lymphocyte ratio, and red cell distribution
width predict prognosis in patients with laryngeal carcinoma. Eur
Arch Otorhinolaryngol. 274:535–542. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
van Kessel KE, de Haan LM, Fransen van de
Putte EE, van Rhijn BW, de Wit R, van der Heijden MS, Zwarthoff EC
and Boormans JL: Elevated derived neutrophil-to-lymphocyte ratio
corresponds with poor outcome in patients undergoing pre-operative
chemotherapy in muscle-invasive bladder cancer. Bladder Cancer.
2:351–360. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Edge SB and Compton CC: The American joint
committee on cancer: The 7th edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tanaka K, Miyata H, Sugimura K, Kanemura
T, Hamada-Uematsu M, Mizote Y, Yamasaki M, Wada H, Nakajima K,
Takiguchi S, et al: Negative influence of programmed
death-1-ligands on the survival of esophageal cancer patients
treated with chemotherapy. Cancer Sci. 107:726–733. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen K, Cheng G, Zhang F, Zhang N, Li D,
Jin J, Wu J, Ying L, Mao W and Su D: Prognostic significance of
programmed death-1 and programmed death-ligand 1 expression in
patients with esophageal squamous cell carcinoma. Oncotarget.
7:30772–30780. 2016.PubMed/NCBI
|
|
26
|
Takada K, Toyokawa G, Okamoto T, Akamine
T, Takamori S, Katsura M, Fujishita T, Shoji F, Oda Y and Maehara
Y: An immunohistochemical analysis of PD-L1 protein expression in
surgically resected small cell lung cancer using different
antibodies and criteria. Anticancer Res. 36:3409–3412.
2016.PubMed/NCBI
|
|
27
|
Motzer RJ, Escudier B, McDermott DF,
George S, Hammers HJ, Srinivas S, Tykodi SS, Sosman JA, Procopio G,
Plimack ER, et al: Nivolumab versus everolimus in advanced
renal-cell carcinoma. N Engl J Med. 373:1803–1813. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Taube JM, Anders RA, Young GD, Xu H,
Sharma R, McMiller TL, Chen S, Klein AP, Pardoll DM, Topalian SL
and Chen L: Colocalization of inflammatory response with B7-h1
expression in human melanocytic lesions supports an adaptive
resistance mechanism of immune escape. Sci Transl Med.
4:127ra372012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Grimm M, Rieth J, Hoefert S, Krimmel M,
Rieth S, Teriete P, Kluba S, Biegner T, Munz A and Reinert S:
Standardized pretreatment inflammatory laboratory markers and
calculated ratios in patients with oral squamous cell carcinoma.
Eur Arch Otorhinolaryngol. 273:3371–3384. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lee SM, Russell A and Hellawell G:
Predictive value of pretreatment inflammation-based prognostic
scores (neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte
ratio, and lymphocyte-to-monocyte ratio) for invasive bladder
carcinoma. Korean J Urol. 56:749–755. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu JS, Huang Y, Yang X and Feng JF: A
nomogram to predict prognostic values of various inflammatory
biomarkers in patients with esophageal squamous cell carcinoma. Am
J Cancer Res. 5:2180–2189. 2015.PubMed/NCBI
|
|
32
|
Stotz M, Liegl-Atzwanger B, Posch F, Mrsic
E, Thalhammer M, Stojakovic T, Bezan A, Pichler M, Gerger A and
Szkandera J: Blood-based biomarkers are associated with disease
recurrence and survival in gastrointestinal stroma tumor patients
after surgical resection. PLoS One. 11:e01594482016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang Q, Blank S, Fiel MI, Kadri H, Luan W,
Warren L, Zhu A, Deaderick PA, Sarpel U, Labow DM and Hiotis SP:
The severity of liver fibrosis influences the prognostic value of
inflammation-based scores in hepatitis B-associated hepatocellular
carcinoma. Ann Surg Oncol. 22 Suppl 3:S1125–S1132. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
He G, Zhang H, Zhou J, Wang B, Chen Y,
Kong Y, Xie X, Wang X, Fei R, Wei L, et al: Peritumoural
neutrophils negatively regulate adaptive immunity via the
PD-L1/PD-1 signalling pathway in hepatocellular carcinoma. J Exp
Clin Cancer Res. 34:1412015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bankey PE, Banerjee S, Zucchiatti A, De M,
Sleem RW, Lin CF, Miller-Graziano CL and De AK: Cytokine induced
expression of programmed death ligands in human neutrophils.
Immunol Lett. 129:100–107. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Thorn M, Guha P, Cunetta M, Espat NJ,
Miller G, Junghans RP and Katz SC: Tumor-associated GM-CSF
overexpression induces immunoinhibitory molecules via STAT3 in
myeloid-suppressor cells infiltrating liver metastases. Cancer Gene
Ther. 23:188–198. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Han S, Liu Y, Li Q, Li Z, Hou H and Wu A:
Pre-treatment neutrophil-to-lymphocyte ratio is associated with
neutrophil and T-cell infiltration and predicts clinical outcome in
patients with glioblastoma. BMC Cancer. 15:6172015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lee VH, Lo AW, Leung CY, Shek WH, Kwong
DL, Lam KO, Tong CC, Sze CK and Leung TW: Correlation of PD-L1
expression of tumor cells with survival outcomes after radical
intensity-modulated radiation therapy for non-metastatic
nasopharyngeal carcinoma. PLoS One. 11:e01579692016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Song Z, Yu X and Zhang Y: Altered
expression of programmed death-ligand 1 after neo-adjuvant
chemotherapy in patients with lung squamous cell carcinoma. Lung
Cancer. 99:166–171. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wei Y, Huang H, Qiu Z, Li H, Tan J, Ren G
and Wang X: NLRP1 overexpression is correlated with the
tumorigenesis and proliferation of human breast tumor. Biomed Res
Int. 2017:49384732017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Pang X, Li R, Shi D, Pan X, Ma C, Zhang G,
Mu C and Chen W: Knockdown of Rhotekin 2 expression suppresses
proliferation and induces apoptosis in colon cancer cells. Oncol
Lett. 14:8028–8034. 2017.PubMed/NCBI
|
|
42
|
Lim SH, Hong M, Ahn S, Choi YL, Kim KM, Oh
D, Ahn YC, Jung SH, Ahn MJ, Park K, et al: Changes in tumour
expression of programmed death-ligand 1 after neoadjuvant
concurrent chemoradiotherapy in patients with squamous oesophageal
cancer. Eur J Cancer. 52:1–9. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Demaria S, Golden EB and Formenti SC: Role
of local radiation therapy in cancer immunotherapy. JAMA Oncol.
1:1325–1332. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Takeshima T, Pop LM, Laine A, Iyengar P,
Vitetta ES and Hannan R: Key role for neutrophils in
radiation-induced antitumor immune responses: Potentiation with
G-CSF. Proc Natl Acad Sci USA. 113:pp. 11300–11305. 2016;
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ohigashi Y, Sho M, Yamada Y, Tsurui Y,
Hamada K, Ikeda N, Mizuno T, Yoriki R, Kashizuka H, Yane K, et al:
Clinical significance of programmed death-1 ligand-1 and programmed
death-1 ligand-2 expression in human esophageal cancer. Clin Cancer
Res. 11:2947–2953. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Chen L, Deng H, Lu M, Xu B, Wang Q, Jiang
J and Wu C: B7-H1 expression associates with tumor invasion and
predicts patient's survival in human esophageal cancer. Int J Clin
Exp Pathol. 7:6015–6023. 2014.PubMed/NCBI
|
|
47
|
Leng C, Li Y, Qin J, Ma J, Liu X, Cui Y,
Sun H, Wang Z, Hua X, Yu Y, et al: Relationship between expression
of PD-L1 and PD-L2 on esophageal squamous cell carcinoma and the
antitumor effects of CD8+ T cells. Oncol Rep.
35:699–708. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zhu Y, Li M, Mu D, Kong L, Zhang J, Zhao
F, Li Z, Liu X, Bo C and Yu J: CD8+/FOXP3+ ratio and PD-L1
expression associated with survival in pT3N0M0 stage esophageal
squamous cell cancer. Oncotarget. 7:71455–71465. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Ito S, Okano S, Morita M, Saeki H,
Tsutsumi S, Tsukihara H, Nakashima Y, Ando K, Imamura Y, Ohgaki K,
et al: Expression of PD-L1 and HLA class I in esophageal squamous
cell carcinoma: Prognostic factors for patient outcome. Ann Surg
Oncol. 23 Suppl 4:S508–S515. 2016. View Article : Google Scholar
|