Introduction
Lung cancer is still the leading cause of
cancer-related deaths in China and worldwide (1,2).
Contributing to approximately 85% of lung cancers and including
histological types like adenocarcinoma (ADE), squamous cell
carcinoma (SCC), large cell carcinoma and mixed histologies,
non-small cell lung cancer (NSCLC) is still one of the major
threats to human health. Diagnosis of it often occurs so late that
nearly two-thirds have lost their opportunity for radical surgery.
Against this relentlessly challenging clinical backdrop,
establishment of useful biomarkers to facilitate early diagnosis,
therapeutic planning as well as prognostic prediction turns out as
great essential.
Human runt-related transcription factor (RUNX)
family, encoded by genes RUNX1, RUNX2 and RUNX3, have
been proved to play pivotal roles in activating and repressing the
transcription of principle regulators of growth, survival and
differentiation pathways through binding DNA via partnering with
the cofactor, CBFβ/PEBP2β (core-binding factor beta
subunit/polyomavirus enhancer-binding protein 2 beta subunit)
(3–6).
RUNX3, a remarkable biomarker firstly demonstrated in gastric
epithelial disorders and cancer, has now emerged to exercise its
mainly tumor suppressive activity while partly oncogenic role and
interact with other signaling molecules in the context of
carcinogenesis in a great variety of cancerous entities (7–11). Besides
gastric tumors, RUNX3 had been reported to be epigenetically
inactivated in a wide spectrum of malignancies, including bile duct
cancer, breast cancer (BC), pancreatic cancer, colorectal cancer
(CRC), prostate cancer, lymphoma, and lung cancer as well (12–19). Its
protein deficiency, however, usually an aftermath of hemizygous
deletion, promoter hypermethylation, histone modification as well
as protein mislocalization, often lead to TGF-β1-induced cell
growth inhibition and result in reduction of sensitivity to
cellular apoptosis, ending up as tumorigenesis (9,20–22).
Several studies (23–25) had
demonstrated that nuclear localization of RUNX3 expression was the
authentic pattern for RUNX3 protein to exert its function as tumor
suppressor gene (TSG), any patterns other than nuclear localization
(positive in the nucleus and positive or negative in the cytoplasm)
were defined as dysfunctioning patterns and rendered inactivated
status of RUNX3, associated with worse outcome and shorter overall
survival (OS) in BC, gastric cancer (GC) and CRC. Although
importance of gene promoter hypermethylation concerning RUNX3
expression in NSCLC had ever been demonstrated in some studies,
seldom report had been focused on clinical significance of patient
survival with different expression patterns of RUNX3. We wondered
if the situation was ever true like what it was in BC, GC and CRC,
that NSCLC patients with nuclear localization of RUNX3 would
definitely have better OS than those with non-nuclear localization,
and if not, what the possible underlying mechanisms would be.
In the present study, we investigated the expression
of RUNX3 and proliferation index Ki-67 immunohistochemically and
evaluated the apoptotic index by means of TdT mediated dUTP-biotin
nick end labelling (TUNEL) method in 188 NSCLCs and analyzed the
patients' OS with different expression patterns of RUNX3 mentioned
in other cancer types, and try to interpret its correlations within
the clinicopathologic parameters and their clinical
significance.
Materials and methods
Patients and tissue samples
Archival formalin-fixed, paraffin-embedded tissue
sections from a series of 5 normal lung tissue and 188 patients
(128 males and 60 females; age range: 36–78 years old) undergone
surgery for NSCLC at the Department of Thoracic Surgery of Fujian
Cancer Hospital and Fujian Medical University Cancer Hospital
during 2010–2011 were selected. None had received chemo- or
radio-therapy prior to tissue collection. The histopathologic
features of cancerous specimens and TNM staging was determined
according to the 8th AJCC guidelines for NSCLC. Grading of SCC into
well-, moderately- and poorly-differentiated types is based on an
assessment of the degree of differentiation and cellular atypia via
hematoxylin and eosin (H&E) staining. Sheets of cells adopting
a pavement-like architecture with prominent intercellular bridges
characterize well-differentiated tumors. Keratinization is
generally present and may lead to the formation of keratin
‘pearls’. More pronounced cytologic atypia, increased mitotic
activity, and frequent areas of necrosis and hemorrhage
characterized moderately differentiated tumors. While those with
the appearance of anaplastic large cell or small cell as well as
even more obviously cytologic atypia and increased mitotic activity
is defined as ‘poorly differentiated SCC’. The final follow-up was
February 5, 2017 and all patients were available of their survival
data. Patients' survival data were censored if they were still
alive or dead of disease other than lung cancer at the date of
surveillance. The study protocol was approved by the Human Ethics
Review Committee of Fujian Cancer Hospital and Fujian Medical
University Cancer Hospital, and a signed informed consent was
obtained from each patient.
Tissue microarray building
A fresh section was cut from each donor block,
stained with H&E, and used as a guide to select the
morphologically most-representative regions of the tumor from which
to sample the individual core needle biopsies (26,27). A
duplicate of 1.0 mm diameter cores were then punched from tumor
areas of each donor tissue block and introduced into previously
prepared recipient paraffin blocks (12×10 Matrix of 1 mm cores),
after having made hosting holes in the blocks with a Tissue
Microarrayer (Unitma Co., Ltd., Seoul, Korea). We constructed 4
recipient blocks with a maximum of 10×12 dots. With a microtome, 4
µm sections were cut from the TMA blocks and placed onto
3-aminopropyltriethoxysilane-coated glass slides to generate TMA
slides for molecular analyses. Some sections were stained with
H&E in a routine manner for histological examination.
Immunohistochemical detection of RUNX3
and Ki-67
Immunohistochemistry was performed with the indirect
enzyme-labeled antibody method, as described previously (28,29). For
detection of RUNX3, mouse anti-human monoclonal (2B3) RUNX3
antibody (dilution 1:500; Abcam, Cambridge, CA, USA) was used. For
detection of Ki-67, rabbit anti-human monoclonal (30–9)
anti-Ki-67 antibody purchased from Roche Applied Science (Penzberg,
Germany) was used. TMA sections were deparaffinized with toluene
and rehydrated in graded alcohols. After autoclaved for 15 min at
120°C in 10 mM citrate buffer (pH 6.0) for antigen retrieval,
endogenous peroxidase was inactivated with 0.3% hydrogen peroxide
in methanol for 15 min. The sections were then pre-incubated with
500 µg/ml normal goat IgG dissolved in 1% BSA in PBS (pH 7.4) for 1
h, reacted with primary antibodies for 16 h, washed with 0.075%
Brij 35 in PBS, and then incubated with HRP-conjugated goat
anti-mouse/rabbit (RUNX3/Ki-67) in 1% BSA in PBS for 1 h. After
washing with 0.075% Brij 35 in PBS, the sites of HRP were
visualized with DAB and H2O2. As a negative
control, some sections were reacted with normal mouse IgG instead
of the specific antibodies. The stained slides were analyzed under
a laser scanning microscope (LSM 5 PASCAL; Carl Zeiss AG,
Oberkochen, Germany).
TUNEL staining for apoptotic cells in
NSCLCs
To identify nuclei with DNA strand breaks at a
cellular level, TUNEL was performed according to the method of
Gavrieli et al (30), with a
slight modification. Paraffin sections (5 µm) were cut onto
silane-coated glass slides, dewaxed with toluene, and rehydrated in
an ethanol series. After washing with PBS, the sections were
treated with 5 µg/ml of proteinase K in PBS at 37°C for 15 min. The
sections were then rinsed once with deionized distilled water and
incubated with TdT buffer (25 mM Tris/HCl buffer, pH 6.6,
containing 0.2 M potassium cacodylate and 0.25 mg/ml BSA) alone at
room temperature for 30 min. After incubation, the slides were
reacted with 200 U/ml TdT dissolved in TdT buffer supplemented with
5 µM biotin-16-dUTP, 20 µM dATP, 1.5 mM CoCl2, and 0.1
mM dithiothreitol at 37°C for 1 h. The reaction was terminated by
washing with 50 mM Tris/HCl buffer (pH 7.4) for 15 min. Endogenous
peroxidase activity was inhibited by immersing the slides in 0.3%
H2O2 in methanol at room temperature for 15
min. The signals were detected immunohistochemically with
HRP-conjugated goat anti-biotin antibody, as described previously
(28,31). For statistical analysis, more than
10,000 cancer cells/patient were counted, and the number of
TUNEL-positive cells was expressed per 1,000 of the total cells
(mean ± SEM). Data for different groups were compared for
statistical difference using Student t-test. P<0.05 was
considered to indicate a statistically significant difference.. The
IHC and TUNEL scoring was performed by a single pathologist (Y.
SHI) following consultation with another pathologist (G. CHEN) and
in the absence of information on patient's outcome or
pathology.
Statistical analysis
The X-tile software program (v3.6.1; Yale University
School of Medicine, New Haven, CT, USA) as described previously
(32) was used to determine the best
cutoffs of RUNX3 by dichotomizing them into high and low expression
subgroups. The SPSS v24.0 statistical software package (SPSS Inc,
Chicago, IL, USA) was employed for all analyses. The association
between tested marker and different clinicopathologic parameters of
the patients, including age, gender, histology, ECOG PS, smoking
status, differentiation SCC, lymphatic vessels invasion, nerve
invasion, pleural invasion, vascular invasion, T-staging,
N-staging, M-staging, TNM-staging, resectibility, depth of
invasiveness, postoperative regional relapse, postoperative
metastasis, serum CEA level and expression of Ki-67 were evaluated
by Pearson's chi-square test, Yates' continuity correction
chi-square test, Fisher's exact test or Spearman's rank correlation
as appropriate. The Kaplan-Meier method with log-rank test was used
to estimate probability of OS. P<0.05 was considered to indicate
a statistically significant difference.
Results
Clinicopathological data of
patients
As shown in Table I,
the diagnosis of 5 normal lung specimen was identically
pneumothorax. A total of 3 males and 2 females were enrolled, with
an average age of 60.2 years old. The NSCLC patient population
included 128 males and 60 females and had a mean age of 58 years
old. By histological classification, 75 cases were SCC and 113 were
ADE. In the SCC group, the well-, moderately- and poorly
differentiated numbers were 4, 57 and 14, respectively. In the ADE
group, the predominant growth pattern numbers for lepidic, acinar,
papillary, micropapillary and solid were 10, 75, 10, 1 and 17,
respectively. As for ECOG performance score the number of <2 and
≥2 in ADE and SCC was 86 and 27, as well as 59 and 16,
respectively. In ADE group, 56 were smokers and 57 were nonsmokers,
while in SCC group, the number was 60 and 15. Serum CEA level was
found abnormal in 55 ADE and 19 SCC patients. Pleural involvement
was positive in 92 ADE and 44 SCC patients. Thirteen ADE and 4 SCC
patients were positive of vascular invasion. Fifty-four ADE and 31
SCC patients were positive of lymphatic vessel involvement, while
others all negative. Nerve invasion was positive in 1 case in ADE
patients, while none was found in SCC patients. Number of negative,
nuclear, cytoplasmic and whole-cell localization of RUNX3
expression in ADE was 35, 3, 38, 37 and 20, 7, 14, 34 in SCC
respectively. Number of nuclear and non-nuclear expression in ADE
was 40 and 73 while in SCC was 41 and 34. As for TNM-staging, the
number of stage I through IV was 43, 21, 43 and 6 in ADE, and 25,
20, 30 and 0 in SCC. For T-staging, the number of stage 1a, 1b, 1c,
2a, 2b, 3 and 4 was 1, 7, 13, 58, 15, 12 and 7 in ADE, and 1, 6, 5,
24, 8, 14 and 17 in SCC. For N-staging, the number of stage from 0
through 3 was 54, 17, 31 and 11 in ADE, and 43, 15, 15 and 2 in
SCC. For M-staging, the number of stage 0, 1a, 1b and 1c was 107,
3, 2 and 1 in ADE, and 75, 0, 0 and 0 in SCC. Twenty-three ADE and
14 SCC patients received postoperative radiotherapy. Fifty-seven
ADE and 41 SCC patients received postoperative chemotherapy.
Nineteen ADE and 13 SCC patients received postoperative
chemoradiation. Postoperative regional relapse was present in 27
ADE and 20 SCC patients, while postoperative distant metastasis was
found in 43 ADE and 24 SCC patients. Postoperative follow-up data
were available for all patients, and the median follow-up time in
ADE and SCC groups were 60.8 and 60.6 months, respectively.
 | Table I.Clinicopathological parameters of
patients. |
Table I.
Clinicopathological parameters of
patients.
|
| No. of cases
(%) |
|---|
|
|
|
|---|
| Variables | Adenocarcinoma | SCC |
|---|
| Median age
(years) | 57.5 | 59.0 |
| Median follow-up
(months) | 60.8 | 60.6 |
| Age (years) |
|
|
|
≤58 | 64 (56.6) | 39 (52.0) |
|
>58 | 49 (43.4) | 36 (48.0) |
| Gender |
|
|
|
Male | 63 (55.8) | 65 (86.7) |
|
Female | 50 (44.2) | 10 (13.3) |
|
Histology | 113 (60.1) | 75 (39.9) |
| ECOG PS |
|
|
|
<2 | 86 (76.1) | 59 (78.7) |
| ≥2 | 27 (23.9) | 16 (21.3) |
| Smoking status |
|
|
|
Yes | 56 (49.6) | 60 (80.0) |
| No | 57 (50.4) | 15 (20.0) |
| Serum CEA
(ng/ml) |
|
|
|
<4.7 | 58 (51.3) | 56 (74.7) |
|
≥4.7 | 55 (48.7) | 19 (25.3) |
| Pleural
invasion |
|
|
|
Yes | 92 (81.4) | 44 (58.7) |
| No | 21 (18.6) | 31 (41.3) |
| Vascular
invasion |
|
|
|
Yes | 13 (11.5) | 4 (5.3) |
| No | 100 (88.5) | 71 (94.7) |
| Lymphatic vessels
invasion |
|
|
|
Yes | 54 (47.8) | 31 (41.3) |
| No | 59 (52.2) | 44 (58.7) |
| Nerve invasion |
|
|
|
Yes | 0 (0) | 0 (0) |
| No | 113 (100) | 75 (100) |
| Localization of
RUNX3 |
|
|
|
Negative | 35 (31.0) | 20 (26.7) |
|
Nucleus | 3 (2.7) | 7 (8.9) |
|
Cytoplasm | 38 (33.6) | 14 (18.7) |
|
Whole-cell | 37 (32.7) | 34 (45.3) |
| Nuclear RUNX3
expression |
|
|
|
Yes | 40 (35.4) | 41 (54.7) |
| No | 73 (64.6) | 34 (45.3) |
| TNM staging |
|
|
| I | 43 (38.1) | 25 (33.3) |
| II | 21 (18.6) | 20 (26.7) |
|
III | 43 (38.1) | 30 (40.0) |
| IV | 6 (5.2) | 0 (0) |
| T-staging |
|
|
| 1a | 1 (0.9) | 1 (1.2) |
| 1b | 7 (6.2) | 6 (8.0) |
| 1c | 13 (11.5) | 5 (6.7) |
| 2a | 58 (51.3) | 24 (32.0) |
| 2b | 15 (13.3) | 8 (10.7) |
| 3 | 12 (10.6) | 14 (18.7) |
| 4 | 7
(6.2) | 17 (22.7) |
| N-staging |
|
|
| 0 | 54 (47.8) | 43 (57.3) |
| 1 | 17 (15.0) | 15 (20.0) |
| 2 | 31 (27.4) | 15 (20.0) |
| 3 | 11 (9.8) | 2
(2.7) |
| M-staging |
|
|
| 0 | 107 (94.7) | 75 (100) |
| 1a | 3 (2.7) | 0 (0) |
| 1b | 2 (1.8) | 0 (0) |
| 1c | 1 (0.8) | 0 (0) |
| Differentiation
(SCC) |
|
|
|
Well | / | 4 (5.3) |
|
Moderately | / | 57 (76.0) |
|
Poorly | / | 14 (18.7) |
| Predominant growth
pattern |
|
|
|
Lepidic | 10 (8.8) | / |
|
Acinar | 75 (66.4) | / |
|
Papillary | 10 (8.8) | / |
|
Micropapillary | 1 (1.0) | / |
|
Solid | 17 (15.0) | / |
| PORT |
|
|
|
Yes | 23 (20.4) | 14 (18.7) |
| No | 90 (79.6) | 61 (81.3) |
| Postoperative
chemotherapy |
|
|
|
Yes | 57 (50.4) | 41 (54.7) |
| No | 56 (49.6) | 34 (45.3) |
| Postoperative
chemoradiation |
|
|
|
Yes | 19 (16.8) | 13 (17.3) |
| No | 94 (83.2) | 62 (82.7) |
| Postoperative
regional relapse |
|
|
|
Yes | 27 (23.9) | 20 (26.7) |
| No | 86 (76.1) | 55 (73.3) |
| Postoperative
distant metastasis |
|
|
|
Yes | 43 (38.1) | 24 (32.0) |
| No | 70 (61.9) | 51 (68.0) |
Expression level of RUNX3 in NSCLC
tissues and its correlation with clinicopathologic variables and
OS
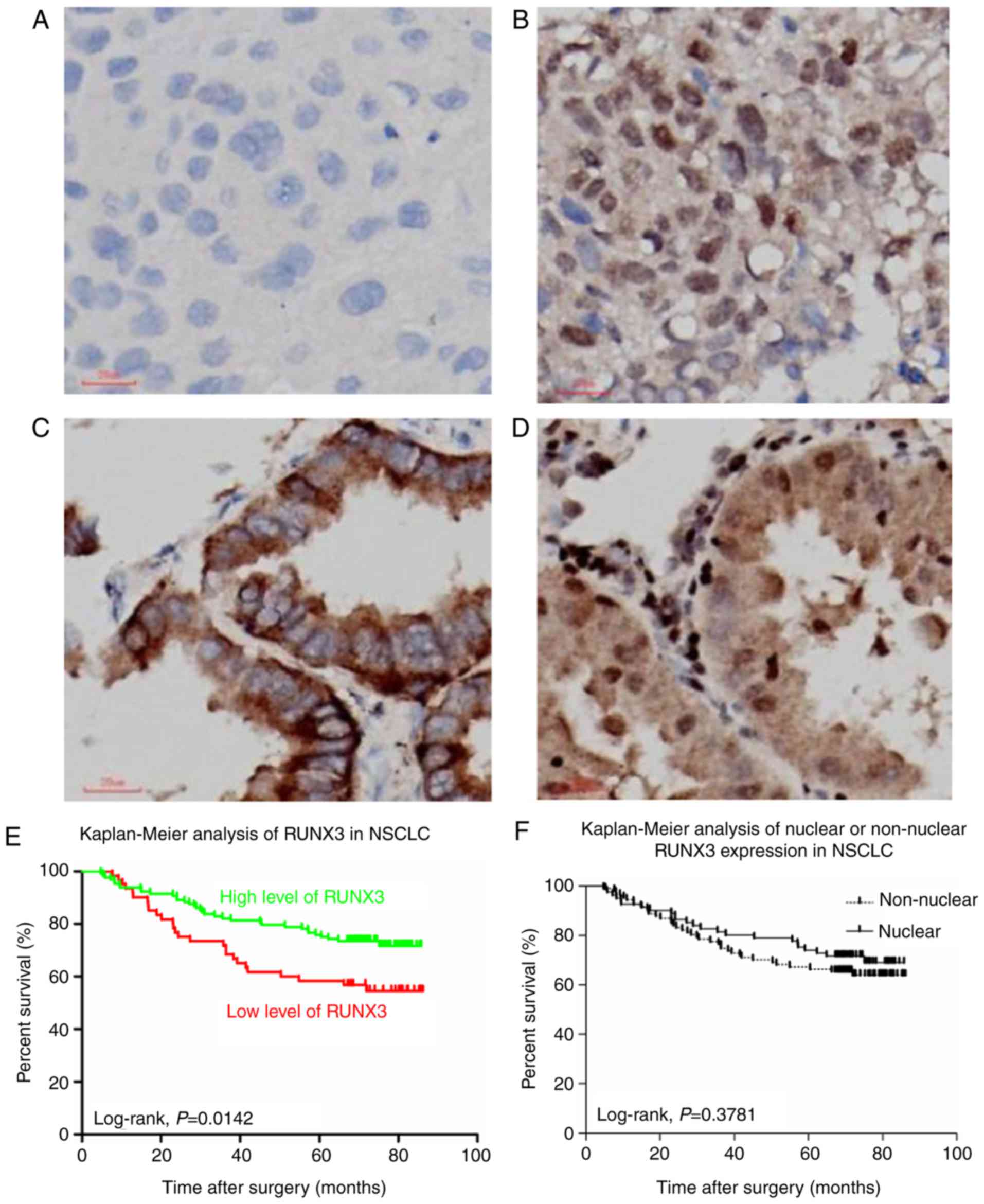
H&E staining of normal lung and NSCLC tissue
demonstrated the nomaly of histological status used in our study
(Fig. 1A, C, E). RUNX3 was only
localized in nuclei of alveolar type II pneumocytes or Clara cells
in normal lung tissue (33), while in
NSCLCs, however, besides nuclei, it could also be localized in
cytoplasm or both (Fig. 1B, D, F).
First of all, we divided the cases into subgroups of high and low
expression of RUNX3 based on the cutoff point determined by X-tile
software, regardless of the localization of its expression and
analyzed the correlation between expression levels and
clinicopathologic parameters. Later on, we set out to analyze the
association between localization of RUNX3 expression and the
clinicopathologic parameters, still using the same cutoff to
differentiate positive from negative expression. Calculated
staining score of immunopositive cells for RUNX3 ranged from 0 to
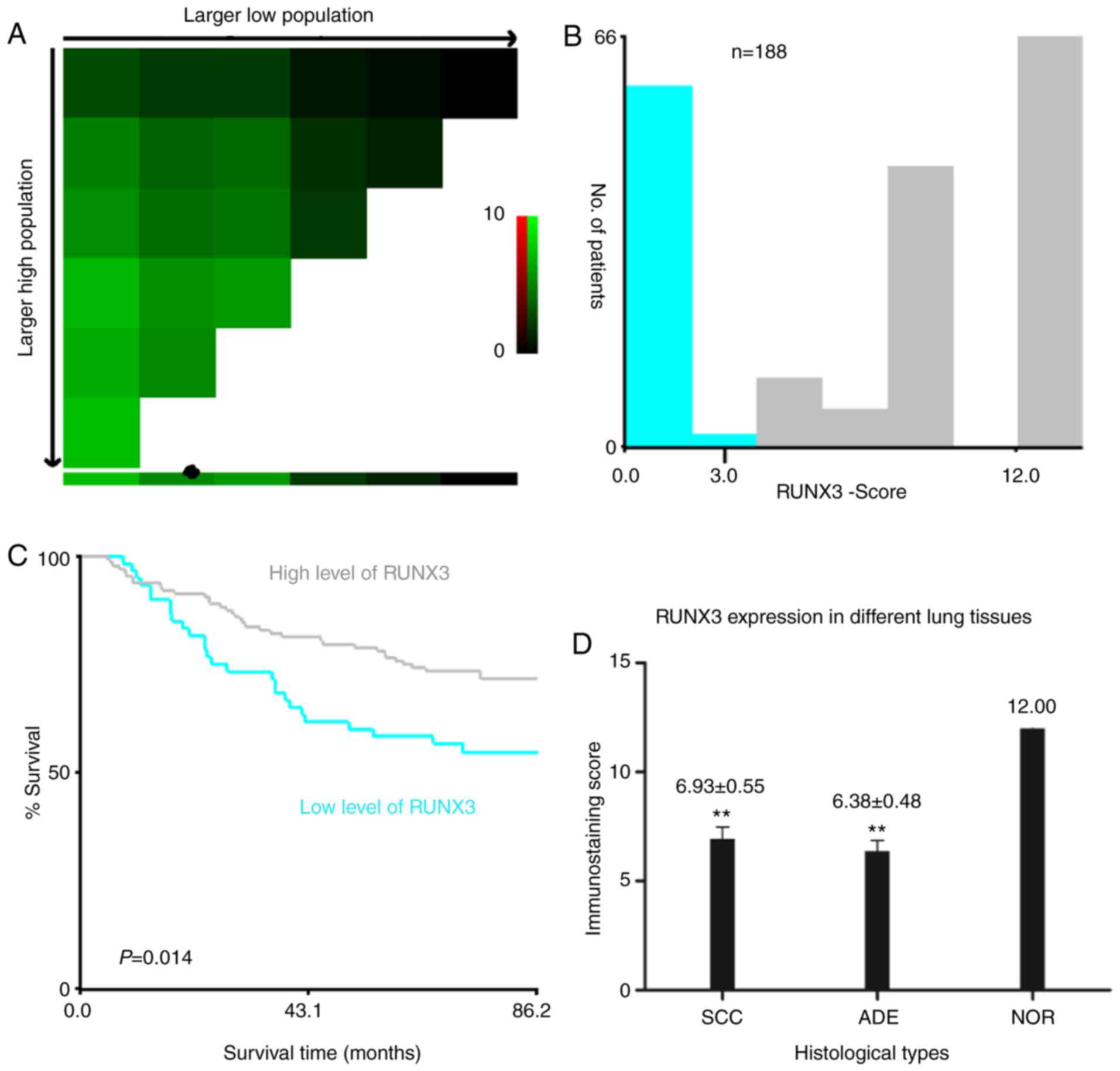
12 in all tested tissues. According to the X-tile plots (Fig. 2A-C), we categorized the cancerous
samples into low (IHC score ≤3) and high (IHC score >3)
expression subgroups for RUNX3 based on a cutoff point determined
by X-tile software related to survival time and status. As shown in
Fig. 2D, the staining score of RUNX3
was significantly higher in normal lung tissue compared to SCC or
ADE (12.00 vs. 6.93±0.55 vs. 6.38±0.48, **P<0.01). Among all 188
NSCLC cases, 55 (29%) were negative of RUNX3 expression and the
other 133 were positive, either in nucleus, cytoplasm or both.
Among the 133 positive individuals, 10 (5%) were localized in
nucleus, 52 (28%) in cytoplasm, and 71 (38%) in both nucleus and
cytoplasm (Fig. 3A-D, Table I).
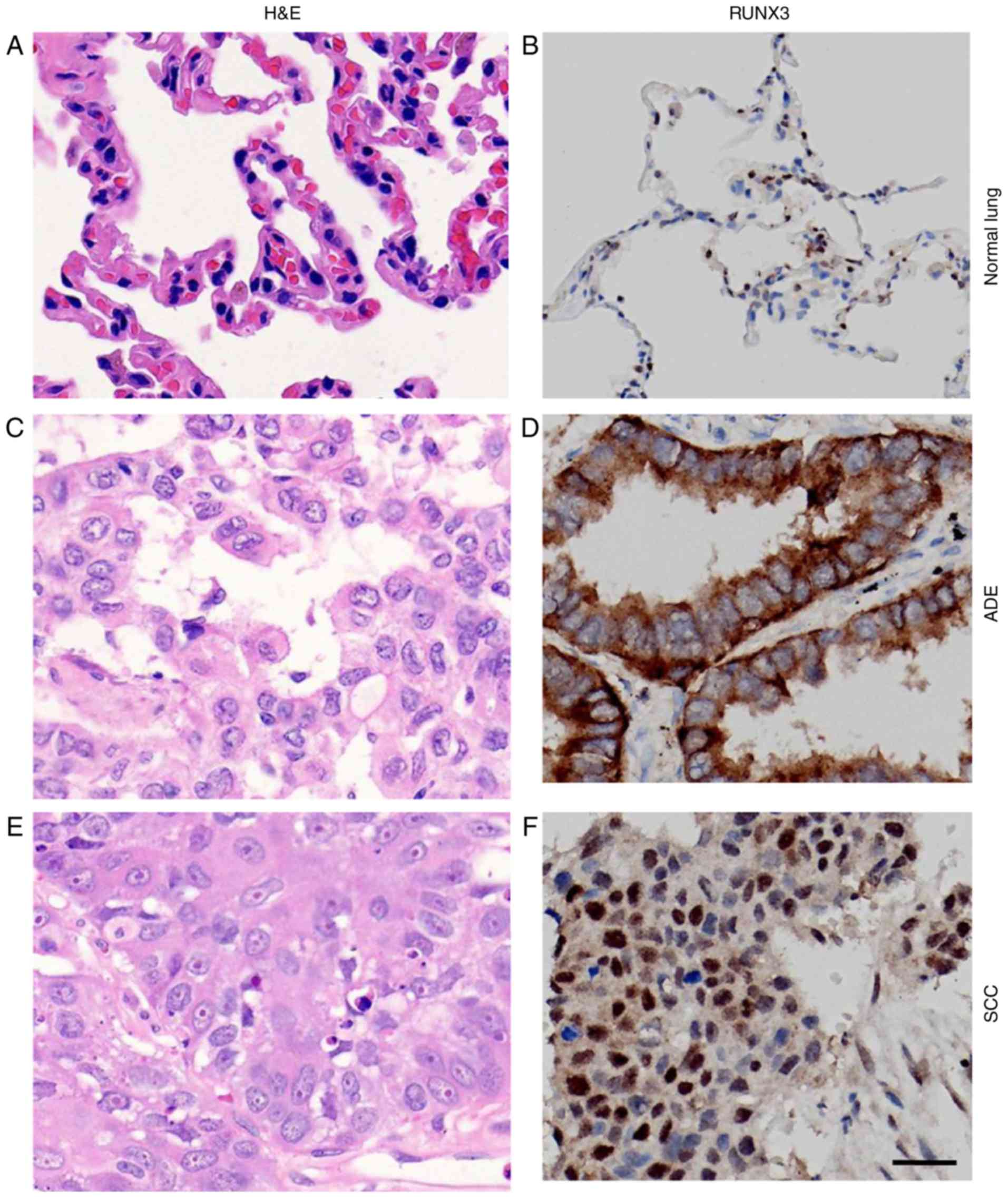 | Figure 1.Immunostaining for RUNX3 in normal
lung tissue and NSCLCs. (A, C and E) H&E staining and (B, D and
F) immunostaining for RUNX3 in (A, B) normal lung tissue, (C, D)
ADE tissues and (E, F) SCC tissues (magnification, ×400; scale
bar=20 µm). RUNX3, runt-related transcription factor 3; NSCLC,
non-small cell lung cancer; H&E, hematoxylin and eosin; ADE,
adenocarcinoma; SCC, squamous cell carcinoma. |
Correlation of RUNX3 expression and
clinicopathologic parameters were determined by means of
χ2 analysis (Table II),
revealing that higher RUNX3 expression was significantly associated
with patients with advanced age (P=0.025), lower ECOG PS (P=0.019)
as well as absence of postoperative metastasis (P=0.002). In
addition, higher RUNX3 expression was observed to have a trend to
correlate with absence of LN metastasis (P=0.062) as well as
absence of distant metastasis at diagnosis (P=0.083). Eventually,
no association had been discovered between expression of RUNX3 and
gender (P=0.196), histology (P=0.536), smoking status (P=0.995),
cellular differentiation (SCC only, P=1.000), lymphatic vessels
invasion (P=0.223), nerve invasion (P=0.492), pleural invasion
(P=0.364), vascular invasion (P=0.437), T-staging (P=0.488),
mediastinal LN involvement (P=0.464), TNM-staging (P=0.230 or
P=0.579), degree of resectibility (P=0.788), depth of invasion
(P=0.757), serum CEA level (P=0.160), postoperative regional
relapse (P=0.148) or Ki-67 expression (P=0.701). Survival analysis
by Kaplan-Meier method with log-rank test indicated that patients
with high level of RUNX3 exhibited much better outcome and longer
OS than those with low RUNX3 expression (P=0.0142; Fig. 3E), regardless of various localizations
of RUNX3 expression.
 | Table II.Associations between expression level
or localization of RUNX3 and clinicopathological parameters in
patients with NSCLC. |
Table II.
Associations between expression level
or localization of RUNX3 and clinicopathological parameters in
patients with NSCLC.
|
| RUNX3b level | RUNX3
localization |
|---|
|
|
|
|
|---|
| Parameters | All | H | L | P-value | All | Nuclear | Non-nuclear | P-value |
|---|
| Agea (years) |
|
|
| 0.025 |
|
|
| 0.911 |
|
≤58 | 103 | 63 | 40 |
| 103 | 44 | 59 |
|
|
>58 | 85 | 65 | 20 |
| 85 | 37 | 48 |
|
| Gender |
|
|
| 0.196 |
|
|
| 0.125 |
|
Female | 60 | 37 | 23 |
| 60 | 21 | 39 |
|
|
Male | 128 | 91 | 37 |
| 128 | 60 | 68 |
|
| Histology |
|
|
| 0.536 |
|
|
| 0.125 |
|
ADE | 113 | 75 | 38 |
| 113 | 40 | 73 |
|
|
SCC | 75 | 53 | 22 |
| 75 | 41 | 34 |
|
| ECOG PS |
|
|
| 0.019 |
|
|
| 0.125 |
|
<2 | 145 | 105 | 40 |
| 145 | 64 | 81 |
|
| ≥2 | 43 | 23 | 20 |
| 43 | 17 | 26 |
|
| Smoker |
|
|
| 0.995 |
|
|
| 0.360 |
|
Yes | 116 | 79 | 37 |
| 116 | 53 | 63 |
|
| No | 72 | 49 | 23 |
| 72 | 28 | 44 |
|
| Differentiation
(SCC) |
|
|
| 1.000c |
|
|
| 0.697 |
|
Poorly | 14 | 10 | 4 |
| 14 | 7 | 7 |
|
|
Well+moderately | 61 | 43 | 18 |
| 61 | 34 | 27 |
|
| Lymphatic vessels
invasion |
|
|
| 0.223 |
|
|
| 0.050 |
|
Yes | 85 | 54 | 31 |
| 85 | 30 | 55 |
|
| No | 103 | 74 | 29 |
| 103 | 51 | 52 |
|
| Nerve invasion |
|
|
| 0.492c |
|
|
| 0.431c |
|
Yes | 1 | 1 | 0 |
| 1 | 1 | 0 |
|
| No | 187 | 127 | 60 |
| 187 | 80 | 107 |
|
| Pleural
invasion |
|
|
| 0.364 |
|
|
| 0.236 |
|
Yes | 136 | 90 | 46 |
| 136 | 55 | 81 |
|
| No | 52 | 38 | 14 |
| 52 | 26 | 26 |
|
| Vascular
invasion |
|
|
| 0.437c |
|
|
| 0.496 |
|
Yes | 17 | 13 | 4 |
| 17 | 6 | 11 |
|
| No | 171 | 115 | 56 |
| 171 | 75 | 96 |
|
| T-staging |
|
|
| 0.488 |
|
|
| 0.137 |
|
T1-2 | 138 | 92 | 46 |
| 138 | 55 | 83 |
|
|
T3-4 | 50 | 36 | 14 |
| 50 | 26 | 24 |
|
| N-staging-1 |
|
|
| 0.062 |
|
|
| 0.034 |
| N0 | 97 | 72 | 25 |
| 97 | 49 | 48 |
|
|
N1-3 | 91 | 56 | 35 |
| 91 | 32 | 59 |
|
| N-staging-2 |
|
|
| 0.464 |
|
|
| 0.652 |
|
N0-1 | 129 | 90 | 39 |
| 129 | 57 | 72 |
|
|
N2-3 | 59 | 38 | 21 |
| 59 | 24 | 35 |
|
| M-staging |
|
|
| 0.083c |
|
|
| 0.701c |
| M0 | 182 | 126 | 56 |
| 182 | 79 | 103 |
|
| M1 | 6 | 2 | 4 |
| 6 | 2 | 4 |
|
| TNM staging-1 |
|
|
| 0.230 |
|
|
| 0.774 |
|
I–II | 109 | 78 | 31 |
| 109 | 46 | 63 |
|
|
III–IV | 79 | 50 | 29 |
| 79 | 35 | 44 |
|
| TNM staging-2 |
|
|
| 0.579 |
|
|
| 0.602 |
| I | 68 | 48 | 20 |
| 68 | 31 | 37 |
|
|
II–IV | 120 | 80 | 40 |
| 120 | 50 | 70 |
|
| Resectibility |
|
|
| 0.788 |
|
|
| 0.868 |
| R0 | 145 | 98 | 47 |
| 145 | 62 | 83 |
|
|
R1-2 | 43 | 30 | 13 |
| 43 | 19 | 24 |
|
| Invasiveness |
|
|
| 0.757 |
|
|
| 0.240 |
|
IS/MI | 24 | 17 | 7 |
| 24 | 13 | 11 |
|
|
Invasive | 164 | 111 | 53 |
| 164 | 68 | 96 |
|
| Serum CEA
(µg/ml) |
|
|
| 0.160 |
|
|
| 0.790 |
|
≤4.7 | 114 | 82 | 32 |
| 114 | 50 | 64 |
|
|
>4.7 | 74 | 46 | 28 |
| 74 | 31 | 43 |
|
| Postoperative
regional relapse |
|
|
| 0.148 |
|
|
| 0.671 |
|
Yes | 47 | 28 | 19 |
| 47 | 19 | 28 |
|
| No | 141 | 100 | 41 |
| 141 | 62 | 79 |
|
| Postoperative
metastasis |
|
|
| 0.002 |
|
|
| 0.035 |
|
Yes | 67 | 36 | 31 |
| 67 | 22 | 45 |
|
| No | 121 | 92 | 29 |
| 121 | 59 | 62 |
|
| Ki-67 |
|
|
| 0.701 |
|
|
| 0.377 |
|
≤10% | 108 | 73 | 36 |
| 109 | 44 | 65 |
|
|
>10% | 79 | 55 | 24 |
| 79 | 37 | 42 |
|
Localization of RUNX3 expression in NSCLC tissues
and its correlation with clinicopathologic variables and OS. In
accordance with Ito et al (23), after determining the cutoff value of
RUNX3 expression, we also categorized all cases into nuclear
(positive in the nucleus and positive or negative in the cytoplasm)
and non-nuclear groups (including: i) negative in both nucleus and
cytoplasm and ii) positive in the cytoplasm while negative in the
nucleus) (Fig. 3A-D). As a result,
among 188 NSCLC patients, nuclear localization of RUNX3 was
observed in 40 cases in ADE histology and 41 in SCC histology,
while non-nuclear was 73 in ADE and 34 in SCC (Table I).
As shown in Table II,
correlation of localization of RUNX3 expression and
clinicopathologic parameters were analyzed and found that
non-nuclear localization was significantly associated with ADE
histology (P=0.009), lymphatic vessels invasion (P=0.050), lymph
node involvement (P=0.034) and postoperative metastasis (P=0.035).
No correlation had been determined between localization of RUNX3
expression and age (P=0.911), gender (P=0.125), ECOG PS (P=0.592),
smoking status (P=0.360), cellular differentiation (SCC only,
P=1.000), nerve invasion (P=0.431), pleural invasion (P=0.236),
vascular invasion (P=0.496), T-staging (P=0.137), mediastinal LN
involvement (P=0.652), M-staging (P=0.701), TNM-staging (P=0.774 or
P=0.602), degree of resectibility (P=0.868), depth of invasion
(P=0.240), serum CEA level (P=0.790), postoperative regional
relapse (P=0.671) or Ki-67 expression (P=0.377). However, when
focusing on the effect that different localization of RUNX3
expression might have on OS via Kaplan-Meier analysis, we found
that no statistical significance had been discovered between
patient groups of nuclear and non-nuclear localization of RUNX3
expression (P=0.3781; Fig. 3F). It
had to be noticed that when categorized into 4 subgroups, i.e.,
negative, nuclear, cytoplasmic and whole-cell expression of RUNX3,
patients with negative expression in both nuclear and cytoplasm
showed the worst outcome and shortest OS, while the other 3
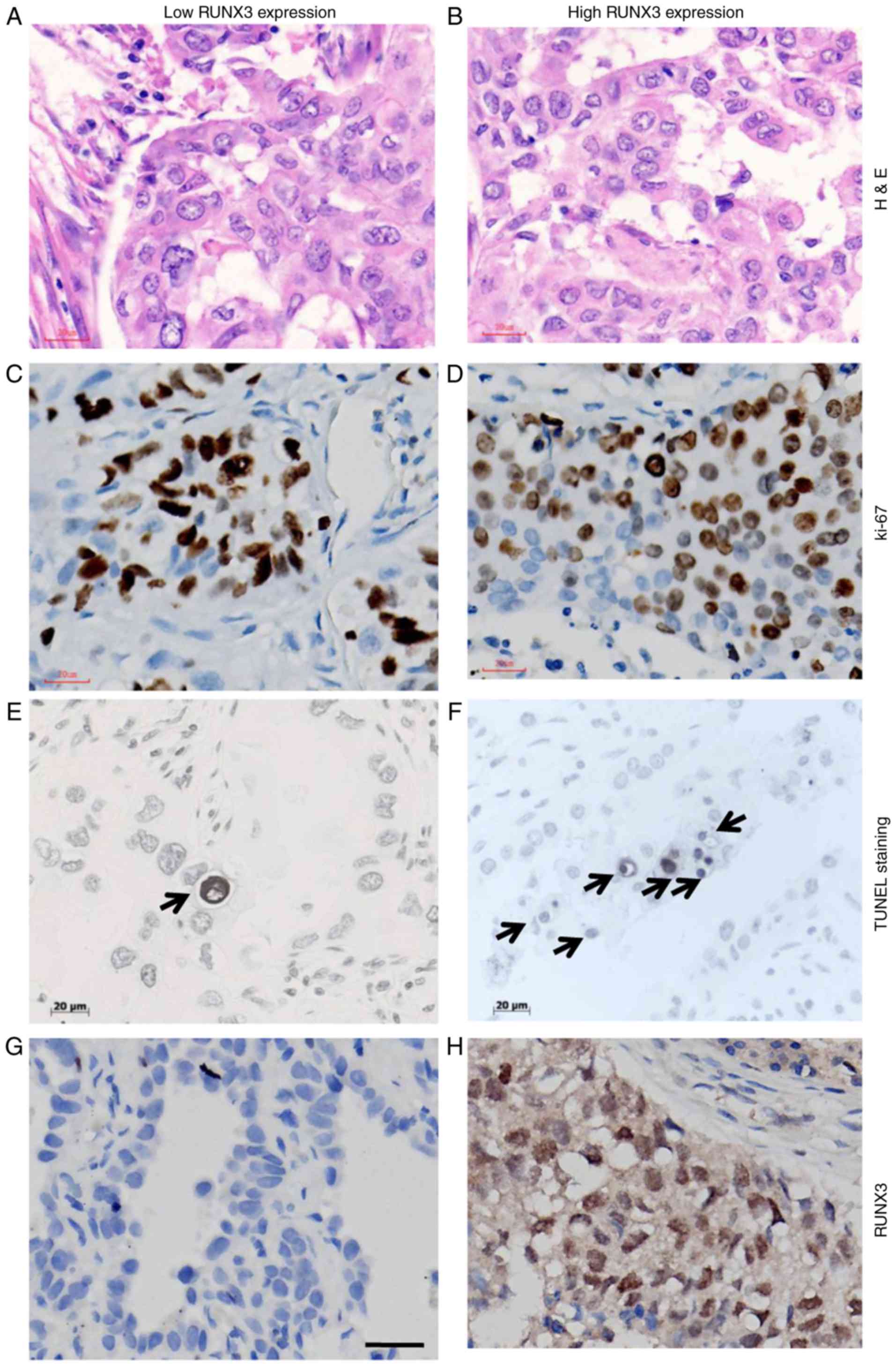
subgroups had no difference in OS with one other (Fig. 4A).
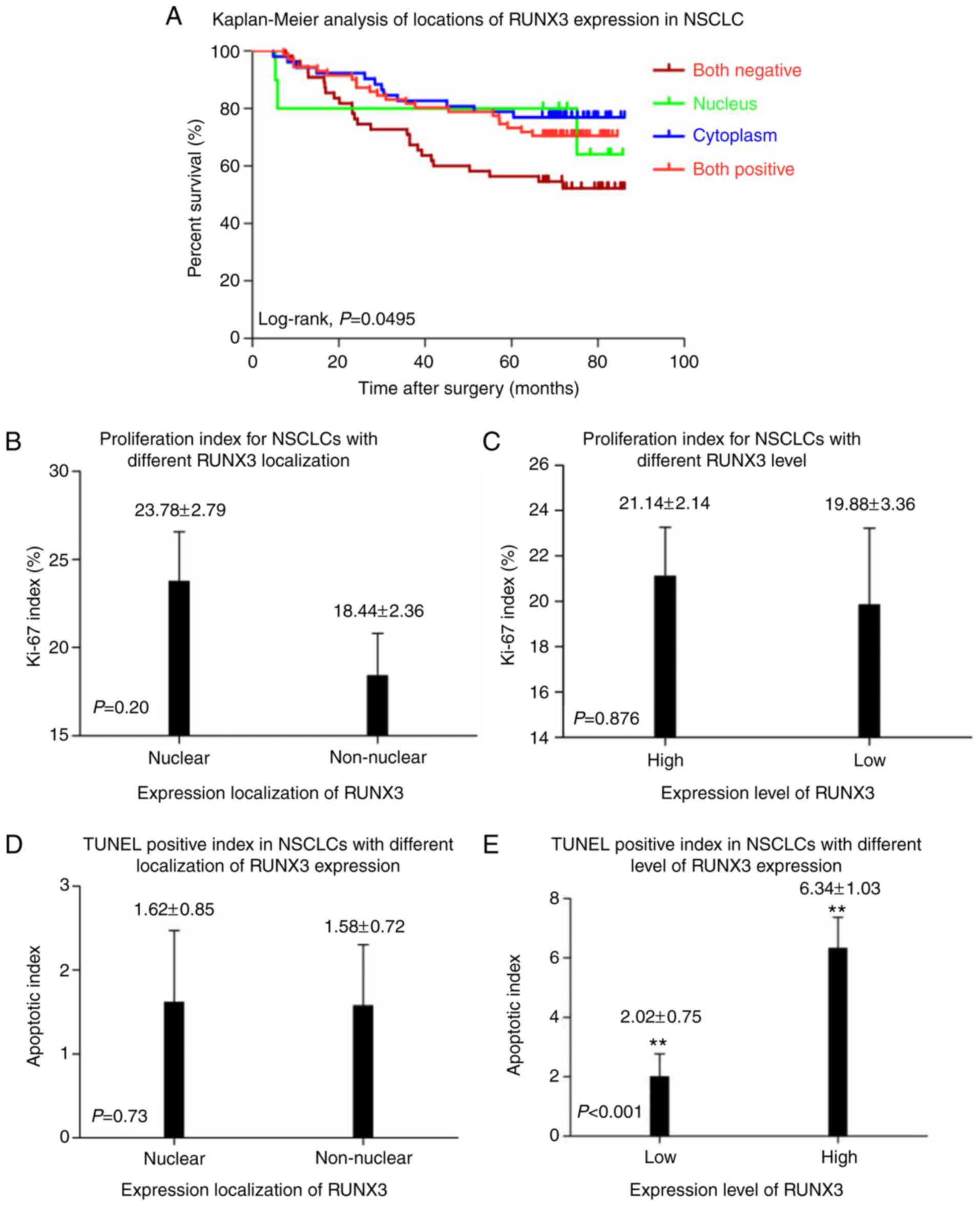 | Figure 4.(A) Kaplan-Meier curves of overall
survival in different localizations of RUNX3 expression in patients
NSCLC: Negative, nucleus, cytoplasm, and nucleus and cytoplasm
(P=0.0495). Patients with negative expression exhibited the worst
survival, while no statistical significance of overall survival was
observed among nuclear, cytoplasmic and whole-cell expression of
RUNX3 (P>0.05). In patients with (B) nuclear and non-nuclear
expression of RUNX3 or (C) different expression levels of RUNX3,
Ki-67 expression demonstrated no statistical difference
(P>0.05). (D) In patients with nuclear and non-nuclear
expression of RUNX3, TUNEL positive index demonstrated no
statistical difference (P=0.73). (E) Apoptotic index in patients
with higher expression level of RUNX3 prevailed significantly over
those with low expression level of RUNX3 (P<0.001). **P<0.01.
Overall survival, OS; NSCLC, non-small cell lung cancer; RUNX3,
runt-related transcription factor 3; TUNEL, terminal
deoxynucleotidyl transferase mediated dUTP-biotin nick end
labelling. |
Proliferative and apoptotic evaluation
in NSCLC patients with different levels or patterns of RUNX3
expression
As shown in Fig. 4B, C
and Table II, IHC score of Ki-67 for
nuclear and non-nuclear localization as well as high and low
expression of RUNX3 subgroups were 23.78±2.79% vs. 18.44±2.36% and
21.14±2.14% vs. 19.88±3.36%, respectively, and no statistical
significance had been determined between them (P=0.377 and 0.701
respectively). No statistical significance had been demonstrated on
apoptotic index between patients with nuclear (n=81) and
non-nuclear (n=107) localization of RUNX3 expression (1.62±0.85 vs.
1.58±0.72, P=0.73; Fig. 4D). However,
in comparison to NSCLC patients with low RUNX3 level (n=129),
higher apoptotic index had been observed in patients with high
RUNX3 expression (n=59) (6.34±1.03 vs. 2.02±0.75, P<0.001;
Fig. 4E).
The nomaly of tissue used for IHC staining of Ki-67
and TUNEL evaluation is shown in Fig. 5A
and B. Ki-67 staining was illustrated in Fig. 5C and D. As for apoptotic staining by
TUNEL method (Fig. 5E and F), cells
with the morphological characteristics of apoptosis were identified
as chromatin condensation and nuclear fragmentation, which were
accompanied by rounding up of the cell, reduction in cellular
volume (pyknosis) and retraction of pseudopodes. In addition,
formation of crescentic caps of condensed chromatin at the nuclear
periphery, and formation of apoptotic bodies could also be observed
(Arrows). The immunostaining of RUNX3 with low and high expression,
based on which patients were divided into different subgroups is
shown in Fig. 5G and H.
Discussion
Loss of expression or protein mislocalization had
both been indicated as patterns of dysfunction regarding RUNX3
expression in malignant tumors like GC, CRC and BC (23–25), which
was correlated with worse prognosis and shorter OS in these cancer
entities. Other studies also demonstrated that methylation-related
transcriptional silencing of RUNX3 played pivotal roles in the
onset and progression of malignancies like esophageal cancer,
hepatocellular carcinoma, pancreatic cancer, prostate cancer, and
lung cancer as well (34–36).
Expression RUNX3 is one of the most interesting
topics that deserves further study in a variety of cancer types. In
order to identify the significance of RUNX3 expression on NSCLC
patients, we immunohistochemically detected the expression level as
well as expression patterns like protein localization of RUNX3 in
188 patients. In the present study, 4 expression patterns were
demonstrated, i.e., negative, nuclear, cytoplasm and whole-cell
localization of RUNX3 protein. Obvious difference had been
discovered of the OS for these 4 categories of patient population,
with the negative subgroup the worst, while other 3 subgroups
almost identical in OS (P=0.0495; Fig.
4A).
Since accumulated evidences had revealed that
patients with different RUNX3 expression patterns like nuclear and
non-nuclear localization in BC, CRC and GC manifested quite
different OS, similar analysis was carried out in NSCLC in the
present study, demonstrating that non-nuclear localization of RUNX3
(including: i) negative in both nucleus and cytoplasm and ii)
positive in the cytoplasm while negative in the nucleus) was
significantly associated with ADE histology (P=0.009), lymphatic
vessels invasion (P=0.050), lymph node involvement (P=0.034) and
postoperative metastasis (P=0.035). However, Kaplan-Meier survival
analysis with log-rank test didn't indicate any statistical
difference between these two patient cohorts (P=0.3781, Fig. 3F), which was quite different from what
Soong et al (25), Kang et
al (37) and Ito et al
(23) had reported in BC, CRC and GC,
that patients with nuclear expression of RUNX3 (positive in the
nucleus and positive or negative in the cytoplasm) would definitely
have better outcome and longer survival than those with non-nuclear
expression.
Since no survival discrepancy had been demonstrated
between nuclear and non-nuclear RUNX3 localization in NSCLC, we
thus set out to analyze the data by dividing patients into
subgroups of high and low RUNX3 expression with the cutoff value
determined via X-tile software, irrespective of its expression
localization, and evaluated the associations between RUNX3
expression level and clinicopathologic parameters, showing that
higher RUNX3 expression was significantly associated with patients
with advanced age (P=0.025), lower ECOG PS (P=0.019) and absence of
postoperative metastasis (P=0.002). Surprisingly, Kaplan-Meier
survival analysis demonstrated that, in comparison to NSCLC
patients with lower RUNX3 level, those with higher level of RUNX3
exhibited much better outcome and longer OS (P=0.0142; Figs. 2A-C, 3E), as was quite different from the survival
analysis between patient subgroups with different expression
localization.
Interestingly, further analyses on the correlations
between RUNX3 expression level/pattern and clinicopathologic
parameters demonstrating that both expression level and
localization of RUNX3 were strongly correlated with postoperative
distant metastasis in NSCLC patients, that's, patients with higher
level of RUNX3 stood a less chance of metastasis (P=0.002) while
those with non-nuclear pattern of RUNX3 expression showed higher
probability of distant metastasis after surgery (P=0.035). In
addition, in analyzing the correlation between different expression
level/pattern of RUNX3 with LN status which was one of the major
prognostic factors in NSCLC, we found that non-nuclear RUNX3
expression was obviously correlated with positive LN involvement
(N1-3 vs. N0, P=0.034) and marginally correlated with lymphatic
vessels invasion (P=0.050), while no statistical relationship had
been determined when referred to expression level of RUNX3
(Table II). Further, higher level of
RUNX3 was found to be correlated with older patients (P=0.025) and
better ECOG PS (P=0.019), while neither of them significantly
correlated with expression localization (both P>0.05).
Interestingly, non-nuclear RUNX3 expression was majorly found in
patients with ADE histology (P=0.009) while expression level of
RUNX3 didn't make any difference between ADE and SCC (P=0.536). Our
previous study had demonstrated that tissue type, smoking status,
ECOG PS, postoperative relapse and postoperative distant metastasis
were several prognostic factors correlated with NSCLC patients' OS
(28), and expression level of RUNX3
was testified to be closely correlated with the some major
prognostic factors of OS like postoperative metastasis, ECOG PS,
and etc. Several recent study indicated that loss of RUNX3
expression promoted cell migration (38), cancerous angiogenesis and increased
microvessel density (39) in cancers.
Taken together, these could partially explain why RUNX3 expression
level could be the influencing factor for OS.
As had been well-documented that RUNX3 played a
pivotal role in the management of cellular proliferation as well as
apoptosis in a variety of cancer cell types, we then set out to
evaluate the proliferating as well as apoptotic index in NSCLC
patients with different level or localization of RUNX3 expression.
No statistical difference in Ki-67 expression had been demonstrated
either between patient subgroups of different expression level or
between subgroups with different localization of RUNX3 expression
(both P>0.05; Figs. 4B and C;
5C and D; Table II). However, as far as the apoptotic
index was concerned, compared to those with low level of RUNX3,
NSCLC patients with high RUNX3 level exhibited significantly higher
apoptotic index (P<0.001; Fig. 4E,
5E and F), while no statistical
significance had been determined in relation to the apoptotic index
between subgroups of nuclear and non-nuclear localization of RUNX3
expression (P=0.73; Fig. 4D). This
could partially explain why NSCLC patients with high RUNX3 would
have better outcome than those with low level, for cancer tissue
with higher RUNX3 would probably suffer from higher apoptotic index
and be more sensitive to therapeutic regimens.
The mechanism of transcription silencing or protein
mislocalization of RUNX3 had in part been illustrated as gene
mutation, hypermethylation of the promoter or exon of RUNX3 gene as
well as alteration of some specific upstream or downstream proteins
like src kinase, transforming growth factor beta (TGF-β) and AT
motif binding factor 1 (ATBF1) as well. Goh et al (40), demonstrated that overexpression of src
kinase resulted in the tyrosine phosphorylation and cytoplasmic
localization of RUNX3 in kidney and cervical cancer cell lines, and
knockdown of src or inhibition of its kinase activity resulted in
re-localization of RUNX3 to the nucleus in these cell lines.
Mabuchi and colleagues revealed that just like RUNX3, ATBF1 was
also able to shuttle between cytoplasm and nucleus in GC cells and
there was a close connection in nuclear localization between them.
It's verified that there was a physical association between ATBF1
and RUNX3, both of them could translocate from cytoplasma to
nucleus in response to TGF-β signal transduction (41). The statement above could partially
illustrate the phenomenon of different localization of RUNX3 in
some cancerous types, however, whether the same activity and
underlying mechanism would also exist and work in the context of
NSCLC or not might still need further verification in the
future.
Recently, a great number of studies demonstrated
that hypermethylation of RUNX3 promoter was mostly cancer-specific
and more frequent in ADE than in SCC histology (13,42) and
could be utilized as a molecular diagnostic marker for NSCLC. Fujii
et al (43), reported that
EZH2 was able to bind directly to the promoter of RUNX3, boosting
the methylating level of histone H3 lysine 27 (H3K27), resulting in
expression loss of RUNX3 in gastric, breast, prostate, colon and
pancreatic cancer cell lines. EZH2 can also direct DNA methylation
by recruitment of DNA methyltransferases (DNMTs) to target gene
promoters, suggesting that it may be a significant causative factor
of aberrent methylation in cancer. Our previous study (28), demonstrated that reduced EZH2
expression in NSCLC was correlated with ADE histology, non-smoking
status, low DNA methylation level at CCGG sites and decreased
cancerous proliferating activity. Interestingly, further analyses
on our previous data indicated that there was obvious difference in
expression direction of EZH2 and its catalytic substrate histone H3
lysine 27 among NSCLC patients with different smoking status (data
not shown). That is, trend of H3K27me3 and EZH2 expression was in
the same direction in the majority of smokers, while in nonsmokers,
their expression trend was mostly in the opposite direction, which
was partly consistent with Zhang et al's findings (44). Taken together, we hypothesized that
the underlying mechanism of different expression level and
localization of RUNX3 in NSCLC might probably be connected with the
epegenetic markers like H3K27me3 and its methyltransferase EZH2 in
part, and further investigation on these markers in different
expression patterns of RUNX3 might probably shed some light on the
detailed roles that RUNX3 might play in onset as well as
progression in patients with NSCLC.
Conclusively, our study indicated loss of expression
rather than cytoplasmic mislocalization of RUNX3 predicted worse
outcome in NSCLC, and could probably be used as a good biomarker in
predicting the prognosis in lung cancer patients.
Acknowledgements
The present study was supported in part by a
Grant-in-Aid for Youth Research Project from Health Administration
of Fujian Province (grant no. 2013-1-10 to X. Chen), Fujian
Provincial Foundation of Natural Science (grant no. 2016J01515 to
X. Chen), Miaopu Research Foundation of Fujian Medical University
(grant no. 2015MP032 to Y. Deng) and Startup Research Project of
Fujian Medical University (grant no. 2016QH040 to Y. Deng).
References
|
1
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ito Y and Miyazono K: RUNX transcription
factors as key targets of TGF-beta superfamily signaling. Curr Opin
Genet Dev. 13:43–47. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Coffman JA: Runx transcription factors and
the developmental balance between cell proliferation and
differentiation. Cell Biol Int. 27:315–324. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cameron ER, Blyth K, Hanlon L, Kilbey A,
Mackay N, Stewart M, Terry A, Vaillant F, Wotton S and Neil JC: The
Runx genes as dominant oncogenes. Blood Cells Mol Dis. 30:194–200.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ito Y, Osato M and Ito K: RUNX and cancer.
Ann Acad Med Singapore. 32 5 Suppl:S6–S7. 2003.PubMed/NCBI
|
|
7
|
Levanon D, Brenner O, Otto F and Groner Y:
Runx3 knockouts and stomach cancer. EMBO Rep. 4:560–564. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Levanon D, Glusman G, Bettoun D, Ben-Asher
E, Negreanu V, Bernstein Y, Harris-Cerruti C, Brenner O, Eilam R,
Lotem J, et al: Phylogenesis and regulated expression of the RUNT
domain transcription factors RUNX1 and RUNX3. Blood Cells Mol Dis.
30:161–163. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Miyazono K, Suzuki H and Imamura T:
Regulation of TGF-beta signaling and its roles in progression of
tumors. Cancer Sci. 94:230–234. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Otto F, Stock M, Fliegauf M, Fenaux P,
Preudhomme C and Lübbert M: Absence of somatic mutations within the
Runt domain of AML2/RUNX3 in acute myeloid leukaemia. Leukemia.
17:1677–1678. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li QL, Ito K, Sakakura C, Fukamachi H,
Inoue Ki, Chi XZ, Lee KY, Nomura S, Lee CW, Han SB, et al: Causal
relationship between the loss of RUNX3 expression and gastric
cancer. Cell. 109:113–124. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim R, Trubetskoy A, Suzuki T, Jenkins NA,
Copeland NG and Lenz J: Genome-based identification of cancer genes
by proviral tagging in mouse retrovirus-induced T-cell lymphomas. J
Virol. 77:2056–2062. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yanagawa N, Tamura G, Oizumi H, Takahashi
N, Shimazaki Y and Motoyama T: Promoter hypermethylation of tumor
suppressor and tumor-related genes in non-small cell lung cancers.
Cancer Sci. 94:589–592. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Anglin I and Passaniti A: Runx protein
signaling in human cancers. Cancer Treat Res. 119:189–215. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bae SC and Choi JK: Tumor suppressor
activity of RUNX3. Oncogene. 23:4336–4340. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Goel A, Arnold CN, Tassone P, Chang DK,
Niedzwiecki D, Dowell JM, Wasserman L, Compton C, Mayer RJ,
Bertagnolli MM and Boland CR: Epigenetic inactivation of RUNX3 in
microsatellite unstable sporadic colon cancers. Int J Cancer.
112:754–759. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kang GH, Lee S, Lee HJ and Hwang KS:
Aberrant CpG island hypermethylation of multiple genes in prostate
cancer and prostatic intraepithelial neoplasia. J Pathol.
202:233–240. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li QL, Kim HR, Kim WJ, Choi JK, Lee YH,
Kim HM, Li LS, Kim H, Chang J, Ito Y, et al: Transcriptional
silencing of the RUNX3 gene by CpG hypermethylation is associated
with lung cancer. Biochem Biophys Res Commun. 314:223–228. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wada M, Yazumi S, Takaishi S, Hasegawa K,
Sawada M, Tanaka H, Ida H, Sakakura C, Ito K, Ito Y and Chiba T:
Frequent loss of RUNX3 gene expression in human bile duct and
pancreatic cancer cell lines. Oncogene. 23:2401–2407. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tamura G: Promoter methylation status of
tumor suppressor and tumor-related genes in neoplastic and
non-neoplastic gastric epithelia. Histol Histopathol. 19:221–228.
2004.PubMed/NCBI
|
|
21
|
Oshimo Y, Oue N, Mitani Y, Nakayama H,
Kitadai Y, Yoshida K, Ito Y, Chayama K and Yasui W: Frequent loss
of RUNX3 expression by promoter hypermethylation in gastric
carcinoma. Pathobiology. 71:137–143. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fainaru O, Woolf E, Lotem J, Yarmus M,
Brenner O, Goldenberg D, Negreanu V, Bernstein Y, Levanon D, Jung S
and Groner Y: Runx3 regulates mouse TGF-beta-mediated dendritic
cell function and its absence results in airway inflammation. EMBO
J. 23:969–979. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ito K, Liu Q, Salto-Tellez M, Yano T, Tada
K, Ida H, Huang C, Shah N, Inoue M, Rajnakova A, et al: RUNX3, a
novel tumor suppressor, is frequently inactivated in gastric cancer
by protein mislocalization. Cancer Res. 65:7743–7750. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lau QC, Raja E, Salto-Tellez M, Liu Q, Ito
K, Inoue M, Putti TC, Loh M, Ko TK, Huang C, et al: RUNX3 is
frequently inactivated by dual mechanisms of protein
mislocalization and promoter hypermethylation in breast cancer.
Cancer Res. 66:6512–6520. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Soong R, Shah N, Peh BK, Chong PY, Ng SS,
Zeps N, Joseph D, Salto-Tellez M, Iacopetta B and Ito Y: The
expression of RUNX3 in colorectal cancer is associated with disease
stage and patient outcome. Br J Cancer. 100:676–679. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mello RB, Silva MR, Alves MT, Evison MP,
Guimarães MA, Francisco RA, Astolphi RD and Iwamura ES: Tissue
microarray analysis applied to bone diagenesis. Sci Rep.
7:399872017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Eskaros AR, Egloff SA, Boyd KL, Richardson
JE, Hyndman ME and Zijlstra A: Larger core size has superior
technical and analytical accuracy in bladder tissue microarray. Lab
Invest. 97:335–342. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen X, Song N, Matsumoto K, Nanashima A,
Nagayasu T, Hayashi T, Ying M, Endo D, Wu Z and Koji T: High
expression of trimethylated histone H3 at lysine 27 predicts better
prognosis in non-small cell lung cancer. Int J Oncol. 43:1467–1480.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Deng Y, Chen X, Ye Y, Shi X, Zhu K, Huang
L, Zhang S, Ying M and Lin X: Histological characterisation and
prognostic evaluation of 62 gastric neuroendocrine carcinomas.
Contemp Oncol (Pozn). 20:311–319. 2016.PubMed/NCBI
|
|
30
|
Gavrieli Y, Sherman Y and Ben-Sasson SA:
Identification of programmed cell death in situ via specific
labeling of nuclear DNA fragmentation. J Cell Biol. 119:493–501.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Song N, Liu J, An S, Nishino T, Hishikawa
Y and Koji T: Immunohistochemical analysis of histone H3
modifications in germ cells during mouse spermatogenesis. Acta
Histochem Cytochem. 44:183–190. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Camp RL, Dolled-Filhart M and Rimm DL:
X-tile: A new bio-informatics tool for biomarker assessment and
outcome-based cut-point optimization. Clin Cancer Res.
10:7252–7259. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Araki K, Osaki M, Nagahama Y, Hiramatsu T,
Nakamura H, Ohgi S and Ito H: Expression of RUNX3 protein in human
lung adenocarcinoma: Implications for tumor progression and
prognosis. Cancer Sci. 96:227–231. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang C, Li J, Huang T, Duan S, Dai D,
Jiang D, Sui X, Li D, Chen Y, Ding F, et al: Meta-analysis of DNA
methylation biomarkers in hepatocellular carcinoma. Oncotarget.
7:81255–81267. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Whittle MC and Hingorani SR: RUNX3 defines
disease behavior in pancreatic ductal adenocarcinoma. Mol Cell
Oncol. 3:e10765882015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chen F, Liu X, Bai J, Pei D and Zheng J:
The emerging role of RUNX3 in cancer metastasis (Review). Oncol
Rep. 35:1227–1236. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kang KA, Piao MJ, Ryu YS, Maeng YH and
Hyun JW: Cytoplasmic localization of RUNX3 via histone
deacetylase-mediated SRC expression in oxidative-stressed colon
cancer cells. J Cell Physiol. 232:1914–1921. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chen F, Bai J, Li W, Mei P, Liu H, Li L,
Pan Z, Wu Y and Zheng J: RUNX3 suppresses migration, invasion and
angiogenesis of human renal cell carcinoma. PLoS One. 8:e562412013.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Xue J, Wu XL, Huang XT, Qu M, Guo F, Sun
GY, Zhang PC, Han L and Pan LM: Correlation of RUNX3 expression
with microvessel density in colorectal adenocarcinoma tissues and
clinical significance. Asian Pac J Trop Med. 10:98–101. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Goh YM, Cinghu S, Hong ET, Lee YS, Kim JH,
Jang JW, Li YH, Chi XZ, Lee KS, Wee H, et al: Src kinase
phosphorylates RUNX3 at tyrosine residues and localizes the protein
in the cytoplasm. J Biol Chem. 285:10122–10129. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Mabuchi M, Kataoka H, Miura Y, Kim TS,
Kawaguchi M, Ebi M, Tanaka M, Mori Y, Kubota E, Mizushima T, et al:
Tumor suppressor, AT motif binding factor 1 (ATBF1), translocates
to the nucleus with runt domain transcription factor 3 (RUNX3) in
response to TGF-beta signal transduction. Biochem Biophys Res
Commun. 398:321–325. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lee KS, Lee YS, Lee JM, Ito K, Cinghu S,
Kim JH, Jang JW, Li YH, Goh YM, Chi XZ, et al: Runx3 is required
for the differentiation of lung epithelial cells and suppression of
lung cancer. Oncogene. 29:3349–3361. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Fujii S, Ito K, Ito Y and Ochiai A:
Enhancer of zeste homologue 2 (EZH2) down-regulates RUNX3 by
increasing histone H3 methylation. J Biol Chem. 283:17324–17332.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhang H, Fillmore Brainson C, Koyama S,
Redig AJ, Chen T, Li S, Gupta M, Garcia-de-Alba C, Paschini M,
Herter-Sprie GS, et al: Lkb1 inactivation drives lung cancer
lineage switching governed by Polycomb Repressive Complex 2. Nat
Commun. 8:149222017. View Article : Google Scholar : PubMed/NCBI
|