Introduction
Thyroid cancer is one of the most common types of
cancer in the endocrine system, with an increasing incidence since
1980 (1). Papillary thyroid carcinoma
(PTC) accounts for 80% of all types of thyroid cancer (2). However, the molecular mechanisms
underlying PTC remain unclear and biomarkers for PTC are
required.
Long non-coding RNAs (lncRNAs), which are ~200
nucleotides long, do not encode any proteins but instead function
to regulate the expression of associated genes (3). lncRNAs are now recognized as regulators
of tumorigenesis and tumor progression (4,5). BRAF
mutations are the most common type of mutation in the lesions of
patients with PTC, occurring in 45% of cases (6). BRAF-activated non-protein coding RNA
(BANCR) is a 693-bp-long transcript on chromosome 9. It is
frequently overexpressed and may be involved in the migration of
melanoma cells (7). In non-small cell
lung cancer, BANCR promotes the migration and invasion of cancer
cells (8) through the
mitogen-activated protein kinase (MAPK) signaling pathway.
Epithelial-mesenchymal transition (EMT) refers to the process of
transformation of epithelial cells to a mesenchymal cell phenotype,
and serves an important function in tumor invasion and metastasis,
since it may promote cancer cell migration and invasion (9,10). The
association between BANCR and cellular migration, invasion, EMT and
MAPK signaling in PTC remains unclear.
Since BANCR is involved in the proliferation of PTC
cells (11), the aim of the present
study was to investigate the molecular mechanisms underlying BANCR
and EMT in PTC.
Materials and methods
Patients and tissue samples
A total of 27 patients who received surgical
resection for PTC were reviewed from January 2015 to December 2016
at the Department of General Surgery, Zhongshan Hospital of Xiamen
University (Xiamen, China). Following surgery, all tumors and
paired tissues were frozen in liquid nitrogen and stored at −80°C
for future experiments. The study was approved by the Ethics
Committee on Human Research of the Zhongshan Hospital of Xiamen
University and all patients provided written informed consent.
Cell lines and cell culture
The human PTC cell line BCPAP was purchased from the
Chinese Academy of Sciences (Beijing, China). The human
undifferentiated thyroid carcinoma cell line CAL-62 was purchased
from the Chinese Academy of Sciences. The human cell follicular
thyroid carcinoma lines WRO and FTC-133 were purchased from
Shanghai Honsun Biological Technology Co., Ltd. (Shanghai, China).
Cells were cultured in RPMI-1640 medium, 100 U/ml penicillin and
100 mg/ml streptomycin (all from Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) at 37°C in a humidified atmosphere
containing 5% CO2.
Total RNA extraction and reverse
transcription-polymerase chain reaction (RT-qPCR)
Total RNA from tissues and cells was extracted using
RNAiso Plus (Takara Biotechnology Co., Ltd., Dalian, China). RNA
was reverse-transcribed using the PrimeScript RT kit (Takara),
according to the manufacturer's instructions. The cDNA was
amplified using the SYBR Premix Ex TaqII (Takara Biotechnology Co.,
Ltd.). Relative expression values were calculated using the
2−ΔΔCq method (12,13).
Primers were obtained from Sangon Biotech (Shanghai, China) and the
sequences are presented in Table I.
The software used for analysis was LightCycler® 96
(version 1.1.0.1320; Roche Diagnostics GmbH, Mannheim, Germany).
The thermocycling conditions were as follows: Initial denaturation
at 95°C for 10 min, followed by 45 cycles of denaturation at 95°C
for 10 sec, annealing at 60°C for 30 sec, and a final cycle of
denaturation at 95°C for 10 sec, annealing at 65°C for 60 sec and
extension at 97°C for 1 sec.
 | Table I.Primer sequences. |
Table I.
Primer sequences.
| Target | Primers |
|---|
| β-actin | F:
5′-ACTGGAACTGTGAAGGTGAC-3′ |
|
| R:
5′-GTGGACTTGGGCGAGGACTG-3′ |
| Vimentin | F:
5′-GAGAACTTGGCCGTTGAAGC-3′ |
|
| R:
5′-GCTTCCTGTTGGTGGCAATC-3′ |
| E-cadherin | F:
5′-TGCCCAGAAGATGAATAAGG-3 |
|
| R:
5′-GTGTATGTGGCAATGCGTTC-3′ |
| N-cadherin | F:
5′-CTCCTATGAGTGCAACAGGAACG-3′ |
|
| R:
5′-TTGGATCAGTGTCATAATCAAGTGCTGTA-3′ |
| BANCR | F:
5′-CCTTCTTGTAGGGTCTGGATTG-3′ |
|
| R:
5′-CATTGGTGCTGCAGTCTATTTC-3′ |
Establishment of stable cell
lines
The BCPAP cell line was infected by lentivirus
containing BCPAP-NC and BCPAP-BANCR constructs [2×108
transduction units (TU)/50 µl; Genomeditech, Inc., Shanghai,
China]. Then 1×106 BCPAP cells were transfected using
lentivirus (final concentration 4×106 TU/ml) and 6 µg/ml
polybrene (Santa Cruz Biotechnology, Inc., Dallas, TX, USA) for 24
h. The efficiency of infection was evaluated using RT-qPCR. Total
RNA was extracted from BCPCP, BCPAP-NC and BCPAP-BANCR cell lines.
The reference gene was β-actin and relevant primer sequences are
presented in Table I. The instrument
for RT-qPCR and thermocycling conditions were aforementioned. The
method of quantification was 2−ΔΔCq (13).
Cell migration and invasion
assays
For the migration experiments, BCPAP
(1.5×104) cells were suspended in 200 µl serum-free
medium (RPMI-1640 medium; Gibco; Thermo Fisher Scientific, Inc.)
and seeded into the upper chamber, whereas 800 µl medium containing
10% fetal bovine serum was added to the lower chamber (6.5 mm
Transwell® with 8.0 µm Pore Polycarbonate Membrane
Insert, Sterile; cat. no. 3422; Corning Incorporated, Corning, NY,
USA). These cells were incubated for 14 h, washed with PBS and
fixed for between 15 and 20 min. Cells were washed with PBS and
stained with 0.1% crystal violet at room temperature for 10 min.
The images were captured under a light microscope (magnification,
×100; AxioVert.A1; Zeiss GmbH, Jena, Germany). Five random fields
were captured and quantified using a double-blind method. For the
invasion experiments, BCPAP (4×104) cells were cultured
for 14 h and processed as aforementioned.
Western blot analysis
Cells were washed twice with ice-cold PBS and lyzed
using radioimmunoprecipitation assay buffer (Beyotime Institute of
Biotechnology, Shanghai, China) supplemented with a protease
inhibitor (complete Mini EDTA-free tablets; Roche Applied Science,
Pleasanton, CA, USA) and phenylmethylsulfonyl fluoride (Beyotime
Institute of Biotechnology). Proteins were denatured at 100°C for
10 min and equal amounts of sample (30 µg) were separated by
SDS-PAGE (10% gels). Electrophoresed proteins were then transferred
onto polyvinylidene fluoride membranes (EMD Millipore, Billerica,
MA, USA). The membranes were blocked with 5% BSA Blocking Buffer
(Huayueyang Biotechnology Co., Ltd., Beijing, China) at room
temperature for 1 h. The densitometric analysis for the
quantification of the bands was performed using enhanced
chemiluminescence (ECL) chromogenic substrate (WesternBright ECL;
Advansta, Menlo Park, CA, USA) and ChemiDoc™ XRS System (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). β-actin was used as an
endogenous control. Membranes were incubated with following primary
antibodies at 4°C overnight: Epithelial (E)-cadherin (1:1,000; cat.
no. 3195S), neuronal (N)-cadherin (1:1,000; cat. no. 13116S),
vimentin (1:1,000; cat. no. 5741S), c-Raf (1:2,000; cat. no.
53745S), mitogen-activated protein kinase
(MAPK)/extracellular-signal-regulated protein kinase (ERK) kinase
1/2 (MEK1/2) (1:2,000; cat. no. 4694S), ERK1/2 (1:2,000; cat. no.
4370S), phospho (p)-c-Raf (1:2,000; cat. no. 9421S), p-MEK1/2
(1:2,000; cat. no. 2338S), p-ERK1/2 (1:2,000; cat. no. 4370S),
GAPDH (1:2,000; cat. no. 5174S) and β-actin (1:1,000; cat. no.
3700S) (all from Cell Signaling Technology, Inc., Danvers, MA,
USA). The following secondary antibodies used were used:
Horseradish peroxidase (HRP)-conjugated goat anti-mouse and
HRP-conjugated goat anti-rabbit immunoglobulin G antibody (Jackson
ImmunoResearch Laboratories, Inc., West Grove, PA, USA). The
inhibitor, U0126 (S1102; Selleck Chemicals, Houston, TX, USA),
blocked the Raf-MEK-Erk signaling pathway by treating cells at 37°C
for 30 min. U0126 was diluted by DMSO (20688; Thermo Fisher
Scientific, Inc.) to a final concentration of 20 µmol/l
Confocal imaging
BCPAP cells were plated at 3×104
cells/well. Cells were washed with PBS twice and fixed with
methanol for 20 min at room temperature. Cells were washed again
with PBS three times and cells were incubated at 37°C in a sealed
box for 1 h with 5% bovine serum albumin (BSA) in PBS. Cells were
then incubated with primary antibodies E-cadherin (1:150; cat. no.
3195S) and vimentin (1:200; cat. no. 5741S) (both from Cell
Signaling Technology, Inc.) which were diluted with 5% BSA to 80 µl
at 4°C overnight. Cells were then washed with PBS four times and
then incubated with fluorescent-tag-labeled secondary antibodies
(Alexa Fluor 555-phalloidin; 1:600; cat. no. 8953S; Cell Signaling
Technology, Inc.) at 37°C for 1 h in the dark. Cells were washed
with PBS four times and incubated with 100 µl DAPI at room
temperature for 10 min. Cells were washed with PBS four times and
then incubated with 8 µl Mounting medium, antifading (Beijing
Solarbio Science and Technology Co., Ltd., Beijing, China) and
dried. Images of cells were captured using a Zeiss LSM 510 system
(magnification, ×100; Carl Zeiss AG, Oberkochen, Germany).
Statistical analysis
Data were analyzed using GraphPad Prism (version
5.0; GraphPad Software, Inc., La Jolla, CA, USA). The relevant data
are expressed as the mean ± standard error of the mean. Statistical
significance among groups was assessed using Student's t-test,
one-way analysis of variance followed by least significant
difference method. P<0.05 was considered to indicate a
statistically significant difference.
Results
lncRNA BANCR expression levels in PTC
tissues and cell lines
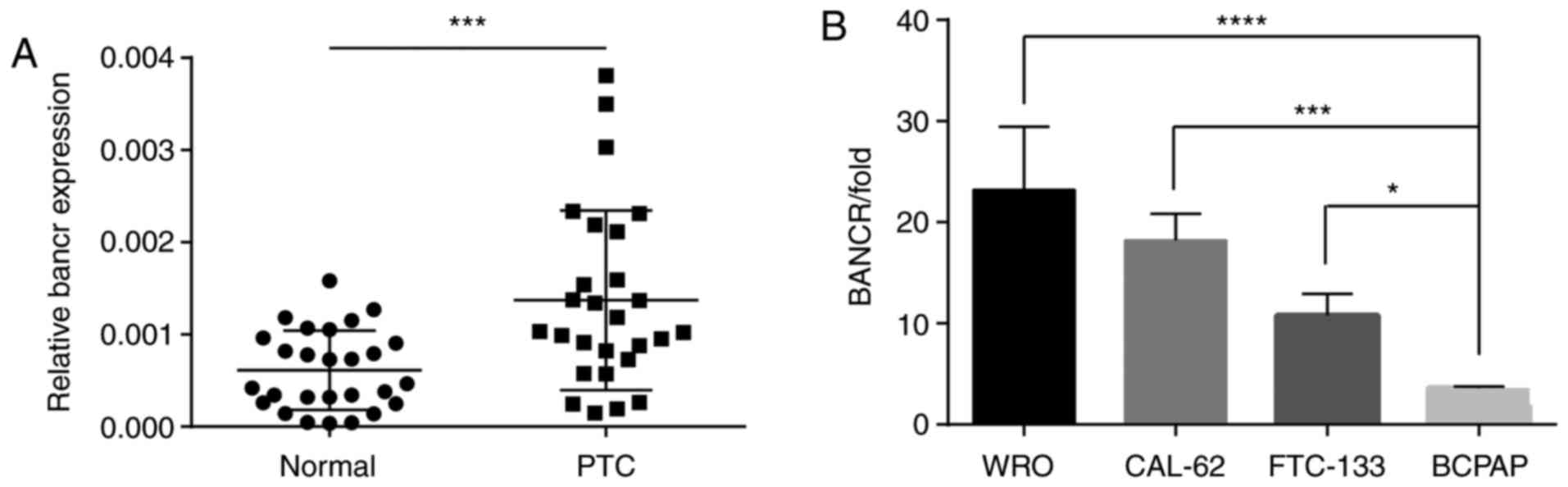
The expression level of BANCR in 27 paired tissue
samples from patients with PTC was evaluated. The BANCR level was
significantly increased in the thyroid tissues compared with the
adjacent normal tissues (Fig. 1A).
The expression of BANCR in four human thyroid cancer cell lines
were also evaluated. The cell lines differed in the expression
levels of BANCR (Fig. 1B). The BCPAP
cell line had the lowest expression level of BANCR and was
therefore employed for subsequent experiments.
lncRNA BANCR promotes migration and
invasion of PTC cells
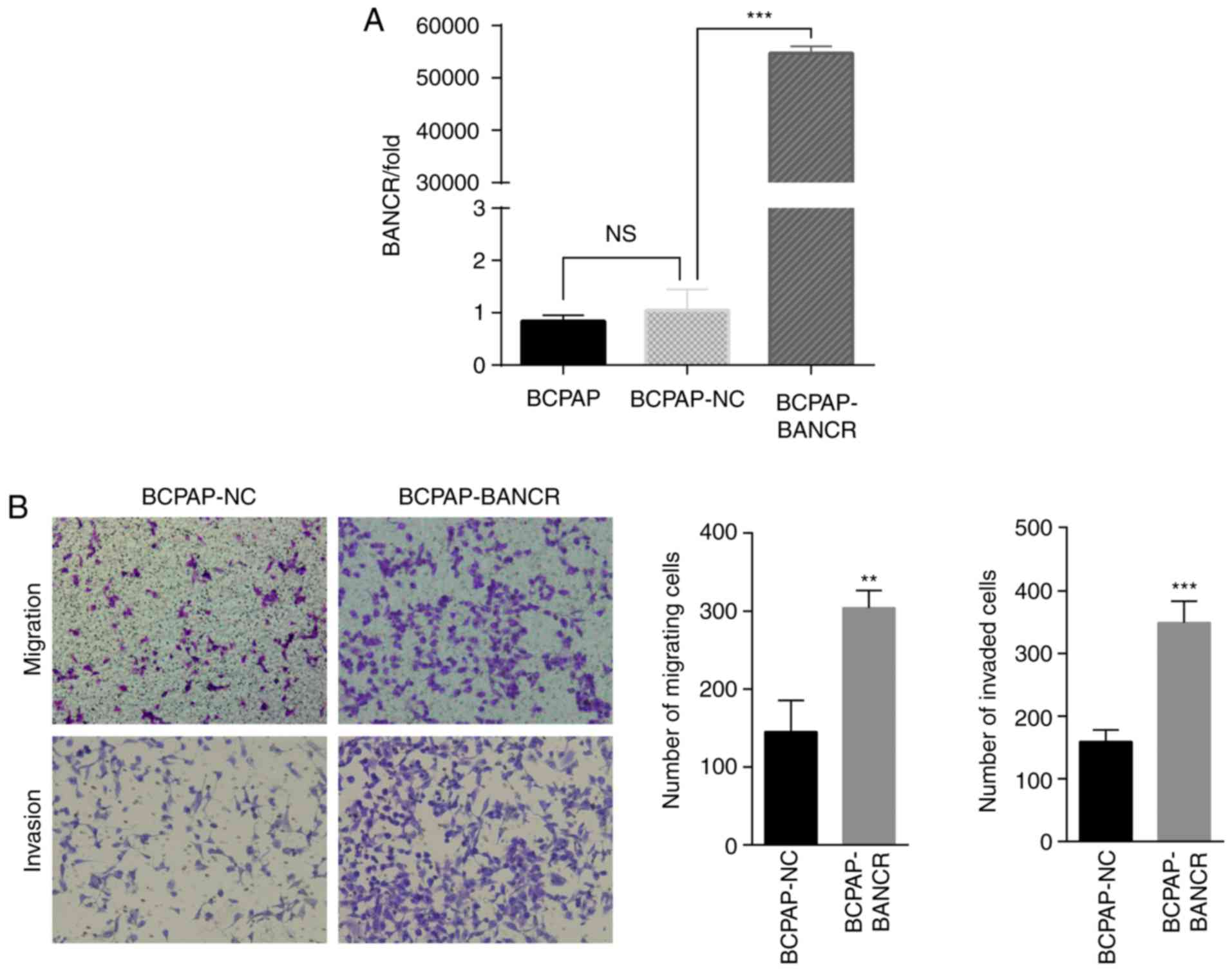
BCPAP cells were transfected with lentivirus and the
expression levels of BANCR were assessed. BANCR was significantly
upregulated in BCPAP-BANCR cells compared with the BCPAP or
BCPAP-NC (Fig. 2A). The migration and
invasion of BCPAP-BANCR cells were significantly increased compared
with the cells transfected with empty vector (Fig. 2B).
lncRNA BANCR promotes EMT in PTC cell
lines
The expression of EMT-induced markers, including
E-cadherin, N-cadherin and vimentin, was assessed in BCPAP cells
using RT-qPCR, western blot analysis and confocal microscopy. The
results indicated that upregulated expression of BANCR increased
the expression levels of N-cadherin and vimentin, but decreased the
expression of E-cadherin (Fig. 3A).
Western blot analysis and confocal microscopy also revealed that
increased BANCR expression upregulated the expression of vimentin
and decreased the expression of E-cadherin in BCPAP-BANCR cells
(Fig. 3B and C).
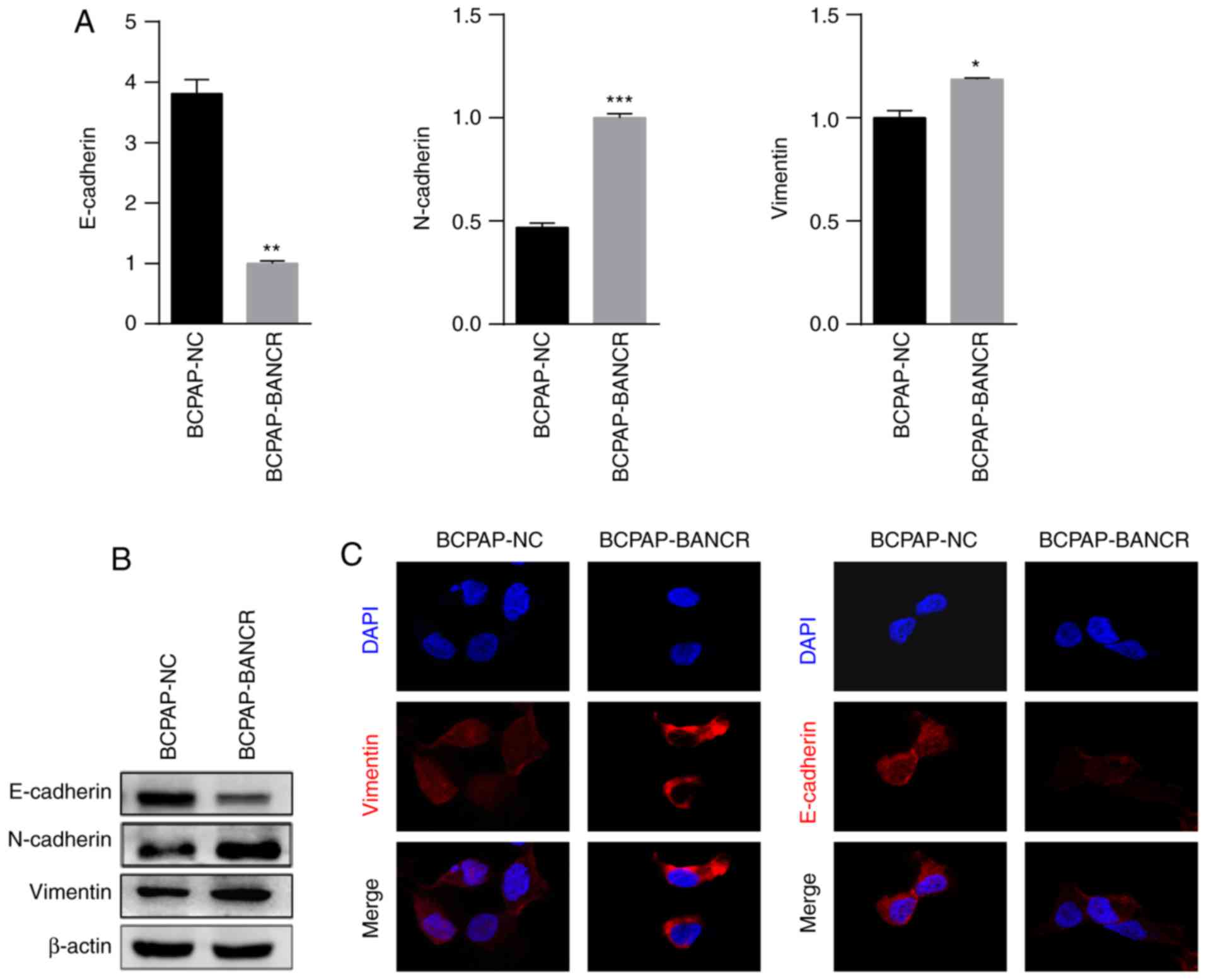 | Figure 3.BANCR overexpression promotes cellular
invasion and metastasis by inducing EMT in PTC. (A) The expression
of N-cadherin and vimentin was upregulated, and the expression of
E-cadherin was downregulated in BCPAP-BANCR cells compared with in
BCPAP-NC cells. (B) Western blot analysis of the expression of
E-cadherin, vimentin and N-cadherin in BCPAP-NC and BCPAP-BANCR
cells. (C) Expression of E-cadherin, vimentin and N-cadherin in
BCPAP-NC and BCPAP-BANCR cells as assessed using confocal
microscopy. DAPI was used to stain the nucleus. Increased BANCR
expression upregulated vimentin and downregulated E-cadherin in
BCPAP-BANCR cells. (n=3; magnification, ×100). *P<0.05,
**P<0.01, ***P<0.001 vs. BCPAP-NC cells. PTC, papillary
thyroid carcinoma; BANCR, BRAF-activated non-protein-coding RNA;
NC, negative control; E-cadherin, epithelial cadherin; N-cadherin,
neuronal cadherin. |
lncRNA BANCR regulates EMT in PTC via
the Raf/MEK/ERK signaling pathway
First, the expression levels of c-Raf, MEK1/2 and
ERK1/2 were evaluated in the BCPAP-BANCR and BCPAP-NC cells using
western blot analysis. The expression levels of p-c-Raf, p-MEK1/2
and p-ERK1/2 were significantly increased in BCPAP-BANCR cells
compared with in BCPAP-NC cells (Fig.
4A). These results suggest an association between BANCR and the
Raf/MEK/ERK signaling pathway. To test this hypothesis, BCPAP cells
were treated with the c-Raf inhibitor U0126 and used the cells
treated with DMSO as the blank control group. The results indicated
that treatment with the c-Raf inhibitor U0126 inactivated p-MEK1/2
and p-ERK1/2 in BCPAP cell lines (Fig.
4B). Therefore, U0126 may inhibit the effect of BANCR on the
Raf/MEK/ERK signaling pathway. In order to clarify the effects of
BANCR in inducing EMT via the Raf/MEK/ERK signaling pathway, the
BCPAP-NC and BCPAP-BANCR cell lines were treated with U0126 and the
expression levels of E-cadherin and vimentin were assessed using
western blot analysis (Fig. 4C). The
results demonstrated that the expression of E-cadherin was
downregulated following overexpression of BANCR in the BCPAP cell
line. However, in response to U0126 treatment, E-cadherin
expression was upregulated in both BCPAP-NC and BCPAP-BANCR cell
lines, vimentin expression was also upregulated following the
overexpression of BANCR; however, the expression was downregulated
when treated with U0126 in both BCPAP and BCPAP-BANCR cell lines
(Fig. 4C). Therefore, BANCR may
induce EMT in PTC via the Raf/MEK/ERK signaling pathway.
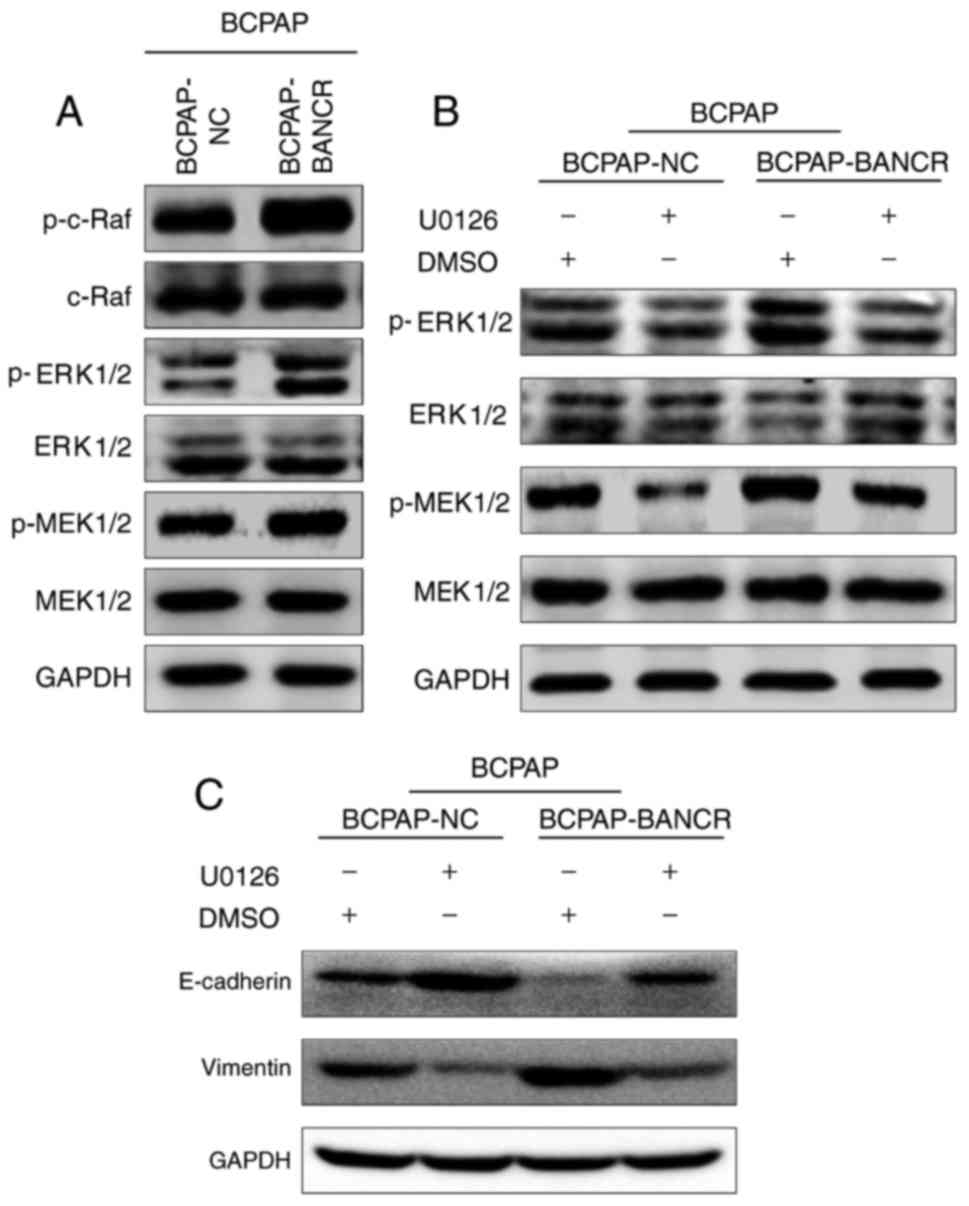 | Figure 4.BANCR induces EMT in PTC via the
Raf/MEK/ERK signaling pathway. (A) Overexpression of BANCR
upregulated the expression of p-c-Raf, p-MEK1/2 and p-ERK1/2. (B)
Following incubation with the c-Raf inhibitor U0126, the ability of
BCPAP-BANCR cells to upregulate p-c-Raf, p-MEK1/2, p-ERK1/2 was
diminished. (C) Following overexpression of BANCR, E-cadherin was
downregulated in BCPAP cells. However, in response to U0126
treatment, E-cadherin expression was upregulated and vimentin
expression was downregulated in BCPAP-BANCR cells compared with
BCPAP-NC cells. PTC, papillary thyroid carcinoma; BANCR,
BRAF-activated non-protein-coding RNA; NC, negative control;
E-cadherin, epithelial cadherin; EMT, epithelial-mesenchymal
transition; p-, phospho-; ERK, extracellular-signal-regulated
protein kinase; MEK, mitogen-activated protein kinase/ERK kinase;
DMSO, dimethyl sulfoxide. |
Discussion
lncRNAs were traditionally considered to exhibit no
cellular function since they do not encode any proteins. Recent
studies have confirmed their function in biological processes,
including regulating or controlling gene expression, and in
pathological processes, including tumorigenesis (14,15). It
has been identified that lncRNAs affect numerous cellular processes
in tumor cells, including the cell cycle, survival rate,
proliferation and migration (16–19).
In thyroid cancer, a number of lncRNAs demonstrate
differential expression between carcinoma and para-cancer tissues
(20). For example, maternal
expressed gene 3 (MEG3) was the first lncRNA demonstrated to act as
a tumor suppressor in melanoma cells (21). In PTC, MEG3 was upregulated in
carcinoma tissues compared with normal tissues and suppressed
migration and invasion by targeting Ras-related C3 botulinum toxin
substrate 1 (22). Papillary thyroid
carcinoma susceptibility candidate 3 acts as a tumor suppressor in
thyroid cancer cells and leads to marked significant inhibition of
proliferation, cell cycle arrest and increased apoptosis (23). Antisense non-coding RNA in the INK4
locus has been demonstrated to promote the invasion and metastasis
of thyroid cancer cells through the transforming growth
factor-β/small mother against decapentaplegic signaling pathway
(24). Nc886 exerts an oncogenic
function in thyroid cancer by suppressing double-stranded
RNA-activated protein kinase (25).
BANCR participates in the proliferation of malignant
melanoma cells (7). BANCR regulates
cellular proliferation and migration via p38 MAPK and c-Jun
N-terminal kinase inactivation in lung carcinoma (LC) (8). In lung cancer cells, BANCR is associated
with poor prognosis and promotes metastasis by inducing EMT
(26). Recently, BANCR has been
demonstrated to promote cell proliferation in PTC (11), However, the association between BANCR
and EMT and the underlying molecular mechanism in PTC remain
unclear. To the best of our knowledge, the present study is the
first to address these key issues.
The results of the present study demonstrated that
the expression of BANCR in 27 paired tissue samples from PTC
patients exhibited a significant increase compared with its
expression in the adjacent normal tissues. The BCPAP cell line was
selected for subsequent experiments due to low expression of BANCR
and it was demonstrated that upregulation of BANCR expression may
promote the migration and invasion of PTC cells. Additionally, the
expression of EMT-induced markers (E-cadherin, N-cadherin and
vimentin) in cells overexpressing BANCR was also assessed using
qPCR, western blot analysis and confocal microscopy. The results
indicated that increased BANCR expression levels were associated
with increased expression of N-cadherin and vimentin. Therefore, it
was hypothesized that BANCR may promote EMT in PTC. In thyroid
cancer, there are two classical cell signaling pathways: The
ERK/MAPK signaling pathway and the phosphoinositide
3-kinase/protein kinase B signaling pathway. A number of studies
have demonstrated that the V600E mutation of BRAF activates the
MAPK signaling pathway (27). BANCR
has previously been reported to be associated with BRAF (V600E) and
BANCR regulated LC proliferation and migration via the MAPK
signaling pathway (8). The results of
the present study indicated that overexpression of BANCR may
upregulate the expression of p-c-Raf, p-MEK1/2 and p-ERK1/2.
Therefore, BANCR may activate the Raf/MEK/ERK signaling pathway.
This effect was reversed by U0126 treatment. In BCPAP-NC and
BCPAP-BANCR cells, the expression of E-cadherin was upregulated,
whereas vimentin expression was downregulated in response to U0126
treatment.
The present study demonstrated that BANCR promotes
the migration, invasion and EMT in PTC via the Raf/MEK/ERK
signaling pathway. Although the BCPAP cell line was the only cell
line employed, the results of the present study of value. In the
future, further tissue samples from patients with PTC and
additional cell lines may be utilized to confirm the results of the
present study. Additionally, in vivo experiments employing
nude mice may also provide new insights into the function of BANCR
on PTC.
Acknowledgements
The present study was supported by the Department of
General Surgery, Zhongshan Hospital. The authors thank the
Digestive Diseases Center of Xiamen City for support.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chen AY, Jemal A and Ward EM: Increasing
incidence of differentiated thyroid cancer in the United States,
1988–2005. Cancer. 115:3801–3807. 2009. View Article : Google Scholar
|
|
2
|
Wang Y, Guo Q, Zhao Y, Chen J, Wang S, Hu
J and Sun Y: BRAF-activated long non-coding RNA contributes to cell
proliferation and activates autophagy in papillary thyroid
carcinoma. Oncol Lett. 8:1947–1952. 2014. View Article : Google Scholar
|
|
3
|
Ponting CP, Oliver PL and Reik W:
Evolution and functions of long noncoding RNAs. Cell. 136:629–641.
2009. View Article : Google Scholar
|
|
4
|
Zhu L and Xu PC: Downregulated lncRNA-ANCR
promotes osteoblast differentiation by targeting EZH2 and
regulating Runx2 expression. Biochem Biophys Res Commun.
432:612–617. 2013. View Article : Google Scholar
|
|
5
|
Nakagawa T, Endo H, Yokoyama M, Abe J,
Tamai K, Tanaka N, Sato I, Takahashi S, Kondo T and Satoh K: Large
noncoding RNA HOTAIR enhances aggressive biological behavior and is
associated with short disease-free survival in human non-small cell
lung cancer. Biochem Biophys Res Commun. 436:319–324. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Xing M: BRAF mutation in thyroid cancer.
Endocr Relat Cancer. 12:245–262. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li R, Zhang L, Jia L, Duan Y, Li Y, Bao L
and Sha N: Long non-coding RNA BANCR promotes proliferation in
malignant melanoma by regulating MAPK pathway activation. PLoS One.
9:e1008932014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jiang W, Zhang D, Xu B, Wu Z, Liu S, Zhang
L, Tian Y, Han X and Tian D: Long non-coding RNA BANCR promotes
proliferation and migration of lung carcinoma via MAPK pathways.
Biomed Pharmacother. 69:90–95. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Przybyla L, Muncie JM and Weaver VM:
Mechanical control of epithelial-to-mesenchymal transitions in
development and cancer. Annu Rev Cell Dev Biol. 32:527–554. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zheng H, Wang M, Jiang L, Chu H, Hu J,
Ning J, Li B, Wang D and Xu J: BRAF-activated long noncoding RNA
modulates papillary thyroid carcinoma cell proliferation through
regulating thyroid stimulating hormone receptor. Cancer Res Treat.
48:698–707. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Schmittgen TD and Livak KJ: Analyzing
real-time PCR data by the comparative C(T) method. Nat Protoc.
3:1101–1108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Flockhart RJ, Webster DE, Qu K,
Mascarenhas N, Kovalski J, Kretz M and Khavari PA: BRAFV600E
remodels the melanocyte transcriptome and induces BANCR to regulate
melanoma cell migration. Cancer Res. 22:1006–1014. 2012.
|
|
15
|
Gupta RA, Shah N, Wang KC, Kim J, Horlings
HM, Wong DJ, Tsai MC, Hung T, Argani P, Rinn JL, et al: Long
non-coding RNA HOTAIR reprograms chromatin state to promote cancer
metastasis. Nature. 464:1071–1076. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xia M, Yao L, Zhang Q, Wang F, Mei H, Guo
X and Huang W: Long noncoding RNA HOTAIR promotes metastasis of
renal cell carcinoma by up-regulating histone H3K27 demethylase
JMJD3. Oncotarget. 8:19795–19802. 2017.PubMed/NCBI
|
|
17
|
Yang F, Bi J, Xue X, Zheng L, Zhi K, Hua J
and Fang G: Up-regulated long non-coding RNA H19 contributes to
proliferation of gastric cancer cells. Febs J. 279:3159–3165. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yang C, Li X, Wang Y, Zhao L and Chen W:
Long non-coding RNA UCA1 regulated cell cycle distribution via CREB
through P13-K dependent pathway in bladder carcinoma cells. Gene.
496:8–16. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Esteller M: Non-coding RNAs in human
disease. Nat Rev Genet. 12:861–874. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lan X, Zhang H, Wang Z, Dong W, Sun W,
Shao L, Zhang T and Zhang D: Genome-wide analysis of long noncoding
RNA expression profile in papillary thyroid carcinoma. Gene.
569:109–117. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gibb EA, Brown CJ and Lam WL: The
functional role of long non-coding RNA in human carcinomas. Mol
Cancer. 10:382011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang C, Yan G, Zhang Y, Jia X and Bu P:
Long non-coding RNA MEG3 suppresses migration and invasion of
thyroid carcinoma by targeting of Rac1. Neoplasma. 62:541–549.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fan M, Li X, Jiang W, Huang Y, Li J and
Wang Z: A long non-coding RNA, PTCSC3, as a tumor suppressor and a
target of miRNAs in thyroid cancer cells. Exp Ther Med.
5:1143–1146. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhao JJ, Hao S, Wang LL, Hu CY, Zhang S,
Guo LJ, Zhang G, Gao B, Jiang Y, Tian WG and Luo DL: Long
non-coding RNA ANRIL promotes the invasion and metastasis of
thyroid cancer cells through TGF-β/Smad signaling pathway.
Oncotarget. 7:57903–57918. 2016.PubMed/NCBI
|
|
25
|
Lee EK, Hong SH, Shin S, Lee HS, Lee JS,
Park EJ, Choi SS, Min JW, Park D, Hwang JA, et al: nc886, a
non-coding RNA and suppressor of PKR, exerts an oncogenic function
in thyroid cancer. Oncotarget. 7:75000–75012. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sun M, Liu XH, Wang KM, Nie FQ, Kong R,
Yang JS, Xia R, Xu TP, Jin FY, Liu ZJ, et al: Downregulation of
BRAF activated non-coding RNA is associated with poor prognosis for
non-small cell lung cancer and promotes metastasis by affecting
epithelial-mesenchymal transition. Mol Cancer. 13:682014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fraser S, Go C, Aniss A, Sidhu S,
Delbridge L, Learoyd D, Clifton-Bligh R, Tacon L, Tsang V, Robinson
B, et al: BRAF(V600E) mutation is associated with decreased
disease-free survival in papillary thyroid cancer. World J Surg.
40:1618–1624. 2016. View Article : Google Scholar : PubMed/NCBI
|