Introduction
Lung cancer is one of the most common malignant
tumors, currently exhibiting the highest mortality rate worldwide
(1). In China, lung cancer has the
second highest morbidity rate among the malignant tumors, with the
highest morbidity in certain cities (2–4).
Overexpression of the anti-apoptosis gene BCL2, apoptosis regulator
(BCL2), and insufficient expression of the pro-apoptosis
gene BCL2 associated X, apoptosis regulator (BAX), may
inhibit cell apoptosis, prolong cell survival and promote cell
proliferation; it is thought to be the most common mechanism for
the tumorigenesis of lung cancer (5,6).
Therefore, examination of the expression of BCL2 and
BAX in pre-cancerous lesions could facilitate the early
diagnosis of lung cancer.
The amino acid 2-aminoethanesulfonic acid, commonly
known as taurine, has a simple chemical structure that contains a
thiol group. Taurine accounts for <0.1% of total human body
weight, and exists in all organs in its free form. Taurine was
reported to be an essential nutrient for cats in 1975 (7). Subsequent studies have revealed that the
endogenous synthesis of taurine in humans is limited and that
humans may suffer from taurine deficiency under certain conditions
where there is insufficient intake; hence, taurine is considered to
be an essential nutrient for humans (8,9). Recent
studies have proposed that changes in systemic taurine levels can
be used to predict the formation and malignant transformation of
certain tumors (10,11). For example, the serum level of taurine
was found to be significantly lower in patients with breast cancer
than in patients in the high-risk breast cancer group or the
healthy control group (7). Thus,
taurine is considered a novel biomarker for early diagnosis of
breast cancer (10). High levels of
taurine were also detected in the urine of patients with non-muscle
invasive bladder cancer, indicating that taurine could also serve
as a novel indicator for the diagnosis of bladder cancer (11). Taurine has been shown to exert an
inhibitory effect on dimethylbenzanthracine-induced breast cancer
in rats; it was also demonstrated that taurine could induce
apoptosis and suppress proliferation in colorectal and breast
cancer cells (12,13). Okamoto et al (14) reported that taurine exerted a
protective effect against chemical-induced tumorigenesis of liver
cancer in male F344 rats, using diethylnitrosamine as the
carcinogen and phenobarbital as the tumor promotor. When
S180 xenograft tumors in nude mice were treated with
taurine, apoptosis was markedly increased: The expression of the
anti-apoptotic protein Bcl-2 was reduced, whereas the expression of
the pro-apoptotic protein Bax was upregulated (15). Taurine can also downregulate the
expression of matrix metalloproteinase-2 and upregulate the
expression of N-acetyl galactosaminyl transferase 2, suppressing
the potential invasion and metastasis of glioma cells (16).
To the best of our knowledge, the effect of taurine
on lung cancer cells has not yet been reported. In this study, the
human non-small cell lung cancer A549 cell line was used to
investigate the pro-apoptotic and anti-proliferation effects of
taurine on lung cancer cells. The underlying molecular mechanism
was also elucidated to provide evidence of the potential clinical
application of taurine in tumor therapy.
Materials and methods
Reagents and antibodies
Taurine was purchased from Sigma-Aldrich; Merck KGaA
(Darmstadt, Germany). RPMI-1640, Dulbecco's Modified Eagle's Medium
(DMEM) and fetal bovine serum (FBS) were purchased from Gibco;
Thermo Fisher Scientific, Inc. (Waltham, MA, USA). The anti-p53
upregulated modulator of apoptosis (PUMA) polyclonal antibody (cat.
no. 55120-1-AP) was purchased from ProteinTech Group, Inc.
(Chicago, IL, USA); the anti-Bcl-2 monoclonal antibody (cat. no.
sc-783), anti-Bax monoclonal antibody (cat. no. sc-526),
anti-β-actin monoclonal antibody (cat. no. sc-130300) and anti-HA
monoclonal antibody (cat. no. sc-393579) were all purchased from
Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). Horseradish
peroxidase (HRP)-labeled goat anti-mouse IgG (cat. no. ZDR-5307),
HRP-labeled goat anti-rabbit IgG (cat. no. ZDR-5306), trypsin, SDS
and TEMED were all purchased from Origene Technologies, Inc.
(Beijing, China). Cisplatin (DDP) was purchased from Shandong Qilu
Pharmaceutical Co., Ltd. (Jinan, China).
Cell lines and animals
The human non-small cell lung cancer A549 cell line
was purchased from the Type Culture Collection of the Chinese
Academy of Sciences (Shanghai, China). A total of 40 4-week-old
BALB/C nude mice (half from each sex), weighing ~20 g (SPF grade;
certificate no. HNASLKJ20121369), were purchased from Hunan SJA
Laboratory Animal Co., Ltd. (Hunan, China), and maintained in
individually ventilated cages (20–26°C and 40–70% humidity), in the
Animal Science Center of Nanchang University (Nanchang, China). The
mice were fed with SPF grade aseptic mice feed purchased from
Beijing Keao Xieli Feed Co., Ltd. (Beijing, China) and sterilized
water, 12/12 h light/dark cycle. All animal experiments were
approved by the Ethics Committee of Nanchang University.
Grouping and treatment
The human non-small cell lung cancer cell line A549
were cultured in RPMI-1640 complete culture solution supplemented
with 10% FBS and stored at 37°C in a 5% CO2 incubator.
A549 cells at the logarithmic growth phase were randomly divided
into 6 groups: Control, taurine 20 mM, taurine 40 mM, taurine 80
mM, taurine 160 mM and the positive control group, DDP (10 mg/ml).
The cells were then treated with the respective agents for 24, 48
and 72 h.
MTT assay for cell proliferation
A549 cells at the logarithmic growth phase were
collected, and the concentration of the cell suspension was
adjusted based on the Trypan blue method. In total, 200 µl of cell
suspension was added to each well of a 96-well plate, to a final
density of 3,000-8,000 cells per well. Each assay point was
performed in four replicates, and the empty wells at the edges of
the plates were filled with sterile PBS. The cells were cultured at
37°C in a 5% CO2 incubator. The following day, the cells
were treated with taurine at the various concentrations or with DDP
10 g/ml. After 48 h incubation, 20 µl of MTT solution (5 mg/ml) was
added to each well for and incubated for 4 h. The supernatant in
each well was then gently aspirated, and 150 µl dimethyl sulfoxide
was added to each well to dissolve the crystals, and the plate was
shaken on a horizontal shaker for 10 min. The optical density (OD)
at 490 nm were measured using a microplate reader, and the
inhibition ratio was calculated using the following equation:
Inhibition ratio (%) = (1 - OD value of the experimental group/OD
value of the control group) ×100.
Hoechst 33342 staining for cell
apoptotic morphology
A549 cells at the logarithmic growth phase were
collected and seeded into 12-well plates, at a density such that
the adherent cells reached 70–90% confluence by the following day.
The cells were then divided into five groups: Control, taurine 40
mM, taurine 80 mM, taurine 160 mM and DDP 10 g/ml, with 3
replicates in each group. The cell lines were treated for 48 h at
37°C in a 5% CO2 incubator. The medium was then removed,
the cells were washed twice with PBS and 500 µl Hoechst 33342 stain
solution (5 µg/ml) was added into each well to completely cover the
cells. The cells were then incubated in the dark at 37°C in a 5%
CO2 incubator for 20–30 min. Subsequently, the Hoechst
33342 stain was removed, the cells were washed twice with PBS and
apoptotic morphology was observed under a fluorescence microscope
(×200 magnification) and images were captured. A total of ten
random high magnification fields were selected to calculate cell
apoptosis ratio as follows: Cell apoptosis ratio = apoptotic cell
number/total cell number ×100.
Flow cytometry analysis of
apoptosis
Following a 48 h treatment, as aforementioned,
1–5×105 cells were collected and resuspended in 500 µl
Binding Buffer (Annexin V-FITC/PI double staining cell apoptosis
detection kit; Jiangsu Keygen Biotechnology Co., Ltd., Nanjing,
China). Annexin V-fluorescein isothiocyanate (FITC; 5 µl) and 5 µl
propidium iodide (PI) were added successively into the cell
suspension, mixed well and incubated for 5–15 min at room
temperature in the dark. The cells were then analyzed for apoptotic
status using a FACSCalibur flow cytometer with the BD CellQuest™
Pro Analysis system (version 6.1; BD Biosciences, Franklin Lakes,
NJ, USA).
Western blot analysis of expression of
PUMA, Bax and Bcl-2
Following the aforementioned 48 h treatments, A549
cells were collected and total protein was extracted with cell
lysis buffer (cat. no. P0013; Beyotime Institute of Biotechnology,
Haimen, China) and denatured. Standard bovine serum albumin
solution was used to produce the standard curve of protein content
to measure the concentration of protein. A total of 30 µg total
protein per lane from each sample was subjected to 10% SDS-PAGE.
Following electrophoresis, protein was transferred to a
polyvinylidene fluoride membrane using a current of 330 mA for 60
min. The membrane was blocked by 1% bovine serum albumin (cat. no.
ST023; Beyotime Institute of Biotechnology) at room temperature for
2 h, and then incubated with specific antibodies against PUMA, Bax
or Bcl-2 (1:200) at 4°C overnight, followed by incubation with the
appropriate HRP-conjugated secondary antibody (1:2,000) for 1.5 h.
The western blots were developed using pierce™ ECL western blotting
substrate (Pierce; Thermo Fisher Scientific, Inc.), and the protein
bands analyzed for protein density using Image-Pro Plus 6 software
(Media Cybernetics, Inc., Rockville, MD, USA). We used the ratio of
the gray value of the target protein to the gray value of the
β-actin as the final result, to produce a semi-quantitative
analysis.
Establishment of a xenograft tumor
model in nude mice
A549 cells were inoculated subcutaneously in the
left and right flanks of the abdomen of 40 5-week-old nude mice.
Once the diameter of the tumors reached between 2–3 mm, the mice
were randomly divided into four groups: i) Control group, treated
with 0.2 ml saline was injected at multiple sites inside and around
the tumor; ii) taurine treatment group, treated with 0.2 ml taurine
solution (100 mg/kg) injected at multiple sites inside and around
the tumor; iii) exogenous PUMA treatment group, treated with 0.2 ml
mixture of pCEP4-(HA)2-PUMA plasmid (100 µg; Institute
of Cancer Research, University of Pittsburgh, Pittsburgh, PA, USA)
and liposome (Invitrogen; Thermo Fisher Scientific, Inc.) injected
at multiple sites inside and around the tumor which have been
sterilized with 75% alcohol; iv) taurine and exogenous PUMA
treatment group (combined treatment group), treated with 0.1 ml
taurine solution (100 mg/kg) and 0.1 ml mixture of
pCEP4-(HA)2-PUMA plasmid (100 µg) and liposome injected
simultaneously at multiple sites inside and around the tumor. The
treatment was applied seven times, with an interval of 72 h between
each treatment. The diameter of the tumors was measured every 72 h
for the calculation of tumor volumes, and tumor growth curves were
plotted accordingly. The mice were euthanized on day 27 and the
tumors were photographed and weighed. The tumor suppression ratio
was then calculated as follows: Tumor volume (cm3)
VT=1/2× a × b2 (a is the longer arm, b is the
shorter arm); Tumor suppression ratio (%) = (average tumor weight
of control group-average tumor weight of treatment group)/average
tumor weight of control group ×100.
Immunohistochemical analysis
When the mice were euthanized, the tumors were
collected and any blood was washed off with PBS. Immediately
following photography, the tumors were fixed with 4%
paraformaldehyde for 24 h at 4°C, and examined for expression
levels of PUMA, Bax and Bcl-2 by immunohistochemistry. Tissue
samples were paraffin embedded, sectioned (3 µm), heated at 65°C
for 2 h, dewaxed with conventional xylene (xylene I for 15 min, the
xylene II 15 min), and dehydrated with a gradient alcohol series
(100% ethanol for 2 min, 100% ethanol for 2 min, 95% ethanol for 2
min, 95% ethanol for 2 min, 80% ethanol for 2 min then 70% ethanol
for 2 min), and then washed 1 time with tap water for 3 min.
Antigen retrieval was performed using citric acid buffer (0.01 M,
pH 6.0, 100°C) and boiled for 20 min in a microwave oven (wattage,
800 W; time, 20 min). Endogenous peroxidase was inactivated with 3%
H2O2 at 37°C for 10 min in a water bath.
Sections were incubated with specific antibodies against PUMA, Bax
or Bcl-2 (1:200) at 4°C for 6 h, followed by incubation with a
horseradish peroxidase-conjugated secondary antibody at 37°C for 1
h (dilution, 1:2,000; cat. no. ZDR-5307) and stained with
3,3N-diaminobenzidine tertrahydrochloride (0.5 mg/ml) and
hematoxylin (4 mg/ml) for 5 min at 25°C. Under an Olympus BX-41
binocular light microscope (at ×40 magnification), images from five
fields on each section of two non-consecutive sections of each
tumor were captured. The images were then analyzed using HMIAS-2000
image analyzing software (version no. V1.0; Wuhan Qianping Imaging
Technology Co., Ltd., Wuhan, China) for automatic calculation of
integrated optical density to determine protein expression.
Statistical analysis
SPSS 17.0 statistical software (SPSS, Inc., Chicago,
IL, USA) was used to analyze the experimental data, and expressed
as the mean ± standard deviation. One-way analysis of variance
followed by LSD post hoc test or unpaired t-test was performed for
comparisons between multiple groups, and a Q-test was used to
compare two groups among multiple groups. P<0.05 was considered
to indicate a statistically significant difference.
Results
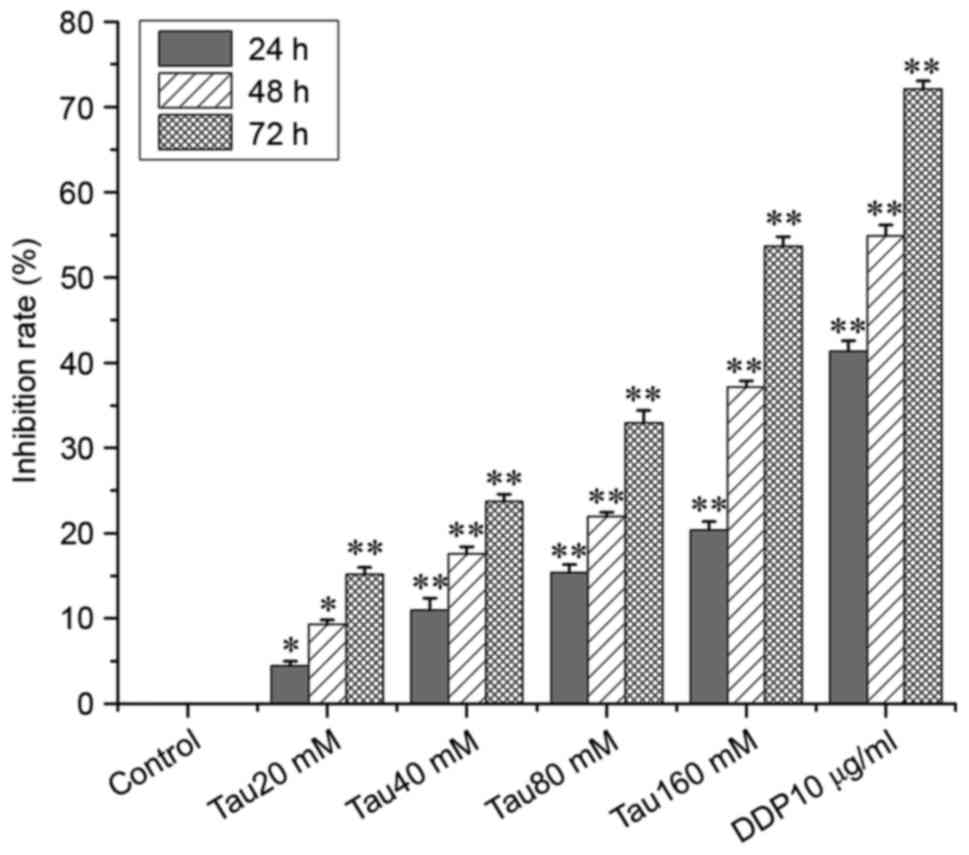
Taurine inhibits the proliferation of
the human lung cancer A549 cell line
To investigate the effect of taurine on cell
proliferation, A549 cells were treated with various taurine
concentrations. As shown in Fig. 1,
the inhibitory effect of taurine on the proliferation of A549 cells
significantly increased in a time- and concentration-dependent
manner when compared with the control group.
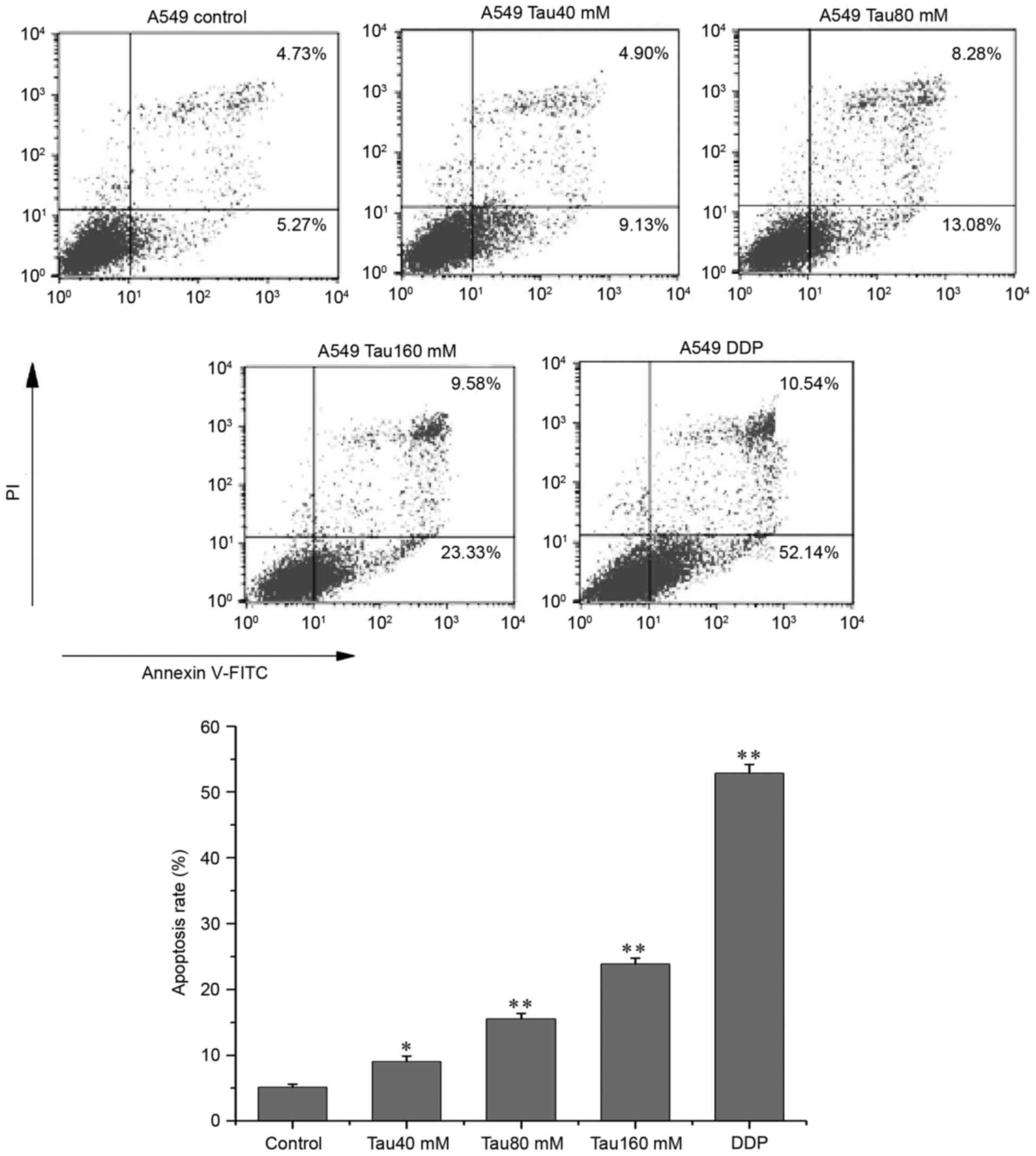
Taurine promotes apoptosis in the
human lung cancer A549 cell line
A549 cells were treated with different
concentrations of taurine (range, 40–160 mM) for 48 h, and then
double-stained with anti-Annexin V-FITC antibody and PI. The
apoptosis ratio (Annexin V-FITC+/PI−, the
lower right quadrant) of A549 cells corresponded with increasing
concentrations of taurine (Fig. 2),
and the apoptosis ratios of all taurine groups were significantly
different when compared with the control group (P<0.05,
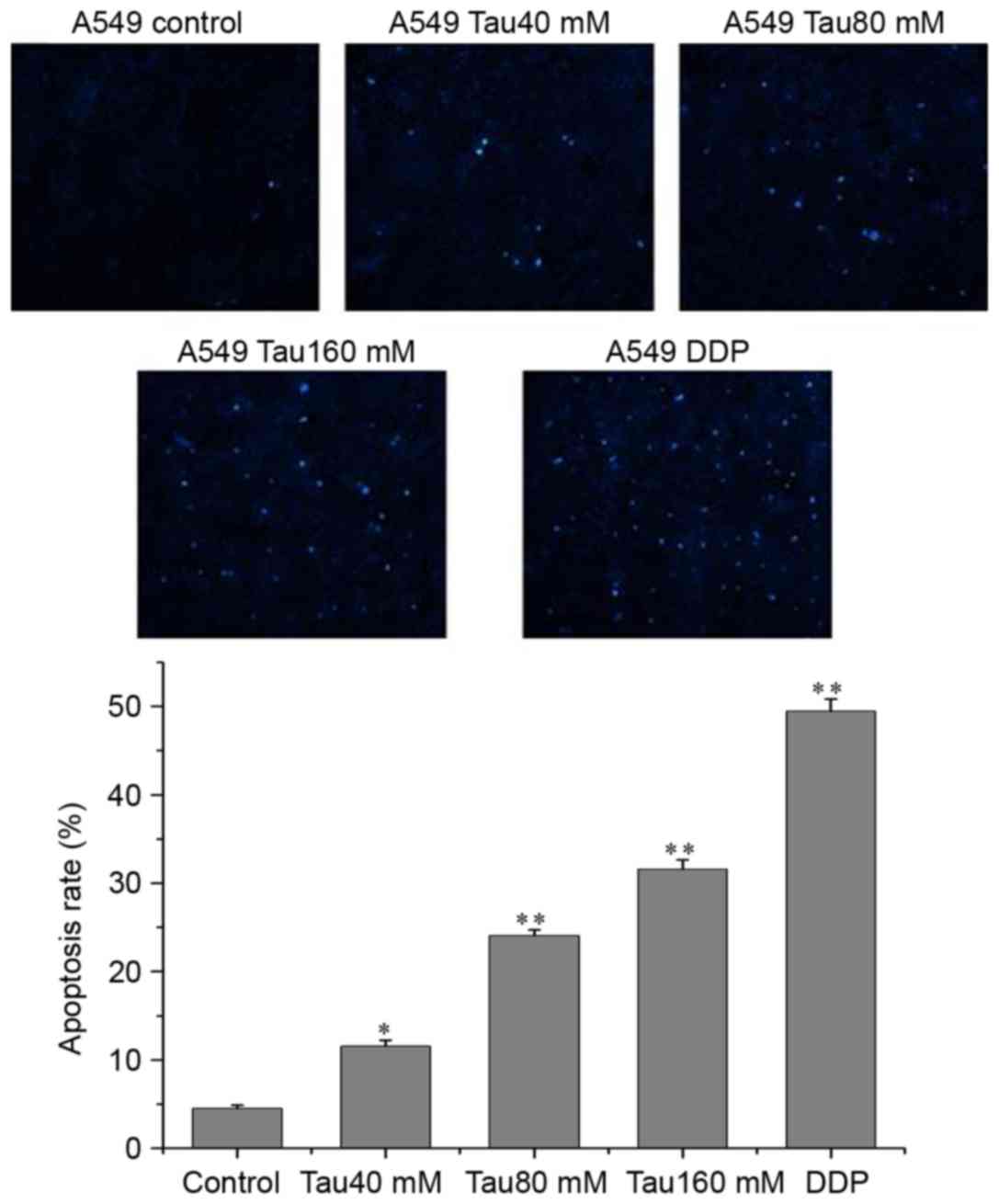
P<0.01). In addition, Hoechst 33342 fluorescence staining
revealed that the numbers of apoptotic cells were also increased as
taurine concentration increased, and that the number of apoptotic
cells were significantly higher than in the control group (Fig. 3; P<0.01).
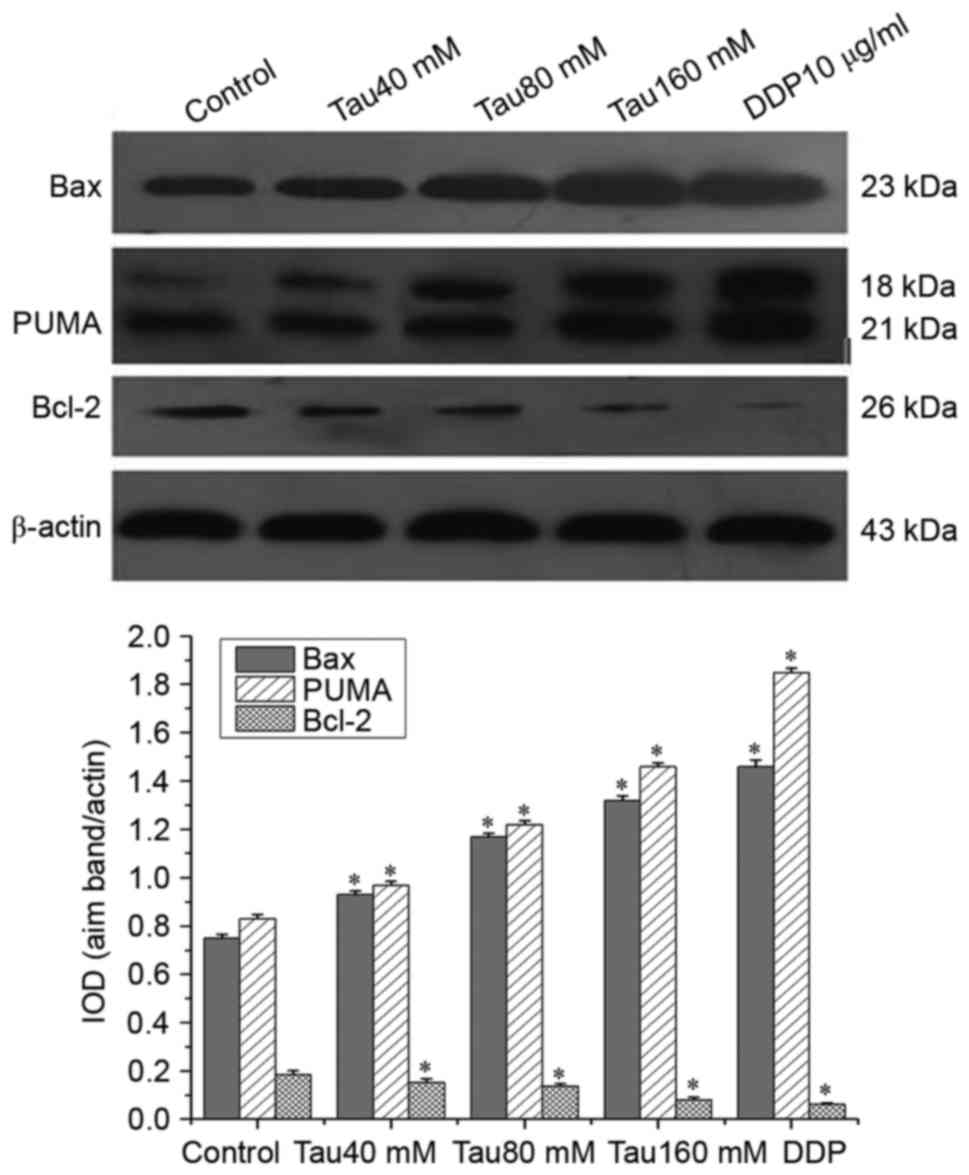
Taurine regulates the expression of
PUMA, Bax and Bcl-2 in the human lung cancer A549 cell line
Western blot analysis revealed that the expression
of PUMA and Bax was significantly upregulated, whereas that of
Bcl-2 was significantly downregulated in A549 cells. This trend
continued with increasing concentrations of taurine, compared with
the control group (Fig. 4;
P<0.05). Taurine altered the expression levels of PUMA, Bax and
Bcl-2 in a dose-dependent manner.
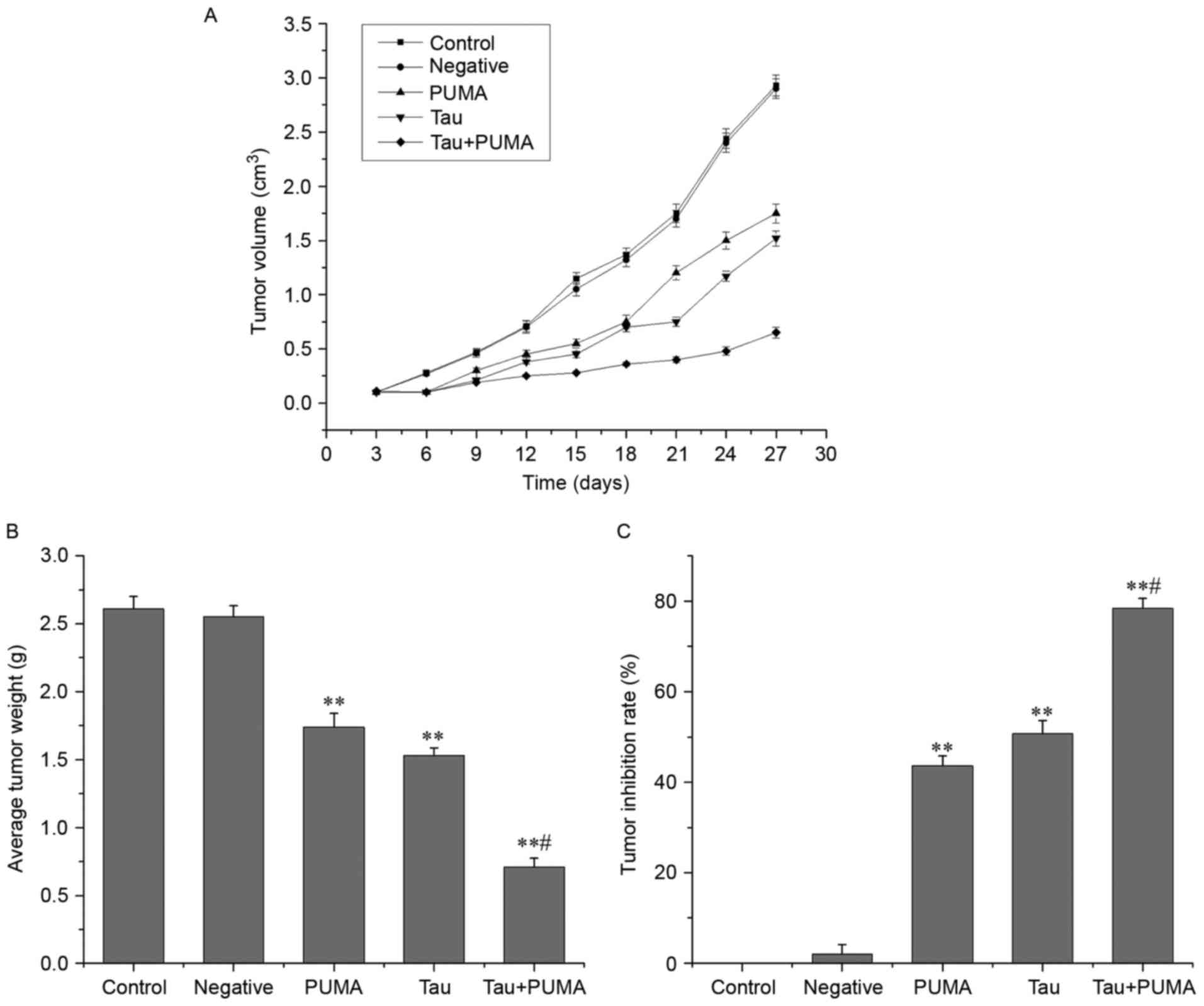
Taurine suppresses the ectopic growth
of lung cancer cell-derived xenograft tumors in nude mice
As shown in Fig. 5,
treatment with exogenous PUMA alone, taurine alone and combined
treatment with exogenous PUMA and taurine gave an average tumor
suppression ratio of 42.94±1.99, 50.09±2.35 and 78.52±1.46%,
respectively. These ratios all differed significantly from that of
control group (P<0.01). The average tumor volumes and weights of
all treatment groups were significantly different from that of
control group (P<0.01). In addition, the tumor suppression ratio
of the combined-treatment group was much higher than that of the
taurine or PUMA single-treatment groups, indicating that combined
treatment with taurine and exogenous PUMA act synergistically to
suppress the growth of xenograft tumors in nude mice.
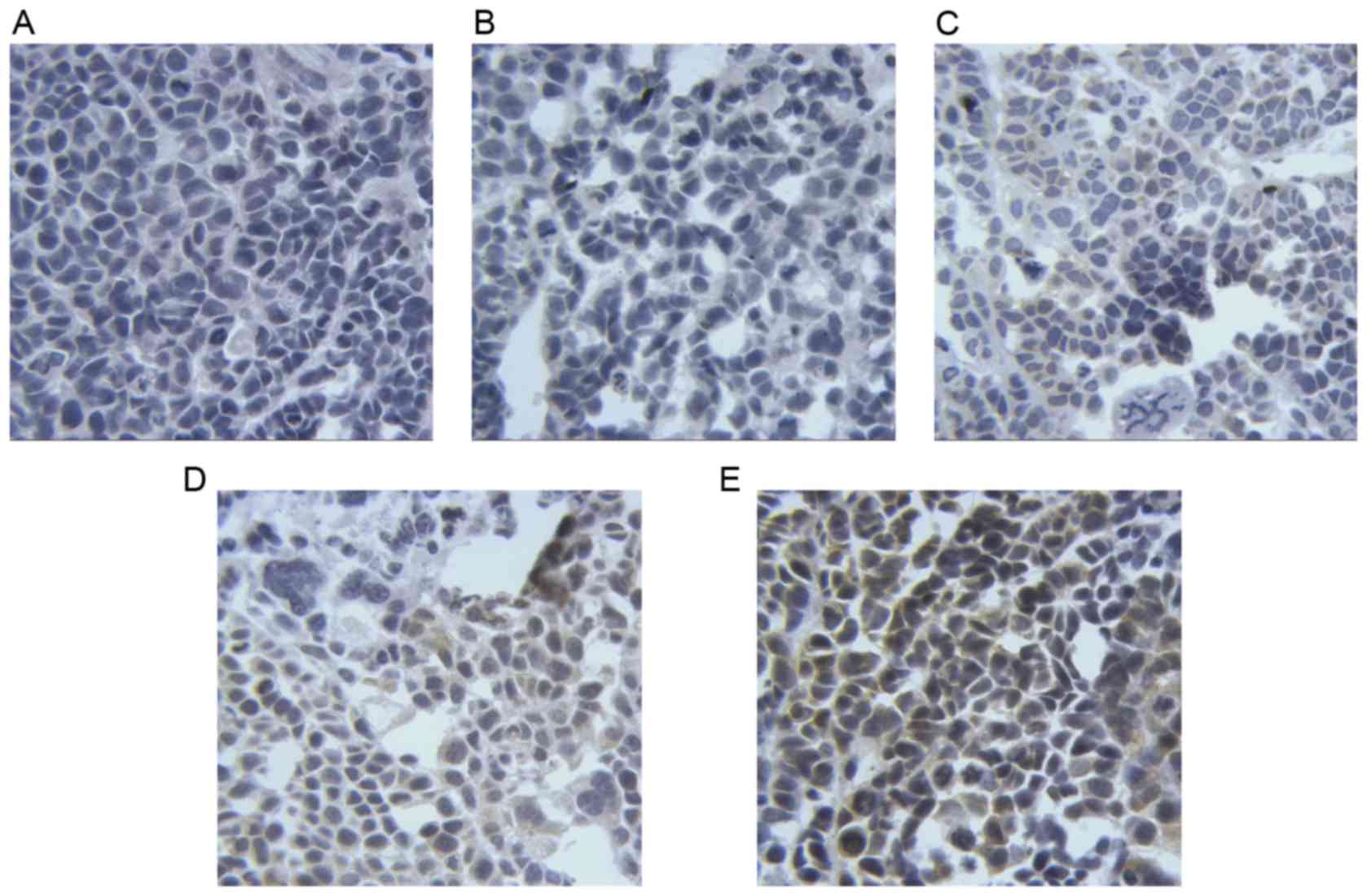
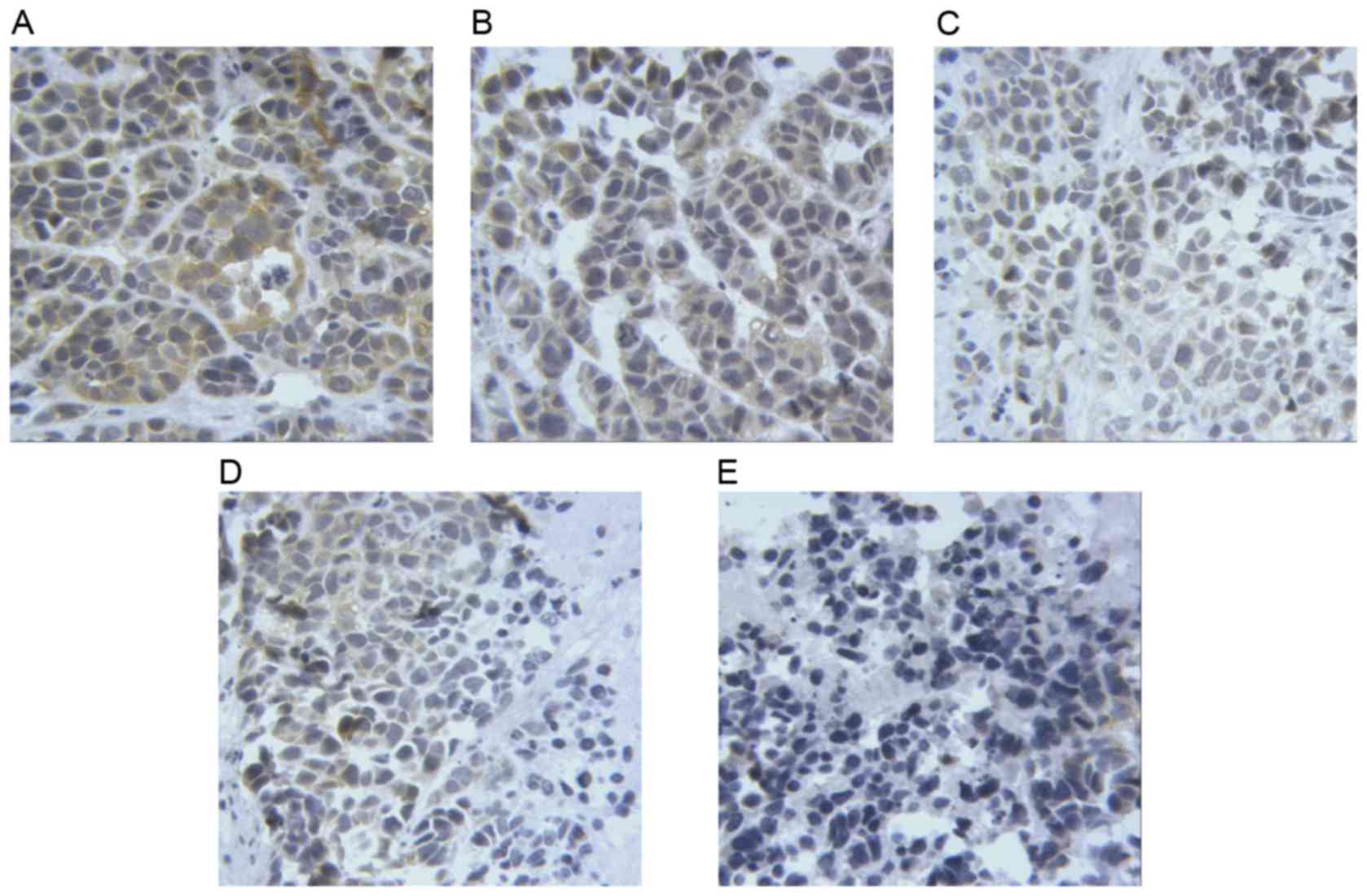
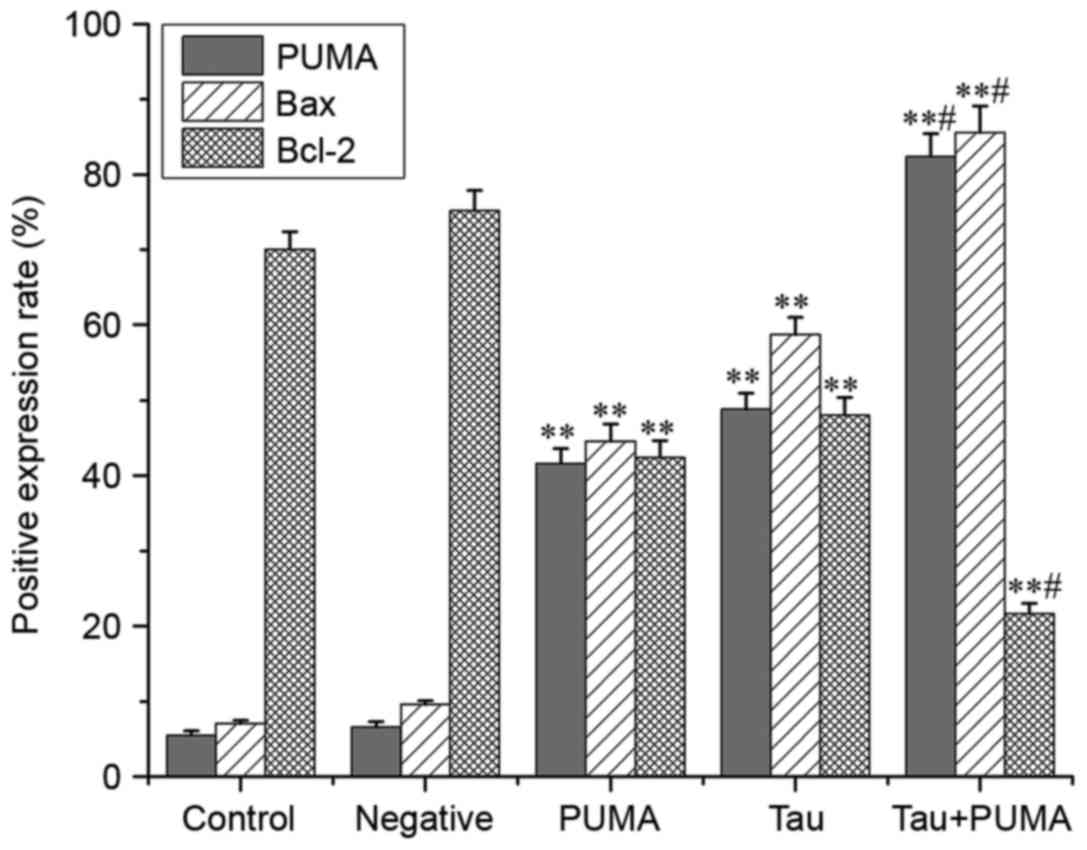
Taurine regulates the expression of
PUMA, Bax and Bcl-2 in xenograft tumors in nude mice
The results of immunohistochemistry (Figs. 6–8)
revealed that the levels of PUMA and Bax were significantly
elevated in the cytoplasm of tumor cells treated with taurine, PUMA
and taurine and exogenous PUMA, when compared with the control
group (P<0.01). Moreover, the levels of proteins in the
cytoplasm were significantly higher in the taurine and exogenous
PUMA group, than in the PUMA or taurine single-treatment groups
(P<0.01). Compared with the control group, the level of
cytoplasmic Bcl-2 was significantly decreased in the taurine,
exogenous PUMA and taurine and exogenous PUMA treatment groups
(P<0.01). In addition, the level of cytoplasmic Bcl-2 was the
lowest in the taurine and exogenous PUMA group (Fig. 9), and was significantly different to
that of the taurine or PUMA single-treatment groups
(P<0.01).
Discussion
Taurine is an amino sulfonic acid that naturally
exists in the human body. It is an endogenous anti-injury
substance, including oxidative stress injury, alcoholic liver
injury and injury of endotoxin to myocardium that possesses a wide
spectrum of physiological and pharmacological functions, including
anti-oxidative activities and modulation of calcium homeostasis
(12,17). Taurine has been used for treatment of
inflammation, hepatobiliary diseases, cardiovascular diseases
(18), diabetes mellitus (19), cataracts (20) and a number of other diseases. Taurine
treatment reportedly leads to downregulation of the anti-apoptotic
protein Bcl-2 and upregulation of the pro-apoptotic protein Bax in
nude mice S180 xenograft tumors, as revealed by
immunohistochemical analysis (15).
This therefore leads to enhanced apoptosis in the xenograft tumors.
In addition, taurine can also induce apoptosis in human colorectal
cancer cells independently of tumor protein p53 (hereafter p53), by
upregulating PUMA (12). However,
studies on the effect of taurine on lung cancer are limited. Hence,
this study focused on the effect of taurine on the proliferation
and apoptosis of the human lung cancer A549 cell line.
The in vitro experiments of the present study
revealed that taurine at a concentration of 20–160 mmol/l could
inhibit the proliferation of and promote apoptosis in A549 cells,
and that the effect was dose- and time-dependent (Figs. 1–3).
With increasing concentration of taurine, the expression of PUMA
and Bax was significantly upregulated, whereas that of Bcl-2 was
downregulated (Fig. 4). The
regulatory effect of taurine on the expression of PUMA, Bax and
Bcl-2 in A549 cells was dose-dependent. This dose-dependent
activity may represent the major mechanism for the pro-apoptotic
and anti-proliferative effect of taurine on A549 cells.
Tumorigenesis is a multi-factorial and multi-stage
process, whereby the activation of oncogenes or loss of tumor
suppressor genes leads to dysregulation of cell proliferation,
differentiation and apoptosis. Studies have shown that PUMA affects
cell proliferation and induces apoptosis more effectively than p53,
meaning that the administration of exogenous PUMA may represent a
promising method of tumor therapy (21,22). The
ectopic expression of PUMA has been demonstrated to effectively
suppress the proliferation of A549 lung cancer cells (23).
In the present study, pCEP4-(HA)2-PUMA
recombinant plasmids were injected into the A549 cell-derived
xenograft tumors in nude mice to investigate the effect of PUMA on
taurine treatment for A549-derived xenograft tumors. The results of
this treatment indicated that taurine exerted a strong inhibitory
effect on the growth of A549 cell-derived xenograft tumors in nude
mice (Fig. 5). In addition, the
treatment with taurine and PUMA combined exerted a better
therapeutic effect than the individual treatment group of taurine
and exogenous PUMA on the xenograft tumors in terms of suppression
of tumor growth, upregulation of PUMA and Bax and downregulation of
Bcl-2. The potency of the combined treatment was much greater than
each single treatment with taurine or PUMA (P<0.05).
Therefore, PUMA serves a critical role in the action of taurine
against lung cancer, and may represent a novel target for gene
therapy in lung cancer.
PUMA is a member of BH3-only family protein,
belonging to the Bcl-2 superfamily. PUMA contains a BH3 domain,
which binds to anti-apoptotic proteins, including Bcl-2, Bcl-extra
large (Bcl-xL), Bcl-2-like protein 2, induced myeloid leukemia cell
differentiation protein Mcl-1 and Bcl-2-related protein A1
(24), exerting potent anti-apoptotic
functions. PUMA can enhance mitochondrial membrane permeability and
release pro-apoptotic factors to induce cell apoptosis by the
following mechanisms: i) PUMA binds to the anti-apoptotic molecule
Bcl-2/Bcl-xL on the mitochondrial membrane to abolish their
inhibitory effect on the pro-apoptotic Bax/Bcl-2 homologous
antagonist/killer (Bak) dimer; ii) PUMA binds directly to the
Bax/Bak dimer on the mitochondrial membrane, inducing a
conformational change that leads to their translocation from the
cytoplasm to mitochondrial outer membrane and oligomerization, so
as to alter the original ‘membrane channel protein’; iii) PUMA
binds to Bcl-xL in the p53/Bcl-xL complex, releasing p53 and
further activating Bax (25–27).
The mechanism of the antitumor effect of taurine is
associated with its ability to boost anti-oxidatitive capacity,
promote immunity, enhance synergy with chemotherapeutic agents and
reduce chemotherapeutic toxicity (28). To protect cells from oxidative injury
and promote tumor cell apoptosis, taurine can enhance the
anti-oxidative capacity of the body (28,29).
Taurine can upregulate the activity of superoxide dismutase,
glutathione peroxidase and hydrogen peroxidase, thereby reducing
the levels of reactive oxygen species and suppressing the growth of
tumor cells (30). The combined use
of taurine and the chemotherapeutic agent cisplatin enhanced the
antitumor effect of DDP (31), and
also activated caspase-3, −6, −7 and −9, activating of the
mitochondrial apoptotic pathway. Moreover, Abd-Rabou et al
(32) found that taurine in
combination with curcumin could enhance immunity in and induce
lysis of Huh-7 tumor cells. The results of the present study is
consistent with that of a previous publication (33), and reinforces the finding that taurine
can upregulate the expression of the pro-apoptotic proteins PUMA
and Bax, downregulate the expression of the anti-apoptotic protein
Bcl-2, and increase the ratio of Bax/Bcl-2, thus promoting
apoptosis.
In conclusion, taurine can inhibit the proliferation
of human lung cancer cells A549 and the growth of transplanted
tumors in nude mice, and promote the apoptosis of A549 cells by
increasing the protein level of PUMA, Bax and decreasing the
protein level of Bcl-2. In nude mice transplanted tumors, PUMA
serves a critical role in the action of taurine against lung
cancer, and may represent a novel target for gene therapy in lung
cancer.
Acknowledgements
The present study was supported by a grant from the
National Natural Science Foundation of China (grant no. 81360032)
and the Natural Science Foundation of Jiangxi Province (grant no.
20161BAB205206).
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Shi L, Xu ZZ, Wu G, Chen XT, Huang YY,
Wang YJ, Jiang WQ and Bin Ke: Up-regulation of miR-146a increases
the sensitivity of non-small cell lung cancer to DDP by
downregulating cyclin J. BMC Cancer. 17:1382017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rouibaa F, Bakkar M, Seddik H, Addioui T,
Filali FZ, Akka R, Desla H and Aourarh A: Interest of expandable
metallic stents in the management of colonic tumor occlusion:
Experience of a Moroccan hospital. Pan Afr Med J. 2:245–269.
2013.
|
|
3
|
Watanabe A, Taniguchi F, Izawa M, Suou K,
Uegaki T, Takai E, Terakawa N and Harada T: The role of survivin in
the resistance of endometriotic stromal cells to drug-induced
apoposis. Hum Reprod. 24:3172–3179. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zaffaroni N, Costa A, Pennati M, De Marco
C, Affini E, Madeo M, Erdas R, Cabras A, Kusamura S, Baratti D, et
al: Survivin is highly expressed and promotes cell survival in
malignant peritoneal mesothelioma. Cell Oncol. 29:453–466.
2007.PubMed/NCBI
|
|
5
|
Meng J, Fang B, Liao Y, Chresta CM, Smith
PD and Roth JA: Apoptosis induction by MEK inhibition in human lung
cancer cells is mediated by Bim. PLoS One. 5:e130262010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Perez BA, Ghafoori AP, Lee CL, Johnston
SM, Li Y, Moroshek JG, Ma Y, Mukherjee S, Kim Y, Badea CT and
Kirsch DG: Assessing the radiation response of lung cancer with
different gene mutations using genetically engineered mice. Front
Oncol. 3:722013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hayes KC, Carey RE and Schmidt SY: Retinal
degeneration associated with taurine deficiency in the cat.
Science. 188:949–951. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Huxtable RJ: Physiological action of
taurine. Physiol Rev. 72:101–163. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Han XB: Human condition essential
nutrients-taurine. Foreign Med (Hygienic Branch). 1–132. 1987.
|
|
10
|
El Agouza IM, Eissa SS, El Houseini MM,
El-Nashar DE and Abd El Hameed OM: taurine: A novel tumor marker
for enhanced detection of breast cancer among female patients.
Angiogenesis. 14:321–330. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Srivastava S, Roy R, Singh S, Kumar P,
Dalela D, Sankhwar SN, Goel A and Sonkar AA: Taurine-a possible
fingerprint biomarker in non-muscle invasive bladder cancer: A
pilot study by 1H NMR spectroscopy. Cancer Biomark. 6:11–20. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang X, Tu S, Wang Y, Xu B and Wan F: The
mechanism of taurine-induced apoptosis in human colon cancer cells.
Acta Biochim Biophys Sin (Shanghai). 46:261–272. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang X, Lu H, Wang Y, Liu C, Zhu W, Zheng
S and Wan F: Taurine induces apoptosis of breast cancer cells by
regulating apoptosis-related proteins of mitochondria. Int J Mol
Med. 35:218–226. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Okamoto K, Sugie S, Ohnishi M, Makita H,
Kawamori T, Watanabe T, Tanaka T and Mori H: Chemopreventive
effects of taurine on diethylnitrosamine and phenobarbital-induced
hepatocarcinogenesis in male F344 rats. Jpn J Cancer Res. 87:30–36.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang HR: Experimental study of the effect
of taurine on sarcoma 180(S180) in mices. PhD
dissertationQingdao University Qingdao: 2008
|
|
16
|
Neary PM, Hallihan P, Wang JH, Pfirrmann
RW, Bouchier-Hayes DJ and Redmond HP: The evolving role of
taurolidine in cancer therapy. Ann Surg Oncol. 17:1135–1143. 2010.
View Article : Google Scholar
|
|
17
|
Schaffer SW, Jong CJ, Ito T and Azuma J:
Effect of taurine on ischemia-reperfusion injury. Amino Acids.
46:21–30. 2014. View Article : Google Scholar
|
|
18
|
Yin Y, Wen K, Wu Y, Kang Y and Lou J:
Inhibition of sodium current by taurine magnesium coordination
compound prevents cesium chloride-induced arrhythmias. Bio Trace
Elem Res. 146:192–198. 2012. View Article : Google Scholar
|
|
19
|
El Zahraa Z, EI Ashry F, Mahmoud MF, EI
Maraghy NN and Ahmed AF: Effect of Cordyceps sinensis and taurine
either alone or in combination on streptozotocin induced diabetes.
Food Chem Toxicol. 50:1159–1165. 2012. View Article : Google Scholar
|
|
20
|
Son HY, Kim H and H Kwon Y: Taurine
prevents oxidative damage of high glucose-induced cataractogenesis
in isolated rat lenses. J Nutr Sci Vitaminol (Tokyo). 53:324–330.
2007. View Article : Google Scholar
|
|
21
|
Sun Q, Sakaida T, Yue W, Gollin SM and Yu
J: Chemosensitization of head and neck cancer cells by PUMA. Mol
Cancer Ther. 6:3180–3188. 2007. View Article : Google Scholar
|
|
22
|
Chen D, Wei L, Yu J and Zhang L:
Regorafenib inhibits colorectal tumor growth through PUMA-mediated
apoptosis. Clin Cancer Res. 20:3472–3484. 2014. View Article : Google Scholar
|
|
23
|
Liu CJ, Zhang XL, Luo DY, Zhu WF, Wan HF,
Yang JP, Yang XJ and Wan FS: Exogenous p53 upregulated modulator of
apoptosis (PUMA) decreases growth of lung cancer A549 cells. Asian
Pac J Cancer Prev. 16:741–746. 2015. View Article : Google Scholar
|
|
24
|
Yee KS and Vousden KH: Contribution of
membrane localization to the apoptotic activity of PUMA. Apoptosis.
13:87–95. 2008. View Article : Google Scholar
|
|
25
|
Letai A: Puma strikes Bax. J Cell Biol.
185:189–191. 2009. View Article : Google Scholar
|
|
26
|
Adams JM and Cory S: The Bcl-2 apoptotic
switch in cancer development and therapy. Oncogene. 29:1324–1337.
2007. View Article : Google Scholar
|
|
27
|
Schuler M and Green DR: Mechanisms of
p53-dependent apoptosis. Biochem Soc Trans. 29:684–688. 2001.
View Article : Google Scholar
|
|
28
|
Mates JM, Segura JA, Alonso FJ and Marquez
J: Sulphur-containing non enzymatic antioxidants: Therapeutic tools
against cancer. Front Biosci (Schol Ed). 4:722–748. 2012.
View Article : Google Scholar
|
|
29
|
Das J, Ghosh J, Manna P and Sil PC:
Taurine suppresses doxorubicin-triggered oxidative stress and
cardiac apoptosis in rat via up-regulation of PI3-K/Akt and
inhibition of p53, p38-JNK. Biochem Pharmacol. 81:891–909. 2011.
View Article : Google Scholar
|
|
30
|
Yu J and Kim AK: Effect of taurine on
antioxidant enzyme system in B16F10 melanoma cells. Adv Exp Med
Biol. 643:491–499. 2009. View Article : Google Scholar
|
|
31
|
Kim T and Kim AK: Taurine enhances
anticancer activity of cisplatin in human cervical cancer cells.
Adv Exp Med Biol. 776:189–198. 2013. View Article : Google Scholar
|
|
32
|
Abd-Rabou AA, Zoheir KM and Ahmed HH:
Potential impact of curcumin and taurine on human hepatoma cells
using Huh-7 cell line. Clin Biochem. 45:1519–1521. 2012. View Article : Google Scholar
|
|
33
|
Zhang X, Sheng J, Zhang C and Zhao F:
Taurine induces apoptosis in pulmonary artery smooth muscle cells.
Zhongguo Zhong Yao Za Zhi. 37:654–657. 2012.(In Chinese).
|