Introduction
At present, lung cancer is the leading cause of
cancer-associated mortality worldwide in men and women (1). Despite the discovery and application of
new standard therapies, the current 5-year relative survival rate
for lung cancer remains at 18% (1).
Lung cancer is classified into two major categories according to
pathological type: Non-small cell lung cancer (NSCLC) and small
cell carcinoma. NSCLC, the most common type of lung cancer,
accounts for ~80–85% of all lung cancer cases (2). The disease stage at diagnosis, including
the T stage, has been reported to predict the prognosis of NSCLC
(3). However, the effects of gene
expression on tumor growth in NSCLC remain far from being
completely understood.
CD44, a 85–90 kDa transmembrane glycoprotein, is
widely expressed on the surface of cells in the majority of normal
and carcinomatous human tissues (4).
In normal human cells, CD44 has been reported to be involved in
multitudinous cellular functions, including proliferation,
adhesion, migration, hematopoiesis, lymphocyte activation, homing
and extravasation (5). In various
types of human malignancy, high levels of CD44 expression have been
demonstrated to be associated with cancer progression (6–10). In lung
cancer, diverse studies have clarified that the expression of CD44
is associated with tumorigenesis and malignant features of NSCLC
(11,12). Tumor and serum expression of CD44 have
been confirmed as prognostic indicators for patients with NSCLC
(13,14). In addition, overexpression of CD44 is
associated with the occurrence and migration of NSCLC (15). Furthermore, accumulating evidence has
demonstrated that lung cancer cells expressing CD44 tend to have
stem cell-like properties (16–18) and
that cancer stem cells are responsible for driving metastasis
(16). CD44 also contributes to drug
resistance in lung cancer (19).
Although evidence demonstrating that CD44 may act as an oncogene in
NSCLC exists, direct cell experiments are still required to further
confirm the effect of CD44 on the malignant phenotype of lung
cancer cells.
The present study aimed to provide more evidence for
the involvement of CD44 in the progression of lung cancer, and so
evaluated the clinical significance of CD44 expression in patients
with NSCLC and investigated the effect of CD44 on the proliferation
of NSCLC cells.
Materials and methods
Cell lines
NSCLC cell lines, including H1975, H1299, H520,
A549, PC-9 and GLC82, were obtained from American Type Culture
Collection (Manassas, VA, USA). The H520 cell line was derived from
lung squamous cell carcinoma, while the other 5 cell lines were all
derived from adenocarcinoma. Among the 5 lung adenocarcinoma cell
lines, H1650 was derived from bronchoalveolar carcinoma cells from
the pleural effusion of a Caucasian male; H1299 was derived from
the lymph nodes of a male patient with lung adenocarcinoma; A549
was also derived from a Caucasian male with lung adenocarcinoma;
PC9 was established by Tokyo University (Tokyo, Japan) and derived
from adenocarcinoma in pleural effusion; and GLC82 was derived from
a female Chinese patient with lung adenocarcinoma.
Cell culture, antibodies, and
reagents
All 6 cell lines were cultured in RPMI-1640 medium
(Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% fetal bovine serum (FBS; Gibco; Thermo Fisher
Scientific, Inc.), 100 U/ml penicillin and 100 µg/ml streptomycin
(Invitrogen; Thermo Fisher Scientific, Inc.). Cells were incubated
at 37°C with 5% CO2 in a humidified atmosphere.
Monoclonal mouse anti-human CD44 antibodies were purchased from
Abcam (cat no. ab6124; Cambridge, UK).
Tissue specimens and
immunohistochemistry
There were 94 cases of NSCLC tissue specimens
excluding adjacent normal tissues acquired from patients who
underwent surgery at Peking University Cancer Hospital and
Institute (Beijing, China) from July 2013 to October 2015 without
any other treatments prior to surgery. Clinicopathological
characteristics of these patients are presented in Table I with 72 cases of squamous carcinoma,
19 cases of adenocarcinoma, 2 cases of adenosquamous carcinoma and
one carcinoid. Written informed consent was obtained from all
patients included in the present study, and this study was approved
by the Ethics Committee of Peking University Cancer Hospital and
Institute.
 | Table I.Associations between CD44 expression
and clinicopathological features in non-small cell lung cancer
cases. |
Table I.
Associations between CD44 expression
and clinicopathological features in non-small cell lung cancer
cases.
|
| CD44 expression |
|
|---|
|
|
|
|
|---|
| Variable | n (+) | (%) | n (−) | (%) | P-value |
|---|
| Age, years |
|
|
|
| 0.532 |
|
<60 | 30 | 71.4 | 12 | 28.6 |
|
| ≥60 | 34 | 65.4 | 18 | 34.6 |
|
| Sex |
|
|
|
| 1.000 |
| Male | 32 | 68.1 | 15 | 31.9 |
|
|
Female | 32 | 68.1 | 15 | 31.9 |
|
| Smoking |
|
|
|
| 0.791 |
|
Yes | 28 | 66.7 | 14 | 33.3 |
|
| No | 36 | 69.2 | 16 | 30.8 |
|
| Pathology |
|
|
|
| 0.211 |
|
Squamous carcinoma | 51 | 70.8 | 21 | 29.2 |
|
|
Adenocarcinoma | 10 | 52.6 | 9 | 47.4 |
|
|
Adenosquamous carcinoma | 2 | 100.0 | 0 | 0.0 |
|
|
Carcinoid | 1 | 100.0 | 0 | 0.0 |
|
| T-stage |
|
|
|
| 0.032 |
| T1 | 33 | 58.9 | 23 | 41.1 |
|
| T2 | 28 | 80.0 | 7 | 20.0 |
|
| T3 | 3 | 100.0 | 0 | 0.0 |
|
| N stage |
|
|
|
| 0.917 |
| N0 | 37 | 68.5 | 17 | 31.5 |
|
|
N1/2/3 | 27 | 67.5 | 13 | 32.5 |
|
| TNM stage |
|
|
|
| 0.279 |
| I | 34 | 70.8 | 14 | 29.2 |
|
| II | 12 | 54.5 | 10 | 45.5 |
|
|
III | 18 | 75.0 | 6 | 25.0 |
|
Tumor tissues were fixed with 10% formaldehyde
solution overnight at room temperature and then paraffin-embedded
tumors were cut into 4-µm thick sections. Sections were routinely
stained, as described in our previous study (20). Briefly, the sections were immersed in
xylene to remove the paraffin, washed in a graded series of
ethanol, immersed in citrate buffer and then incubated in a
high-pressure sterilization oven for antigen retrieval with citrate
buffer at pH 6.0 for 3 min at 100°C. Endogenous peroxidase activity
was blocked in a blocking solution with 3%
H2O2 in PBS for 10 min at room temperature,
and then the sections were incubated with PBS containing 1% bovine
serum albumin (Amresco, Solon, OH, USA) for 10 min at room
temperature to block non-specific binding. Following washing in
PBS, the tissue sections were incubated at room temperature for 1 h
with antibodies against CD44 (cat no. ab6124; dilution, 1:2,000;
Abcam), followed by incubation with horseradish peroxidase
(HRP)-conjugated goat anti-mouse IgG (cat no. A4416; 1:1,000;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) for 1 h at room
temperature. Then, the slides were visualized with 0.1%
3,3-diaminobenzidine (Sigma-Aldrich; Merck KGaA) for 2 min, and
counterstained with one drop of 1% hematoxylin for 10 min at room
temperature. According to the percentage of cells with complete
membrane staining in the tumor tissue section, the section was
classified as follows: Negative, <15%; weak positive, 15–30%;
moderate positive, 30–75%; and strong positive, 75–95%. In the
present study, CD44 expression was either defined as negative, or
positive (either weak, moderate, or strong positive).
Plasmid construction and cell
transfection
Human CD44 open reading frame mammalian expression
plasmids were purchased from YouBio (Chongqing, China, http://www.youbio.cn/). H1299 cells were seeded onto
6-well plates at 80% confluence, and then transfected with 4.0 µg
of CD44 plasmids or pcDNA3.0 empty plasmids (Invitrogen; Thermo
Fisher Scientific, Inc.) using Lipofectamine® 3000
(Invitrogen; Thermo Fisher Scientific, Inc.) following the
manufacturer's protocol. After 24 h, cells were cultured and
propagated in media containing 500 µg/ml G418 (Sigma-Aldrich; Merck
KGaA) at 37°C with an atmosphere containing 5% CO2 in a
humidified incubator until a stable cell line was established.
Small interfering RNAs (siRNAs) targeting CD44 were designed using
BLOCK-iT™ RNAi Designer (Thermo Fisher Scientific, Inc.)
within open reading frame, with the default criteria on the webpage
(http://rnaidesigner.thermofisher.com/rnaiexpress),
and then synthesized by Guangzhou RiboBio Co., Ltd. (Guangzhou,
China). siRNAs (50 nmol/l) were transfected into A549 cells using
Lipofectamine 3000® (Invitrogen; Thermo Fisher
Scientific, Inc.) following the manufacturer's protocol. The CD44
siRNA target sequences were as follows: siRNA-1,
5′-CUCCCAGUAUGACACAUAUTT-3′; siRNA-2, 5′-GGACCAAUUACCAUAACUATT-3′;
siRNA-3, 5′-GCAGUCAACAGUCGAAGAATT-3′; and NC,
5′-UUCUCCGAACGUGUCACGUTT-3′.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
RNA was extracted using TRIzol (Invitrogen; Thermo
Fisher Scientific, Inc.) according to the manufacturer's protocol.
1 µg total RNA was reverse transcribed using
TransScript® All-in-One First-Strand cDNA Synthesis
SuperMix for PCR (TransGene, Beijing, China, http://www.transgen.com.cn), according to the
manufacturer's protocol. qPCR reactions were performed using a
Light-Cycler 480 Real-Time PCR System (Roche Diagnostics GmbH) and
the LightCycler 480 SYBR Green I Master Mix (Roche Diagnostics
GmbH). GAPDH was used as the internal control. The primers
sequences are as follows: CD44, forward 5′-ACACGAAGGAAAGCAGGACC-3′,
and reverse 5′-TTTGCTCCACCTTCTTGACTC-3′; GAPDH, forward
5′-TGAAGGTCGGAGTCAACGG-3′, and reverse 5′-CTGGAAGATGGTGATGGGATT-3′.
The cycling conditions were as follows: 5 min at 95°C, followed by
42 cycles of 10 sec at 95°C, 30 sec at 60°C, and 20 sec at 72°C.
The relative gene expression levels were calculated by the
2−ΔΔCq method (where ΔCq=Cqtarget-
Cqcontrol) (21).
Western blot
Cells were lysed in RIPA buffer (Roche Diagnostics
GmbH) containing a complete protease inhibitor cocktail for 15 min
on ice. The bicinchoninic acid assay method (Beijing Solarbio
Science & Technology Co., Ltd., Beijing, China) was used to
determinate protein quantity and equivalent amounts of total
proteins (30 µg) were separated by 10% SDS-PAGE and transferred
topolyvinylidene fluoride membranes (Millipore, Billerica, MA,
America). Membranes were blocked with 5% nonfat dried milk
dissolved in TBST containing 0.1% Tween 20 for 1 h at room
temperature and then incubated with following specified antibodies
for 1 h at room temperature: Anti-CD44 (cat no. ab6124; dilution,
1:5,000; Abcam) and mouse anti-GAPDH (cat no. 97166; dilution,
1:5,000; Cell Signaling Technology, Inc., Danvers, MA, USA),
followed by HRP-conjugated goat anti-mouse IgG (cat no. A4416;
dilution, 1:5,000; Sigma-Aldrich; Merck KGaA) for 1 h at room
temperature. Signals were visualized using an enhanced ECL
Chemiluminescence reagent (cat no. WBKLS0500; Merck KGaA) and
detected using AI600 version 1.2.0 on Amersham Imager 600 (GE
Healthcare, Chicago, IL, USA).
Flow cytometry
Briefly, a total of 10,000 live cells were incubated
with anti-CD44 (cat no. ab6124; dilution, 1:100; Abcam) at 37°C for
30 min. Following washing with PBS, cells were then incubated with
FITC-labeled goat anti-mouse IgG (cat no. F0257; dilution, 1:100;
Sigma-Aldrich; Merck KGaA) at 37°C for 30 min. Following washing
three times with PBS, the fluorescence intensity was detected with
the BD Accuri™ C6 Flow Cytometer with BD Accuri C6
software version 1.0.264.21 (BD Bioscience, San Diego, CA,
USA).
Cell proliferation assay
Cells were seeded into 96-well plates (1,500
cells/well). To assess cell proliferation, cells were subjected to
Cell Counting Kit-8 (CCK-8) assays (Dojindo, Kumamoto, Japan) at 0,
12, 24, 36, 48, 60 and 72 h. Each time, supernatant was replaced by
RPMI-1640 medium containing 10% CCK-8 reagent. After 2 h incubation
at 37°C, the absorbance at 450 nm was measured to determine the
number of viable cells, according to the manufacturer's protocol.
The experiment was repeated three times independently. The cell
doubling time (DT) was calculated by the equation:
TD=ΔTx[lg2/(lgNt-lgN0)], where ΔT is the time interval; N0 is the
initial cell number and Nt is the end point cell number.
Clonogenic assay
A total of ~500 cells were seeded into 60-mm dishes,
and the culture medium of RPMI-1640 medium supplemented with 10%
FBS was changed every 3 days. Visible colonies were fixed after 8
days in 4% paraformaldehyde for 15 min at room temperature, stained
with 0.1% crystal violet solution for 30 min at room temperature,
and then washed with PBS. The number of colonies number was counted
by naked eye from three independent experiments.
Statistical analysis
All statistical analysis was performed using SPSS
22.0 software (IBM Corp, Armonk, NY, US). Associations between CD44
expression and clinicopathological factors were analyzed using
Pearson's χ2 test and Fisher's exact tests. The mRNA
levels, colony number and size were presented as the mean ±
standard deviation from three independent experiments. The
difference between two groups was assessed using independent
samples t-test. Dunnett's Multiple Comparison tests were used to
compare differences between treatment groups and the control group
following analysis of variance (ANOVA). Two-way ANOVA tests were
used to analyze the difference of cell viability curves for two
groups. P<0.05 (two-tailed) was considered to indicate a
statistically significant difference.
Results
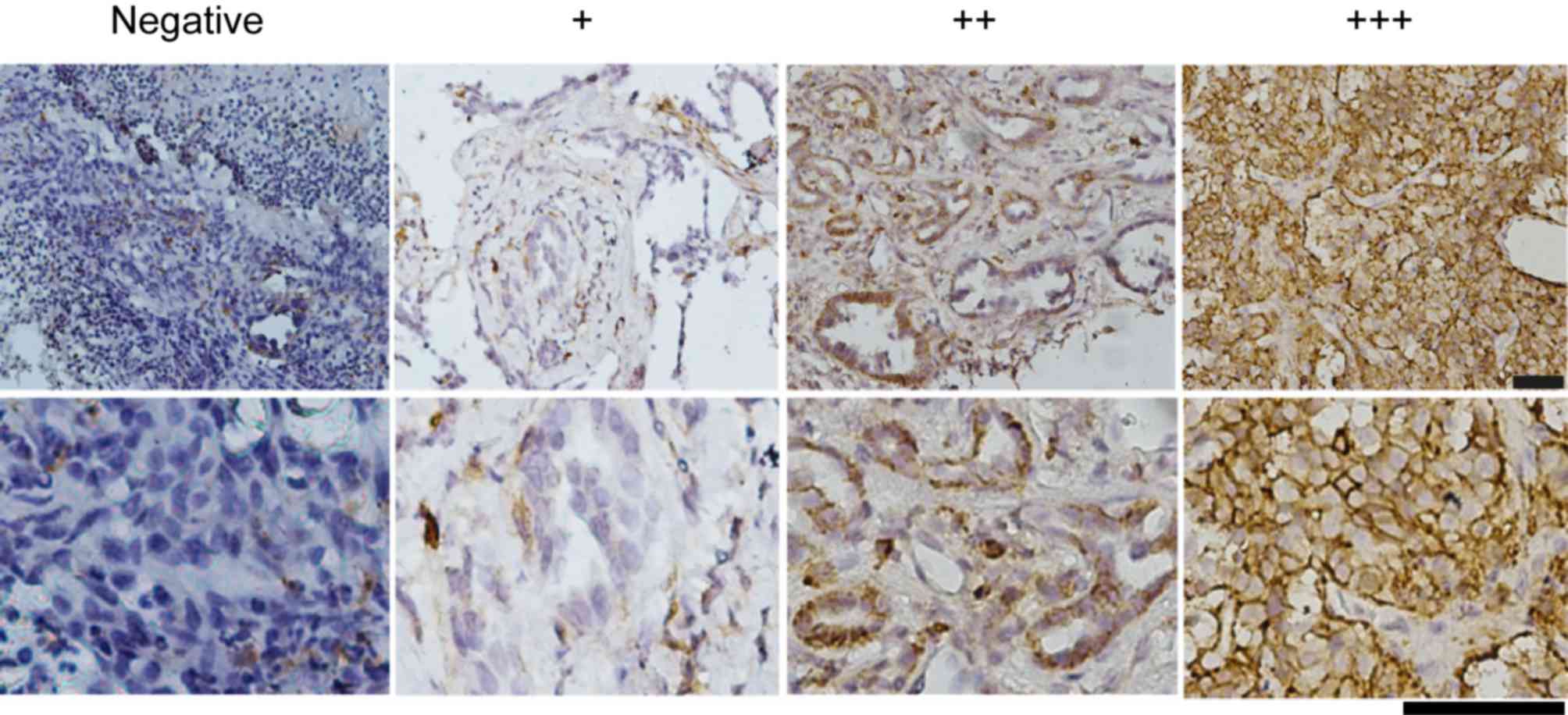
NSCLC tissues express CD44
As presented in Fig.
1, CD44 was predominantly expressed in the cell membrane of
NSCLC cells. CD44 expression was observed in all pathological types
of NSCLC tissue, including squamous cell carcinoma, adenocarcinoma,
adenosquamous carcinoma and carcinoid. The percentage of
CD44-stained tumor cells in the samples ranged from 0–95%. To
facilitate analysis, CD44 expression was classified into two
groups; negative and positive. When >15% of tumor cells stained
positively for CD44 in a sample, it was classified as positive CD44
expression; otherwise it was defined as negative. In total, 64
(68.1%) of 94 NSCLC cases were identified as positive for CD44
expression.
High levels of CD44 expression are
associated with an advanced T stage
To predict the clinical significance of CD44
expression, we analyzed the associations between CD44 expression
and clinicopathological features in NSCLC. There was no significant
difference between CD44 expression and age, sex, smoking status,
pathology type, N classification or TNM stage (22). However, the level of CD44 expression
was positively associated with an advanced T classification
(P<0.05; Table I). A total of
58.9% of cases (33 of 56) stained positive for CD44 in the T1
group, while 80.0% of cases (28 of 35) stained positive for CD44 in
the T2 group, and all cases (3 of 3) stained positive for CD44 in
the T3 group. This result suggested that the higher the T stage
was, the stronger the CD44 staining in the tumor tissue would be,
indicating that CD44 potentially promotes tumor growth.
High levels of CD44 expression are
positively associated with cell proliferation in NSCLC cell
lines
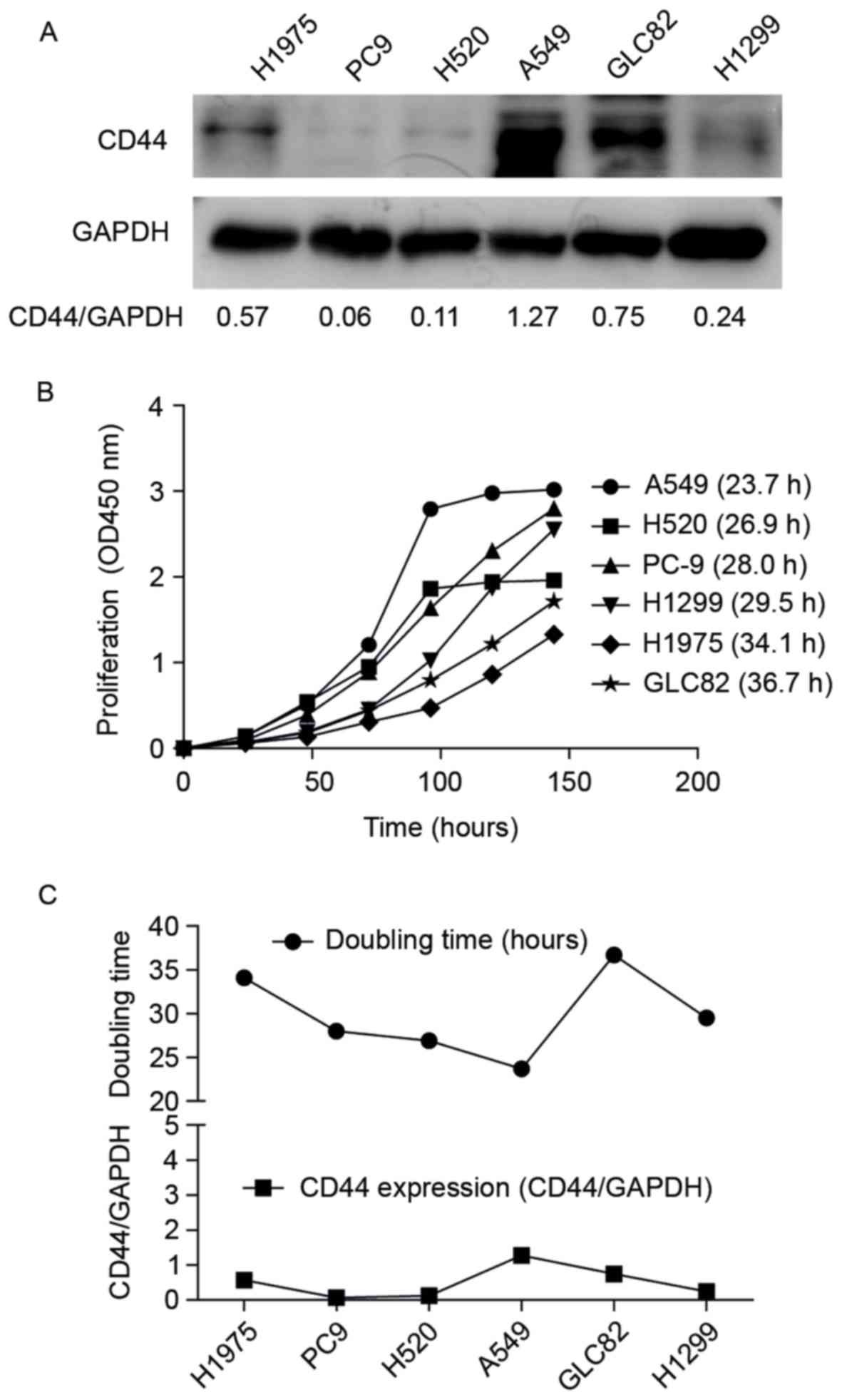
To further investigate the relationship between CD44
expression and cell proliferation, CD44 expression (Fig. 2A) and cell doubling time (Fig. 2B) were determined in NSCLC cell lines.
Among these cells, A549 cells and CLC82 cells had relative high
CD44 expression, while PC9, H520 and H1299 had the relative low
CD44 expression. Thus, H1299 were selected to perform the CD44
over-expression experiments and A549 cells to do the CD44
interference experiments. The data from the present study suggested
that A549 cells had the highest CD44 expression and the shortest
doubling time of the 6 NSCLC cell lines (Fig. 2A and B). The line graph in Fig. 2C also suggested a positive association
between CD44 expression and cell doubling time, indicating that
higher CD44 expression tended to lead to faster cell
proliferation.
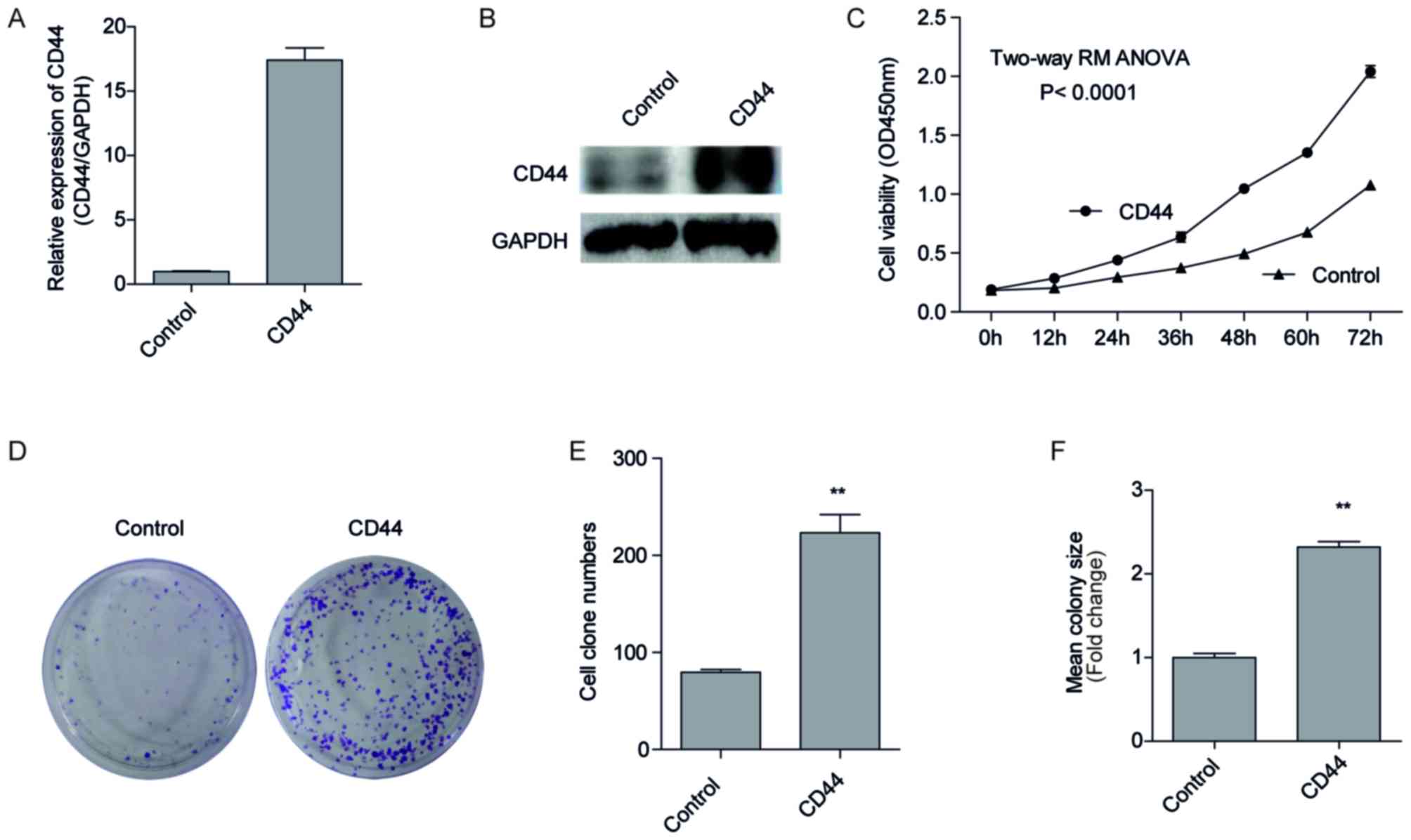
Overexpression of CD44 promotes H1299
cell proliferation
To investigate the forced expression of CD44 on cell
malignancy, we forced expression of CD44 in H1299 cells with a low
endogenous CD44 expression (Fig. 2A).
This was conducted using plasmid transfection and G418 screening, a
pool of H1299 cells that stably overexpressed CD44, named
H1299-CD44, were established, and the negative control cells
transfected with empty vectors were named H1299-control. CD44 mRNA
and protein expression of the two cell lines by RT-qPCR (Fig. 3A) and western blotting (Fig. 3B). As presented in Fig. 3A, the expression of CD44 increased
~17.4 fold in H1299-CD44 cells compared with H1299-control cells.
In addition, the protein expression of CD44 also visibly increased
in H1299-CD44 cells compared with H1299-control cells (Fig. 3B; lane 2), indicating a successful
CD44 overexpression in H1299-CD44 cells. Consistent with this
result, CCK-8 assays suggested that H1299-CD44 cells proliferated
significantly faster than the negative control cells (Fig. 3C; P<0.001). The difference between
H1299-CD44 cells and H1299-control cells became significant 12 h
after seeding the cells. As time increased, the difference became
increasingly significant, and the OD450 value of the
H1299-CD44 cells was almost 2 times that of the H1299-control
cells. These results confirmed our hypothesis that CD44 promoted
cell proliferation in vitro.
Overexpression of CD44 improves the
colony forming ability of H1299 cells
In order to further validate the hypothesis that
CD44 promotes cell proliferation, a clonogenic assay was performed.
A total of 500 cells were seeded on 60-mm plates and cultured for 8
days, with a medium changed each 3 days. As presented in Figs. 3D and E, the number of colonies of
H1299-CD44 cells was significantly increased compared with
H1299-control cells. In addition, the colonies formed by the
H1299-CD44 cells were larger than those formed by the H1299-control
cells (Fig. 3F). These results
suggested that overexpression of CD44 improved the colony forming
ability of H1299 cells.
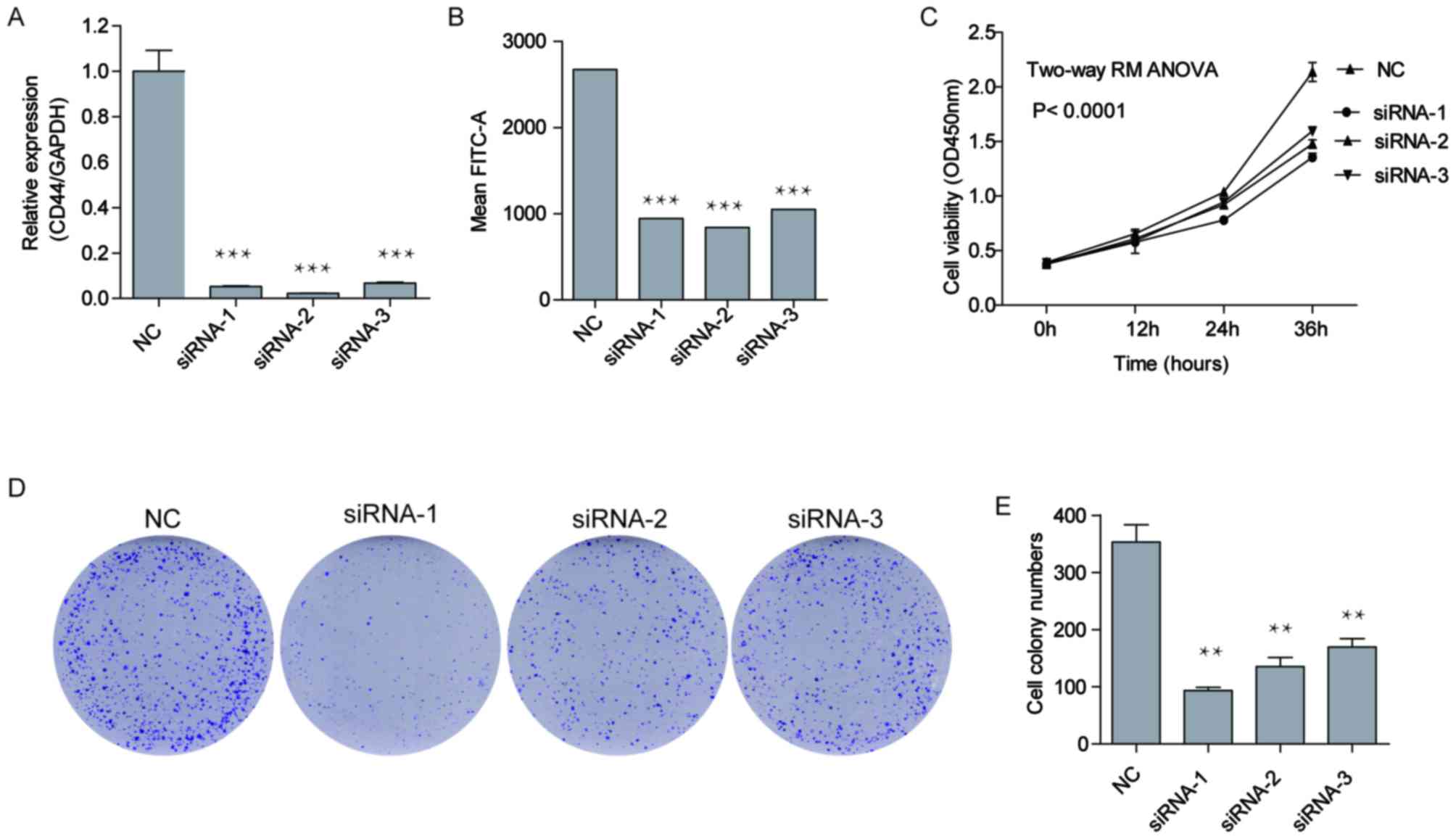
Inhibition of CD44 suppresses the
proliferation and colony formation ability of A549 cells
To further confirm this result, CD44 expression was
knocked down by siRNAs in A549 cells with relatively high levels of
CD44 expression (Fig. 2A). As
presented in Fig. 4A, CD44 expression
was decreased by 94.7, 97.7 and 93.2%, respectively for siRNA-1,
siRNA-2 and siRNA-3, compared with cells transfected with siRNA-NC.
Although the positive CD44 population of the A549 cells did not
alter following siRNA transfection (data not shown), the average
fluorescein intensity of CD44 also decreased by 64.6, 68.5 and
60.7% respectively for siRNA-1, siRNA-2 and siRNA-3, compared with
cells transfected with siRNA-NC (Fig.
4B), indicating that CD44 expression was significantly
inhibited by the siRNAs. As presented in Fig. 4C, A549 cells transfected with CD44
siRNA-1, siRNA-2 and siRNA-3 proliferated more slowly than the
negative control cells (P<0.05). A clonogenic assay was then
performed. The number of cell colonies formed by the
siRNA-transfected cells was significantly decreased compared with
the negative control cells (Fig. 4D and
E; P<0.01). These results suggested that knockdown of CD44
expression suppressed A549 cell proliferation in vitro.
Discussion
In the present study, CD44 expression in NSCLC
tissues was analyzed by immunohistochemistry, and a positive
correlation between CD44 expression and T stage was identified
(P<0.05). A stable CD44-overexpressing cell line, H1299-CD44
cells, and H1299-control cells, were then established. Using
functional experiments, including CCK-8 assays and clonogenic
assays, the data indicated that CD44 promoted NSCLC cell
proliferation.
In previous studies, high expression of CD44 was
hypothesized to be associated with poor prognosis in NSCLC
(14,23). High expression of CD44 was also
reported to be associated with not only occurrence and migration
(15) but also metastasis (24) and drug resistance (19) of NSCLC. In the present study, an
association between a higher CD44-positive expression rate and
higher T stage was observed in NSCLC, which was consistent with the
report from Shinohara et al (14) where significant associations were
observed between CD44 expression and clinicopathological factors
including T stage, N stage, pathological stage and histological
type following immunohistochemical analysis of a cohort consisting
of 261 consecutive patients (12),
but the present study failed to identify a significant association
between CD44 expression and occurrence or metastasis. This may be
due to the small cohort in the present study, so a future study
with a large cohort is still required to confirm the association
between CD44 expression and other clinicopathological prognosis
factors. There are also certain reports indicating that CD44
variant 6 (CD44v6) is associated with disease progression in NSCLC
(13,25,26). Luo
et al (23) reported that high
expression of CD44v6 was associated with histopathological type and
lymph node metastasis by using meta-analysis for a total of 921
patients with NSCLC from ten studies. However, whether the
expression of specific CD44 isoforms predicts prognosis more
effectively than total expression of CD44 is still worthy of future
study. The data from the present study support that CD44 may be a
useful prognostic marker for patients with NSCLC.
The present study provides direct evidence
suggesting that CD44 overexpression increases proliferation, and
knockdown of CD44 reduces proliferation in NSCLC cell lines. A
series of experiments were conducted and the conclusion that CD44
promoted cell proliferation and growth in vitro was drawn.
These results were constant with those of a previous report, where
purified CD44-positive cells were demonstrated to have higher
tumorigenicity than negative cells in vitro and in
vivo (17). With increasing
research focusing on the function of CD44 in cancer, it is
generally accepted that CD44 is a prime therapeutic target for
cancer interventions (27). The
present study supports the hypothesis that CD44 is a potential
therapeutic target for NSCLC. Inhibition of CD44 expression, either
by siRNAs or by CRISPR/Cas9 gene editing targeting NSCLC, may
suppress lung cancer cell proliferation and growth.
Collectively, the results of the present study
suggest that CD44 overexpression is associated with an advanced T
stage, and that CD44 may be a potential candidate target for the
treatment of NSCLC, whereby CD44-dependent cell growth may be
blocked. These results enhance our understanding of the involvement
of CD44 in lung cancer.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81201964, 81772632
and 81773144), the Beijing Municipal Administration of Hospitals
Clinical Medicine Development of Special Funding Support (grant no.
ZYLX201509), Peking University (PKU) 985 Special Funding for
Collaborative Research with PKU Hospitals (grant no. 2013-5-05),
the National High Technology Research and Development Program of
China (863 Program; grant no. 2014AA020602), and the
interdisciplinary medicine Seed Fund of Peking University (grant
no. BMU2018MX019).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Author contributions
BH performed the majority of the experiments. YM,
YY, and LZH participated in certain experiments. HH and JC designed
the experiments and coordinated the project.
Ethics approval and consent to
participate
Written informed consent was obtained from all
patients included in the present study, and this study was approved
by the Ethics Committee of Peking University Cancer Hospital and
Institute.
Consent for publication
All authors consented to publication.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Graham MV, Purdy JA, Emami B, Harms W,
Bosch W, Lockett MA and Perez CA: Clinical dose-volume histogram
analysis for pneumonitis after 3D treatment for non-small cell lung
cancer (NSCLC). Int J Radiat Oncol Biol Phys. 45:323–329. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sterlacci W, Stockinger R, Schmid T,
Bodner J, Hilbe W, Waldthaler C, Oberaigner W, Tzankov A and Fiegl
M: The elderly patient with surgically resected non-small cell lung
cancer-a distinct situation? Exp Gerontol. 47:237–242. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Prochazka L, Tesarik R and Turanek J:
Regulation of alternative splicing of CD44 in cancer. Cell Signal.
26:2234–2239. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Naor D, Sionov RV and Ish-Shalom D: CD44:
Structure, function, and association with the malignant process.
Adv Cancer Res. 71:241–319. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Xu H, Tian Y, Yuan X, Liu Y, Wu H, Liu Q,
Wu GS and Wu K: Enrichment of CD44 in basal-type breast cancer
correlates with EMT, cancer stem cell gene profile, and prognosis.
Onco Targets Ther. 9:431–444. 2016.PubMed/NCBI
|
|
7
|
Aso T, Matsuo M, Kiyohara H, Taguchi K,
Rikimaru F, Shimokawa M, Segawa Y, Higaki Y, Umeno H, Nakashima T
and Masuda M: Induction of CD44 variant 9-expressing cancer stem
cells might attenuate the efficacy of chemoradioselection and
worsens the prognosis of patients with advanced head and neck
cancer. PLoS One. 10:e01165962015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fang M, Wu J, Lai X, Ai H, Tao Y, Zhu B
and Huang L: CD44 and CD44v6 are correlated with gastric cancer
progression and poor patient prognosis: Evidence from 42 studies.
Cell Physiol Biochem. 40:567–578. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sosulski A, Horn H, Zhang L, Coletti C,
Vathipadiekal V, Castro CM, Birrer MJ, Nagano O, Saya H, Lage K, et
al: CD44 splice variant v8-10 as a marker of serous ovarian cancer
prognosis. PLoS One. 11:e01565952016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yikilmaz TN, Dirim A, Ayva ES, Ozdemir H
and Ozkardes H: Clinical use of tumor markers for the detection and
prognosis of bladder carcinoma: A comparison of CD44, cytokeratin
20 and survivin. Urol J. 13:2677–2683. 2016.PubMed/NCBI
|
|
11
|
Jiang H, Zhao W and Shao W: Prognostic
value of CD44 and CD44v6 expression in patients with non-small cell
lung cancer: Meta-analysis. Tumour Biol. 35:7383–7389. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhao S, He JL, Qiu ZX, Chen NY, Luo Z,
Chen BJ and Li WM: Prognostic value of CD44 variant exon 6
expression in non-small cell lung cancer: A meta-analysis. Asian
Pac J Cancer Prev. 15:6761–6766. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Situ D, Long H, Lin P, Zhu Z, Wang J,
Zhang X, Xie Z and Rong T: Expression and prognostic relevance of
CD44v6 in stage I non-small cell lung carcinoma. J Cancer Res Clin
Oncol. 136:1213–1219. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shinohara S, Hanagiri T, Taira A, Takenaka
M, Oka S, Chikaishi Y, Uramoto H, So T, Yamada S and Tanaka F:
Immunohistochemical expression and serum levels of CD44 as
prognostic indicators in patients with non-small cell lung cancer.
Oncology. 90:327–338. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li G, Gao Y, Cui Y, Zhang T, Cui R, Jiang
Y and Shi J: Overexpression of CD44 is associated with the
occurrence and migration of non-small cell lung cancer. Mol Med
Rep. 14:3159–3167. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Su J, Wu S, Wu H, Li L and Guo T: CD44 is
functionally crucial for driving lung cancer stem cells metastasis
through Wnt/β-catenin-FoxM1-twist signaling. Mol Carcinog.
55:1962–1973. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Leung EL, Fiscus RR, Tung JW, Tin VP,
Cheng LC, Sihoe AD, Fink LM, Ma Y and Wong MP: Non-small cell lung
cancer cells expressing CD44 are enriched for stem cell-like
properties. PLoS One. 5:e140622010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Roudi R, Madjd Z, Korourian A, Mehrazma M,
Molanae S, Sabet MN and Shariftabrizi A: Clinical significance of
putative cancer stem cell marker CD44 in different histological
subtypes of lung cancer. Cancer Biomark. 14:457–467. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu J, Xiao Z, Wong SK, Tin VP, Ho KY,
Wang J, Sham MH and Wong MP: Lung cancer tumorigenicity and drug
resistance are maintained through ALDH(hi)CD44 (hi) tumor
initiating cells. Oncotarget. 4:1698–1711. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen J, Xie F, Zhang L and Jiang WG: iASPP
is over-expressed in human non-small cell lung cancer and regulates
the proliferation of lung cancer cells through a p53 associated
pathway. BMC Cancer. 10:6942010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sui X, Jiang W, Chen H, Yang F, Wang J and
Wang Q: Validation of the stage groupings in the eighth edition of
the TNM classification for lung cancer. J Thorac Oncol.
12:1679–1686. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Luo Z, Wu RR, Lv L, Li P, Zhang LY, Hao QL
and Li W: Prognostic value of CD44 expression in non-small cell
lung cancer: A systematic review. Int J Clin Exp Pathol.
7:3632–3646. 2014.PubMed/NCBI
|
|
24
|
Liu Y, Qing H, Su X, Wang C, Li Z and Liu
S: Association of CD44 gene polymorphism with survival of NSCLC and
risk of bone metastasis. Med Sci Monit. 21:2694–2700. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nguyen VN, Mirejovský T, Melinová L and
Mandys V: CD44 and its v6 spliced variant in lung carcinomas:
Relation to NCAM, CEA, EMA and UP1 and prognostic significance.
Neoplasma. 47:400–408. 2000.PubMed/NCBI
|
|
26
|
Afify AM, Tate S, Durbin-Johnson B, Rocke
DM and Konia T: Expression of CD44s and CD44v6 in lung cancer and
their correlation with prognostic factors. Int J Biol Markers.
26:50–57. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jordan AR, Racine RR, Hennig MJ and
Lokeshwar VB: The role of CD44 in disease pathophysiology and
targeted treatment. Front Immunol. 6:1822015. View Article : Google Scholar : PubMed/NCBI
|